Back to Journals » Journal of Experimental Pharmacology » Volume 16
Investigating Bioavailability of Curcumin and Piperine Combination in Comparison to Turmeric Rhizomes: An in vitro Study
Authors Pratti VL, Thomas M, Bhoite R, Satyavrat V
Received 12 August 2023
Accepted for publication 18 November 2023
Published 30 January 2024 Volume 2024:16 Pages 37—47
DOI https://doi.org/10.2147/JEP.S427818
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Varalakshmi Lalithya Pratti, Muthumani Thomas, Rachana Bhoite, Vinita Satyavrat
Dr. Reddy’s Laboratories Ltd, Ameerpet, Hyderabad, India
Correspondence: Varalakshmi Lalithya Pratti, Dr. Reddy’s Laboratories Ltd, Ameerpet, Hyderabad, India, Tel +91 40 49048400, Fax +91 40 49048800, Email [email protected]
Purpose: To assess the permeability of the test item (a combination of curcumin and piperine) and a reference item (dried and crushed turmeric rhizomes) using a combination of Caco-2 cell monolayer permeability assay and liquid chromatography-tandem mass spectrometry.
Methodology: In the Caco-2 cell assay, a transport buffer was prepared, and stock solutions of test and reference items were made. Caco-2 cells were cultured on transwell plates. Permeability assays were conducted for 2 and 6 hours, followed by post-experiment testing for assessing the monolayer integrity. LC-MS/MS (Liquid Chromatography with tandem mass spectrometry) analysis was performed to calculate apparent permeability of each item.
Results: The test item was undetectable at the end of 2 hours of permeability assay. Further, after 6 hours of permeability assay, the permeability of both test and reference item was found to be low.
Conclusion: The results showed that the curcumin and piperine combination had low permeability of curcumin in vitro as compared to the dried and crushed turmeric rhizomes. This could predict the low bioavailability of curcumin in vivo when co-administered with piperine.
Keywords: Caco-2 cells, curcumin, piperine, permeability, bioavailability
Introduction
Curcumin, a naturally occurring yellow polyphenol, represents a prominent bioactive constituent derived from the rhizomes of Curcuma longa L. commonly known as turmeric. Throughout centuries, curcumin has found extensive utilization in the realms of traditional Chinese medicine and Ayurveda.1,2
Curcumin is known for its multiple pharmacologic properties as demonstrated in Figure 1. Curcumin has properties such as immunoregulation, free radical scavenging, and inflammation inhibition. It also exhibits antitumor and antiangiogenic properties and has demonstrated both preventive and therapeutic effects on various types of cancers.3,4 It has also been shown to exhibit antidiabetic properties and may improve glycemic control and blood lipid profiles (Figure 1).5
 |
Figure 1 Health benefits of curcumin. |
The pharmacologic benefits of curcumin have been investigated in various diseases and illness conditions. Some clinical studies have also established its safety and tolerability upon consumption.6–8 However, curcumin being lipophilic in nature and insoluble in water possesses limited bioavailability.6,9 In a clinical study involving healthy individuals, the administration of a high dose of curcumin (12 g/day) resulted in a significantly low increase in serum curcumin levels.9 This may be attributed to its poor absorption, rapid metabolism, and rapid elimination and clearance from the body. Curcumin’s utility as a medicinal agent is hampered by its limited bioavailability.10,11
Researchers globally have undertaken a multitude of endeavors aimed at augmenting the bioavailability of curcumin through the concurrent administration of various bioactive compounds. Adjuvants such as piperine, quercetin, and genistein have demonstrated efficacy in enhancing the bioavailability of curcumin.12 It has been reported that piperine can increase the bioavailability of curcumin by 20-folds.13 Studies also show that the systemic bioavailability of curcumin can be increased by as much as 154% when piperine is administered together with large dosages of oral curcumin (2000 mg/kg).14 As the bioavailability of curcumin is of great interest, the usage of Caco-2 (human adenocarcinoma cell line) experiments for its permeability test looks promising.1,12,15
Caco-2 cells, originating from human colon carcinoma, exhibit spontaneous differentiation, culminating in the formation of a monolayer that emulates key characteristics commonly observed in absorptive enterocytes of the small intestine, including the presence of a brush border layer.16 When cultivated as a single layer, Caco-2 cells undergo this differentiation process and establish tight intercellular junctions, rendering them particularly suitable for investigations into substance permeability across intercellular gaps. Furthermore, Caco-2 cells harbor a diverse array of proteins responsible for the transport, efflux, and enzymatic modification of compounds, thereby allowing them to faithfully simulate a wide spectrum of pathways governing substance transport and metabolic processes. Consequently, Caco-2 cell monolayers offer a highly representative model closely resembling the epithelial lining of the human intestine in multiple facets.17 The Caco-2 cell monolayer permeability model, originally developed by Fogh in 1947, has persisted as an invaluable in vitro methodology for the evaluation of the permeability characteristics of a diverse spectrum of natural and synthetic compounds. This model serves as a predictive tool for estimating the bioavailability of these compounds within the human organism.18 The US Food and Drug Administration has recommended employing this approach to evaluate the bioavailability of drugs/compounds.19 Moreover, the utilization of Caco-2 cell monolayers is commonplace in nutrient absorption investigations for the precise elucidation of essential facets related to nutrient uptake and translocation across epithelial cells.20 Thus, Caco-2 cell lines were selected as the experimental model for the present study.
Liquid chromatography with tandem mass spectrometry (LC-MS/MS) is a common, efficient tool used alongside Caco-2 cell monolayer assays. It reduces chemical usage and enables simultaneous analysis of multiple samples post-permeability testing.21
In this study, the Caco-2 cell monolayer permeability assay was employed, coupled with LC-MS/MS analysis. This combination was utilized to evaluate the in vitro permeability of the test compound (a combination of curcumin and piperine) and the reference substance (dried and crushed turmeric root).
Materials and Methods
Materials
The mixture of curcumin and piperine was used as the test item, and the commercial sample was sourced from the market which had the label claim of 30 mg per 3g of curcumin and 3 mg per 3g of piperine. This choice was made because the product blend was easily available in the market round the year. The molecular weight of the test item was recorded to be 368.38 g/mol. The reference item, namely dried and crushed turmeric rhizomes powder, also had similar molecular weights. The controls—atenolol (molecular weight, 266.336 g/mol) and propranolol (molecular weight, 259.34 g/mol), were sourced from Clearsynth Labs and Sigma Aldrich, respectively. The choice of atenolol and propranolol as controls is made as their chemical structures are well-characterized, enabling precise identification and quantification in complex biological matrices. Further, they have been noted to have clear human absorption of 50% and 90%, respectively.22 The Caco-2 cell line used for the Caco-2 permeability assay was sourced from American Type Culture Collection.
Commercially available Hank’s balanced salt solution (HBSS) sachets (9.7 g/L), Milli Q water, dimethyl sulfoxide (DMSO), 4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid (HEPES), sodium bicarbonate, and glucose were used to prepare transport buffers to prepare primary and secondary working stock solutions.
Additionally, 0.2% formic acid, acetonitrile, curcumin, and curcumin-D6 were used for the LC-MS/MS analysis (Appendix 1).
Methods
Stock Solution Preparation for Caco-2 Cell Membrane Monolayer Assay
A transport buffer with pH 7.4 was prepared by adding a commercially available HBSS sachet (9.7 g/L) to Milli Q water. Additional components including sodium bicarbonate, HEPES, and glucose were also added. The solution was adjusted to pH 7.4 and volume 1L with NaOH and stored at 2°C ─ 8°C until use.
The test item containing the mixture of curcumin and piperine was procured commercially from the shelf and was used in the present study. Briefly, one sachet, that is, 3 g of the test item with a 10:1 ratio of curcumin and piperine, respectively, was added to 200 mL of transport buffer and mixed thoroughly to prepare a primary stock solution of the test item. Similarly, a primary stock solution of the reference item was prepared by adding 50.17 mg of the reference item to 3.345 mL of the transport buffer and mixing thoroughly.
Working stock solutions of both test and reference items (50 μM) were prepared by diluting the primary stocks in the transport buffer. Controls were also prepared, with primary stock solutions of atenolol and propranolol (10 μM) being prepared by dissolving 5.011 mg and 5.006 mg of each substance in 1.881 mL and 1.930 mL of DMSO, respectively. Secondary working stock solutions (1000 μM) were prepared by adding 100 μL of each primary stock to 900 μL of DMSO.
Cell Culture for Assay
Eagle’s minimum essential medium supplemented with 20% fetal bovine serum, 100 M nonessential amino acids, 100 IU/mL penicillin, and 100 g/mL streptomycin was used to culture Caco-2 cells at 37°C ± 1°C in a 5% ± 1% CO2 humidified environment in a tissue culture flask until they reached confluence. Cells were then harvested using 0.25% trypsin/EDTA and seeded at a concentration of −5 × 104 cells/well in a 24-well transwell plate (pore size, 0.4 µm; polycarbonate filter membrane surface area, 0.7 cm2). The cells were allowed to propagate on the filter membranes in a humidified environment with 5% CO2 at 37°C. The culture medium was renewed at least three times per week. On day 19, the cell monolayers from the wells that had a transepithelial electrical resistance (TEER) value of more than 250 ohm.cm2 (omega centimeter) in the permeability study were selected for further experiments.
Permeability Assay
The qualified transwell plates with Caco-2 monolayers were then used for the permeability assay. Rows 1 and 3 had four replicates each for the test item. Similarly, rows 2 and 4 had four replicates each for the reference item. Rows 1 and 2 were used for the 2-hour experiment and rows 3 and 4 for the 6-hour permeability experiment, respectively. Rows 5 and 6 had replicates for the controls and a blank well. Appendix 2 summarizes the transwell plate map.
Working stocks of the test item, reference item, and control were prepared with concentrations of 50 M (molar), 50 M, and 10 M, respectively, using the transport buffer. The sampling volume was 200 L, and it was drawn from the basolateral compartment after each sampling time point. This volume was replenished with fresh HBSS buffer. The permeability experiment was conducted for 2 hours for the test item, reference item, and control. The sampling time points were 30, 60, 90, and 120 minutes. Simultaneously, another permeability experiment was conducted for the test and reference items for 6 hours. The sampling time points were 1, 2, 3, 4, 5, and 6 hours (the sample was drawn and replaced with an HBSS buffer every hour).
The post-experiment (PE) test was conducted after the permeability assay to check the monolayer integrity. The basolateral and apical buffers were replaced with fresh buffers containing Lucifer yellow. Membrane integrity was then measured using TEER values. The PE samples were collected in 60 minutes and analyzed with a suitable analytical mode. The permeability test flowchart is presented in Figure 2.
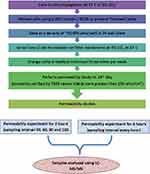 |
Figure 2 Flowchart representing permeability test experiment. |
LC-MS/MS Assay
Sample and Reagent Preparation
Initially prepare 0.2% formic acid by adding 2 mL of formic acid to 500 mL of Milli-Q water and adjusting the volume to 1 L with Milli-Q water. Mobile phase solution was prepared by adding 700 mL of acetonitrile to 300 mL of 0.2% formic acid solution. Sixty percent acetonitrile in Milli-Q water diluent solution was prepared with 60 mL acetonitrile and 40 mL Milli-Q water.
Curcumin standard stock solution was prepared by dissolving 2 mg of curcumin working standard in 1 mL of DMSO in a 2-mL volumetric flask. The volume was adjusted to the mark with DMSO. Similarly, the curcumin-D6 standard stock solution was prepared by dissolving 2 mg of curcumin-D6 working standard in 1 mL of DMSO and adjusting the volume to 2 mL. Both solutions were stored at 2°C–8°C.
A rinsing solution was prepared by mixing 500 mL of methanol with 500 mL of Milli-Q water and stored at ambient temperature.
Methodology
All samples from the permeability assay were analyzed using the LC-MS/MS method. The EXION LC system was paired with a tandem mass spectrometer API 5500. The turbo ion spray ion source was used in negative ion modes. Chromatography was achieved on an Agilent XDB C18, 4.6×150 mm, 3.5-μm column. Samples were chromatographed with the conditions mentioned in Supplementary Table 1. Sample analysis was performed to determine curcumin in samples. The concentration of standards ranged from 0.1 to 50 μM for timepoint samples. The mobile phase was delivered at a flow rate of 0.800 mL/min into the mass spectrometer electrospray ionization chamber. The sample volume was 10 μL. The parameters for the chromatography and spectrometry conditions are listed in Supplementary Table 1 and Supplementary Table 2, respectively.
Extraction Process
To determine the rate of transport of the test and reference item, the extraction procedure was carried out before loading samples onto the LC-MS/MS system.
Calibration curve standard and quality control were spiked in the blank buffer (after it was retrieved from the deep freezer and thawed). Further, 50 mL of blank buffer was added to two vials labeled as blank and blank + ISTD (Internal standard). 50 μL of the corresponding sample was transferred to the vials and the vials were vortexed.
0.150 mL of ISTD mix (25 μM of curcumin-D6) acetonitrile was added to all samples and the samples were kept on the shaker for 5 minutes to ensure the complete mixing of contents. The samples were centrifuged at 4000 rpm at 20°C for 5 minutes. The supernatant layer was transferred into the vials and loaded into autosampler vials and 10 μL was injected into the LC-MS/MS system.
Data Analysis
All the chromatograms obtained from the LC-MS/MS assay were analyzed using the analyst 1.6.3. version software.
The rate of test/reference item/control appearance on the basolateral side of the Caco-2 cell monolayer transwells (dQ/dt) was calculated by plotting a graph for time vs the cumulative amount of test/reference item/control at different sampling time points of the permeability experiment.
Apparent permeability (Papp) was calculated in the unit of centimeters/second (cm/s) using the following equation:
Papp = dQ/dT × 1/Co × 1/A
Co = Initial concentration of the compound in the apical compartment of the well
A = Surface area of the trans well
PE percent Lucifer yellow permeability was calculated using the following formula:
% Permeability = (Sample − Blank/Lucifer Yellow − Blank) x 100
Results
Initial TEER Values
The initial TEER values of the transwell plates used for the test item, reference item, and control (atenolol and propranolol) were found to be more than 250 ohm.cm2. Thus, they were qualified for the permeability experiment. The individual TEER values for the transwell plates used for the 2-hour and 6-hour permeability experiments are mentioned in Figure 3.
 |
Figure 3 Initial TEER values of the transwell plates qualified for the permeability experiment of the test item, reference item, atenolol, and propranolol. |
Permeability Study
The permeability results were obtained using the LC-MS/MS analysis study for the test item, reference item, and control items at different sampling time points. The results were used to calculate Papp values for all the items.
Two-Hour Permeability Experiment
The Papp values of all samples during different time points of the 2-hour permeability experiment are highlighted in Figure 3. For the control items—propranolol and atenolol—the average Papp values were 26.45 E−6 cm/sec and 0.27 E−6 cm/sec, respectively, during the 2-hour sampling time points. Thus, the Papp of propranolol was classified as high and that of atenolol was classified as low.
Till 2 hours after the permeability assay, the permeated amount of the test item was undetectable Hence, the Papp values for the test item were not calculated. The average Papp values of reference items, namely turmeric, demethoxycurcumin, and bisdemethoxycurcumin, were measured until 2 hours of sampling, with values of 0.11 E−6 cm/sec, 0.73 E−6 cm/sec, and 2.34 E−6 cm/sec, respectively. The Papp values of reference items and standards (demethoxycurcumin and bisdemethoxycurcumin) were found to be low.
Six-Hour Permeability Experiment
The average Papp values of the test item, reference item, and standards (demethoxycurcumin and bisdemethoxycurcumin) during the 6-hour permeability experiment are 0 E−6 cm/sec, 0.01 E−6 cm/sec, 1.66 E−6 cm/sec, and 5.23 E−6 cm/sec, respectively (Figure 4). The Papp values of reference items and standards (demethoxycurcumin and bisdemethoxycurcumin) were found to be low during this experiment. The average Papp values for the controls, namely atenolol and propranolol, were not calculated in the 6-hour permeability experiment.
Post-Experiment Test
The PE test was conducted to check the monolayer integrity using Lucifer yellow. The results showed that the percentage of Lucifer yellow transport was <3%, and PE TEER values were more than 250 ohm.cm2 (Figures 5 and 6). This showed that the Caco-2 cell membranes on the transwell plate were intact after the experiment. However, it was observed that the percent Lucifer yellow transport and PE TEER values of the test item replicate 3 and reference item replicate 2 of the 6-hour experiment were found to be more than the cutoff values (percent Lucifer yellow transport, 3%; TEER value, 250 ohm.cm2). Therefore, the data from these wells were not used for Papp calculations.
Discussion
The poor bioavailability of curcumin has been a hurdle in its use as a therapeutic agent for various health benefits. The systemic bioavailability of curcumin after oral intake can be expected to be virtually zero.19 Hence, many attempts are being made to improve the bioavailability of curcumin. One of them has been the co-administration of curcumin and piperine in different formulations.
Recently, a Caco-2 cell monolayer permeability assay was conducted to evaluate the efficacy of two distinct formulations aimed at augmenting curcumin’s bioavailability. These formulations consisted of a curcumin-phospholipid complex and a turmerone/piperine-enhanced curcumin product. Notably, the combination of 25 mg of piperine and 2 g of curcumin utilized in this study revealed a substantial reduction in curcumin efflux within the curcumin and piperine formulation, indicative of enhanced curcumin absorption. Both of these formulations have shown the potential to enhance curcumin bioavailability.23
Several human studies have demonstrated a notable augmentation in curcumin bioavailability achieved through the co-administration of curcumin and piperine in varying ratios. Findings indicate that the concurrent administration of 2 g of curcumin and 5 mg of piperine resulted in approximately a twofold increase in curcumin’s bioavailability. Furthermore, the co-treatment of 2 g of curcumin in conjunction with 20 mg of piperine has exhibited a substantial enhancement in curcumin bioavailability compared to the administration of curcumin alone.13,24
However, the results of one of the most recent investigations were in conflict. Following the concurrent delivery of 12 g of curcumin and 5 mg of piperine, the researchers found no evidence of higher curcumin bioavailability.25
Numerous endeavors have been undertaken to enhance the bioavailability of curcumin through alterations in its formulation when combined with piperine. In one such study, which yielded elevated curcumin bioavailability, emphasis was placed on the co-administration of piperine and curcumin within an oil-in-water formulation stabilized by Poloxamer 407.26 Co-amorphous solids of curcumin and piperine have also been studied to improve the dissolution ability, inhibit rapid metabolism, and facilitate membrane permeability of curcumin, resulting in increased bioavailability of curcumin.27 Further, the co-administration of curcumin with piperic acid compared to piperine has also shown promising potential for increasing curcumin bioavailability.28
In this study, we employed an in vitro Caco-2 cell monolayer permeability assay to extrapolate the in vivo bioavailability of curcumin from formulations containing curcumin and piperine. Specifically, we evaluated the bioavailability of curcumin within a formulation containing 3 mg of piperine per 30 mg of curcumin. Our approach involved a combination of the Caco-2 cell monolayer permeability assay and LC-MS/MS analysis, enabling us to assess the permeability of the test compound (comprising curcumin and piperine) in comparison to a reference material (dried and crushed turmeric rhizomes). Notably, our findings revealed that the curcumin and piperine combination exhibited reduced in vitro curcumin permeability when contrasted with dried and crushed turmeric rhizomes. This outcome suggests a potential limitation in the in vivo bioavailability of curcumin when co-administered with piperine.
With ever evolving researches, new strategies are being developed to improve the bioavailability and absorption of curcumin as compared with the curcumin and piperine combination that was used in the earlier days. Some of the effective strategies include nanoparticle-based delivery systems, liposomal formulations, and absorption enhancers.12 Nanoparticle-based curcumin formulations have exhibited 69.78 times higher bioavailability than free curcumin, whereas liposomal formulations increased bioavailability by 2.35–7.76 times compared to normal suspensions. Additionally, xanthan–chitosan nanofibers increased curcumin bioavailability by three- to fourfold compared to free curcumin.29,30 These approaches have shown promise in enhancing the therapeutic efficacy of curcumin and turmeric.12
As per the published studies/data (in vitro, preclinical, clinical), it has been shown that piperine when added to curcumin improves bioavailability of curcumin. Alternatively, increasing the piperine levels in curcumin and piperine combination might be an effective strategy to improve curcumin bioavailability.14 These results pave the way to study the curcumin and piperine combination in detail for better in vivo bioavailability of curcumin.
Limitations
The present study has been exclusively conducted utilizing Caco-2 cancer cell lines. Nonetheless, the applicability of this experimental approach may be expanded to include various other cancer cell lines and human cellular models. Additionally, exploring the bioavailability of the curcumin-piperine mixture in in vivo settings using animal models is a promising avenue for further investigation.
Conclusion
Thus, the results from this study showed that the curcumin and piperine combination had low permeability of curcumin in vitro as compared to the dried and crushed turmeric rhizomes. This could predict the low bioavailability of curcumin in vivo when co-administered with piperine. With evolving research, new strategies are being developed for improving curcumin bioavailability to use it effectively as a therapeutic agent. Study findings have reported that piperine supplementation enhances the serum concentration, extent of absorption, and bioavailability of curcumin. However, research is still required to obtain the maximum bioavailability of curcumin along with piperine. Studies around this will help to improve the bioavailability of curcumin for better biological applications.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank Vimta labs for conducting the study and BioQuest Solutions for their editorial support.
Funding
Dr. Reddy’s Laboratories Ltd. funded this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yu H, Huang Q. Investigation of the absorption mechanism of solubilized curcumin using Caco-2 cell monolayers. J Agric Food Chem. 2011;59(17):9120–9126. doi:10.1021/jf201451m
2. Sharifi-Rad J, Rayess YE, Rizk AA, et al. Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharmacol. 2020;11:01021. doi:10.3389/fphar.2020.01021
3. Zeng L, Yang T, Yang K, et al. Curcumin and Curcuma longa Extract in the treatment of 10 types of autoimmune diseases: a systematic review and meta-analysis of 31 randomized controlled trials. Front Immunol. 2022;13:896476. doi:10.3389/fimmu.2022.896476
4. Mansouri K, Rasoulpoor S, Daneshkhah A, et al. Clinical effects of curcumin in enhancing cancer therapy: a systematic review. BMC Cancer. 2020;20(1):791. doi:10.1186/s12885-020-07256-8
5. Chien YJ, Chang CY, Wu MY, Chen CH, Horng YS, Wu HC. Effects of curcumin on glycemic control and lipid profile in polycystic ovary syndrome: systematic review with meta-analysis and trial sequential analysis. Nutrients. 2021;13(2):684. doi:10.3390/nu13020684
6. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi:10.1021/mp700113r
7. Pivari F, Mingione A, Piazzini G, et al. Curcumin supplementation (Meriva®) modulates inflammation, lipid peroxidation and gut microbiota composition in chronic kidney disease. Nutrients. 2022;14(1):231. doi:10.3390/nu14010231
8. Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of turmeric (Curcuma longa). J Altern Complement Med N Y N. 2003;9(1):161–168. doi:10.1089/107555303321223035
9. Lao CD, Ruffin MT, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6(1):10. doi:10.1186/1472-6882-6-10
10. Liu W, Zhai Y, Heng X, et al. Oral bioavailability of curcumin: problems and advancements. J Drug Target. 2016;24(8):694–702. doi:10.3109/1061186X.2016.1157883
11. Ahmad S, Hafeez A. Formulation and Development of Curcumin–Piperine-Loaded S-SNEDDS for the Treatment of Alzheimer’s Disease. Mol Neurobiol. 2023;60(2):1067–1082. doi:10.1007/s12035-022-03089-7
12. Faralli A, Shekarforoush E, Ajalloueian F, Mendes AC, Chronakis IS. In vitro permeability enhancement of curcumin across Caco-2 cells monolayers using electrospun xanthan-chitosan nanofibers. Carbohydr Polym. 2019;206:38–47. doi:10.1016/j.carbpol.2018.10.073
13. Sharma V, Nehru B, Munshi A, Jyothy A. Antioxidant potential of curcumin against oxidative insult induced by pentylenetetrazol in epileptic rats. Methods Find Exp Clin Pharmacol. 2010;32(4):227–232. doi:10.1358/mf.2010.32.4.1452090
14. Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi:10.1055/s-2006-957450
15. Frank J, Schiborr C, Kocher A, et al. Transepithelial transport of curcumin in Caco-2 cells is significantly enhanced by micellar solubilisation. Plant Foods Hum Nutr Dordr Neth. 2017;72(1):48–53. doi:10.1007/s11130-016-0587-9
16. van Breemen RB, Li Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin Drug Metab Toxicol. 2005;1(2):175–185. doi:10.1517/17425255.1.2.175
17. Verhoeckx K, Cotter P, López-Expósito I, et al. The impact of food bioactives on health: in vitro and ex vivo models. Expert Opin Drug Metab Toxicol. 2005;1(2):175–185.
18. Ahmed I, Leach DN, Wohlmuth H, De Voss JJ, Blanchfield JT. Caco-2 cell permeability of flavonoids and saponins from Gynostemma pentaphyllum: the immortal herb. ACS Omega. 2020;5(34):21561–21569. doi:10.1021/acsomega.0c02180
19. Dempe JS, Scheerle RK, Pfeiffer E, Metzler M. Metabolism and permeability of curcumin in cultured Caco-2 cells. Mol Nutr Food Res. 2013;57(9):1543–1549. doi:10.1002/mnfr.201200113
20. Glahn R. The use of Caco-2 cells in defining nutrient bioavailability: application to iron bioavailability of foods. In: McClements DJ, Decker EA, editors. Designing Functional Foods. Elsevier; 2009:340–361.
21. Logoyda L, Piponski M, Kovalenko S, et al. Method development for the quantitative determination of captopril from Caco-2 cell monolayers by using LC-MS/MS. Pharmacia. 2021;68(1):61–67. doi:10.3897/pharmacia.68.e52077
22. Zhao YH, Le J, Abraham MH, et al. Evaluation of human intestinal absorption data and subsequent derivation of a quantitative structure-activity relationship (QSAR) with the Abraham descriptors. J Pharm Sci. 2002;91(2):605. doi:10.1002/jps.10118
23. Mc Lellan A. Comparison of Two Commercial Preparations of Curcumin using the Caco-2 in vitro Assay of Human Intestinal Permeability. J Restor Med. 2018;7:1–8. doi:10.14200/jrm.2018.7.0101
24. Majeed M, Badmaev V, Rajendran R Use of piperine to increase the bioavailability of nutritional compounds. U.S. Patent 5,536,506. 1996.
25. Klickovic U, Doberer D, Gouya G, et al. Human pharmacokinetics of high dose oral curcumin and its effect on heme oxygenase-1 expression in healthy male subjects. BioMed Res Int. 2014;2014:458592. doi:10.1155/2014/458592
26. Gülseren I, Guri A, Corredig M. Effect of interfacial composition on uptake of curcumin-piperine mixtures in oil in water emulsions by Caco-2 cells. Food Funct. 2014;5(6):1218–1223. doi:10.1039/c3fo60554j
27. Gopi S, Jacob J, George R, et al. An in-vitro study in the enhancement of curcumin permeability with piperic acid across Caco-2 cells. EJPMR. 2016;3(3):203–205.
28. Wang R, Han J, Jiang A, et al. Involvement of metabolism-permeability in enhancing the oral bioavailability of curcumin in excipient-free solid dispersions co-formed with piperine. Int J Pharm. 2019;561:9–18. doi:10.1016/j.ijpharm.2019.02.027
29. Gupta T, Singh J, Kaur S, Sandhu S, Singh G, Kaur IP. Enhancing bioavailability and stability of curcumin using solid lipid nanoparticles (CLEN): a covenant for its effectiveness. Front Bioeng Biotechnol. 2020;8:879. doi:10.3389/fbioe.2020.00879
30. Li C, Zhang Y, Su T, Feng L, Long Y, Chen Z. Silica-coated flexible liposomes as a nanohybrid delivery system for enhanced oral bioavailability of curcumin. Int J Nanomed. 2012;7:5995–6002. doi:10.2147/IJN.S38043
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



