Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
Immunotherapy Efficacy in Advanced Hepatocellular Carcinoma in a Diverse and Underserved Population in the United States
Authors Bteich F, Desai K, Zhang C, Kaur A, Levy RA, Bioh L, Wang A, Sultana S , Kaubisch A, Kinkhabwala M, Bellemare S, Fidvi S , Kanmaniraja D, Berkenblit R, Moon JY, Adedimeji A, Tow CY, Saenger Y
Received 19 September 2023
Accepted for publication 7 November 2023
Published 3 February 2024 Volume 2024:11 Pages 257—269
DOI https://doi.org/10.2147/JHC.S436804
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr David Gerber
Fernand Bteich,1 Kush Desai,1 Chenxin Zhang,1,2 Anahat Kaur,3 Rachel A Levy,1 Lydia Bioh,1 Aaron Wang,1 Sharmin Sultana,1 Andreas Kaubisch,1 Milan Kinkhabwala,1,4 Sarah Bellemare,1,4 Shabnam Fidvi,5 Devaraju Kanmaniraja,5 Robert Berkenblit,5 Jee-Young Moon,1,2 Adebola Adedimeji,2 Clara Y Tow,6 Yvonne Saenger1
1Montefiore Einstein Comprehensive Cancer Center, Bronx, NY, USA; 2Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA; 3Department of Medicine, Division of Medical Oncology, Jacobi Medical Center, Bronx, NY, USA; 4Department of General Surgery, Division of Abdominal Transplantation, Montefiore Medical Center, Bronx, NY, USA; 5Department of Radiology, Albert Einstein College of Medicine, Bronx, NY, USA; 6Department of Medicine, Division of Transplant Hepatology, Montefiore Medical Center, Bronx, NY, USA
Correspondence: Yvonne Saenger, Montefiore Einstein Comprehensive Cancer Center, Department of Oncology, Albert Einstein College of Medicine, Golding Building, Room 701, 1300 Morris Park Avenue, Bronx, NY, 10461, USA, Tel +1 718-430-2715, Email [email protected]
Background: : Incidence of hepatocellular cancer (HCC) in the Bronx is 61% higher than the rest of New York State. Underserved populations are not well represented in clinical trials of immune checkpoint inhibitors (ICI).
Methods: Demographics were tabulated for 194 patients treated with ICI at the Montefiore-Einstein Comprehensive Cancer Center (MECCC) between 2017 and 2022. Categorical variables were analyzed by Chi-squared test, and survival was analyzed using Kaplan–Meier (KM) curves.
Results: MECCC patients were 40.7% Hispanic and 20.6% Black, compared with 3% and 2%, respectively, in the landmark IMbrave 150 study. Median overall survival (mOS) on ICI was 9.0 months, 25.0 months for the 100 (51.5%) favorable-prognosis Child Pugh A (CPA) patients included in HCC clinical trials. Disease control rate (DCR) was 58.5% among 123 evaluable patients per mRECIST 1.1. Baseline liver function, as defined by CP and the Model for End-Stage Liver Disease-Sodium (MELD-Na), correlated with survival (p < 0.001). Hepatitis C Virus (HCV) and alcoholism were over-represented relative to National Cancer Institute (NCI) data (56.2% vs 4.7% and 38.7% vs 8.2%, respectively). HCV treatment correlated with prolonged survival in infected patients (p = 0.0017). AFP decline correlated with response (p = 0.001). Hispanic patients lived longer when clinical variables were controlled for (mOS 52 vs 23 months; p = 0.011).
Conclusion: In an underserved HCC population, ICI yielded a DCR of 58.5% and low rates of severe toxicity. This work highlights ICI efficacy in minority groups, a need for earlier HCC diagnosis and for studies of genetic and environmental factors in Hispanics with HCC.
Keywords: hepatocellular carcinoma, systemic therapy, immunotherapy, checkpoint inhibitors, minorities
Introduction
Liver cancer is the sixth most common cancer in the world, accounting for 4.7% of all new cancer cases. It is also the third leading cause of cancer-related deaths.1 The most common type of primary liver cancer is hepatocellular carcinoma (HCC), accounting for 75% of all cases.2 The burden of HCC is unevenly distributed based on race and sex with men and racial/ethnic minorities being disproportionately affected. The Bronx, one of the five boroughs in New York City (NYC) with large minority and underserved populations, is a hotspot for liver cancer, with a rate of incidence higher than the national average and the rest of the city.3 Liver cancer in under-represented groups is distinct from majority populations. Under-represented groups have different risk factor profiles for liver disease, a higher incidence of comorbidities, lower enrollment in screening programs, and unequal access to treatment, all culminating in increased mortality.4,5 The advent of immune checkpoint inhibitors (ICI) has revolutionized the treatment of HCC with substantial improvement in outcomes.6,7 However, the efficacy of ICI in underrepresented groups is not well-established due to infrequent inclusion of minority patients in the pivotal trials that led to the widespread adoption of ICI in clinical practice. Therefore, further study of ICI benefit in these populations is urgently needed.
The primary risk factors for HCC include chronic hepatitis C virus (HCV) infection, chronic hepatitis B virus (HBV) infection, alcohol consumption, inherited genetic disorders, and acquired metabolic disorders such as nonalcoholic fatty liver disease (NAFLD), recently renamed metabolic dysfunction-associated steatotic liver disease (MASLD).8 The prevalence of these risk factors is unevenly distributed in the United States. Notably, NAFLD has the greatest contribution to the development of HCC among Whites (34.8%) and Hispanics (39.3%), while HCV remained a stronger contributor among Blacks (36.1%) and Asians (29.7%).9 Furthermore, minorities are diagnosed with more advanced disease than White populations. Despite well-established surveillance guidelines,8 the rate of HCC screening in the US remains low at 18% of eligible patients, based on a meta-analysis encompassing cases between 1990 and 2011.10 In a Veterans Affairs cohort of HCV-infected patients, Blacks were significantly less likely than Whites to receive screening imaging (OR 0.6; 95% CI, 0.45–0.81).11 As would be expected, an eventual consequence of poor screening is advanced disease at the time of diagnosis and poorer outcomes.12,13
Unfortunately, once a diagnosis of advanced liver cancer is made, under-represented groups have less access to effective systemic therapies. Following the approval of ICI, there have been reports of disparities in the systemic treatment based on race/ethnicity and SES.14–19 Ahn et al20 reported disparities in access to ICI therapy, with Latinos (adjusted OR [aOR], 0.63; 95% CI, 0.46–0.83) and Blacks (aOR, 0.71; 95% CI, 0.54–0.89) less likely to receive immunotherapy compared to Whites. The IMbrave 150 trial, studying the combination of atezolizumab and bevacizumab in advanced HCC, included patients who had an excellent performance status (ECOG score 0–1) and liver function no worse than Child-Pugh A, with only 5% of enrolled patients identifying as either Black or Hispanic.21 Several other landmark studies for advanced HCC such as the KEYNOTE-240, CheckMate-459, REACH-2, and HIMALAYA trials had relatively similar inclusion criteria.22–25 Of note, in all of these trials, Black and Hispanic patients were significantly under-represented, with a prevalence ranging from <1% to 8% in the immunotherapy-treated arms.21–29 Several real-world retrospective and database studies have included patients with poorer functional status (ECOG 2) and/or decompensated liver disease (Child-Pugh Class B or C). However, available race and ethnicity data revealed limited prevalence of Blacks and Hispanics.26–28
Characteristics of patients in a minority-rich populations in medically underserved areas of the Bronx are markedly different compared to national or international patient cohorts included in seminal clinical trials.30 The primary objective of this study was to determine the real-world efficacy and tolerability of immune checkpoint inhibitors in HCC patients seen in an underserved predominately Hispanic and African American population in an academic center serving the Bronx. We also sought to define clinico-pathological and demographic features of HCC within The Bronx population and identify predictors of prolonged survival. The results show that ICI yields an overall survival of 25 months in the Bronx population when confounding factors such as poor liver function are accounted for. Notably, many of our patients presented with advanced disease. Using a MELD-Na cutoff rather than CP A identified a larger population of patients with prolonged survival on immunotherapy, potentially eligible for clinical trials. Notably, Hispanics with HCC fared better than non-Hispanics within the Bronx.
Materials and Methods
Data Sources and Definition of the Cohort
A retrospective electronic medical record (EMR) review of all adult patients treated for HCC at MECCC between January 2017 and September 2022 was conducted. All research was performed in accordance with the World Medical Association Declaration of Helsinki. This study protocol was reviewed and approved by the Albert Einstein College of Medicine IRB, protocol #2021-13514. A waiver of informed consent was granted due to the retrospective nature of the work and the absence of direct impact on living patients. A total of 194 patients were identified as having advanced HCC treated with immunotherapy.
Data were retrieved from patients’ medical records. Outcome parameters such as overall survival and disease control rate assessed by radiologists using the Modified Response Evaluation Criteria in Solid Tumors (mRECIST 1.1) were reported. The occurrence of immune adverse events was identified from the available patient charts. Survival was evaluated in light of multiple parameters such as baseline serum alpha-fetoprotein (AFP) level, its evolution at the 3-month re-evaluation mark, the Child–Pugh (CP) class and MELD-Na score at the time of diagnosis.
Statistical Analysis
Continuous data were summarized as median and interquartile range while discrete variables as absolute value and relative frequencies. The main outcome, overall survival, was measured from the date of starting immunotherapy till the date of last follow-up or death from any cause. Kaplan–Meier curves were used to assess the overall survival (OS) of patients in different subgroups, based on their Child-Pugh score, alpha-fetoprotein (AFP) levels, diagnosis methods, ethnicity, etiology of liver disease, and MELD-Na score. The Log rank test was used to compare the OS curves between the subgroups. A multivariate Cox proportional hazard regression model was used to assess the association between different variables and overall survival (OS). The variables were selected based on their significance in a univariate log-rank analysis, with a P-value cutoff of less than 0.05. The proportional hazard assumption for the Cox model was tested using Schoenfeld residuals analysis, and the results showed that the assumption was met (global P-value > 0.05 and P-value > 0.05 for each included variable). Finally, we used a Chi-squared test to compare the population distribution of our data with that of the IMbrave150 clinical trial and the SEER database populations. A P-value of less than 0.05 using a two-tailed test was considered statistically significant. Statistical analysis was performed using R language version 4.1.2 (R Core Team, 2021).
Results
Baseline Patient Characteristics
This retrospective study initially included 1059 patients with HCC who presented to MECCC between January 2017 and September 2022. Of the 1059 patients identified, a total of 194 patients with advanced HCC had received immunotherapy and were subsequently included in the final analysis. Patients’ baseline disease characteristics, demographics, and immunotherapy treatment details are shown in Table 1. Out of the 194 treated patients, 121 (62.2%) received single-agent nivolumab, 44 (22.7%) were treated with atezolizumab and bevacizumab, 22 (11.3%) with pembrolizumab alone, 4 (2.1%) with ipilimumab and nivolumab, while 3 (1.5%) received atezolizumab alone. Of the included patients, 137 (70.6%) were male and the median age at baseline was 65 years. Seventy-nine patients identified as Hispanic (40.7%), 40 as non-Hispanic Black (20.6%), 29 as non-Hispanic White (15.0%), 11 as Asian (5.7%), and 35 were of unknown racial or ethnic background (18.0%). The cohort of HCC patients treated with immunotherapy in The Bronx was significantly enriched for Black and Hispanic patients relative to IMbrave150 (Table 2, p < 0.0001) and the NCI Surveillance, Epidemiology and End Results (SEER database). A review of major ICI studies in the HCC landscape demonstrated that, when racial and ethnic data were included in study results, they were generally similar to the IMbrave 150 data, as shown in Supplemental Table 1.
 |
Table 1 Patient Characteristics (n = 194) |
 |
Table 2 Comparison of Our Bronx Cohort with IMbrave150 and SEER Data |
The leading etiology of chronic liver disease (CLD) within the Bronx population was viral, with 109 patients diagnosed with chronic hepatitis C (56.2%) and 22 with chronic hepatitis B (11.3%). Furthermore, 75 (38.7%), 23 (11.9%), 53 (27.3), and 15 (7.7%) patients were reported as having alcoholic liver disease, non-alcoholic fatty liver disease, multiple co-occurring etiologies, as well as rare or unknown causes of liver disease, respectively. A small subset of 13 patients (6.7%) carried a diagnosis of HIV infection at the time of immunotherapy initiation. We observed a significant over-representation of individuals with chronic hepatitis C compared to both the IMbrave150 clinical trial and NCI SEER database populations (Table 2, p < 0.0001). Further, while alcohol was not documented as a specific cause for cirrhosis as part of IMbrave150, it was more prevalent in the Bronx than in SEER (38.7% vs 8.2%). Therefore, in summary, our patient population differed significantly in racial composition and etiology of liver disease from clinical trial populations on which ICI was tested.
Clinical Outcomes
Median overall survival after immunotherapy initiation was 9.0 months (95% CI, 7.0–14.0) for all participants (Figure 1A). Seventy-five participants were still alive at last follow-up, and their survival data were therefore censored. A total of 123 patients completed both pre-treatment and post-treatment scans, three to six months after initiation of therapy, or at clinical progression, and were therefore evaluable per mRECIST 1.1 criteria by a radiologist. Among evaluable patients, the disease control rate (DCR) was 58.5%. One patient had a radiologically confirmed complete response (CR), while another patient achieved a near CR. Seven patients achieved a surgical CR.
Impact of Baseline Liver Function on Survival
We studied the impact of baseline liver function on survival to better place our Bronx population in the context of national trials. The Child-Pugh (CP) classification at the start of therapy is a well-established predictor of survival. In IMbrave 150, as well as in most other clinical trials, only the better prognostic group, CP A, was included while CP B and CP C were excluded. In the ICI-treated Bronx population, at the start of immunotherapy, CP status was evaluable in 192 patients. A total of 100 (52.0%), 70 (36.5%), and 22 (11.4%) patients were characterized as having Child Pugh class A, B, or C, respectively. Patients in Child-Pugh class A had a median OS of 25.0 months, while those in classes B and C lived a median of 7.0 and 2.0 months, respectively (Figure 1B, p < 0.0001). The Model for End-stage Liver Disease Sodium (MELD-Na) score is an alternative method to evaluate liver function, and we sought to test whether this metric could similarly identify patients with favorable liver function more likely to benefit from immunotherapy. MELD-Na score was calculated for 168 patients with sufficient laboratory and clinical data at the beginning of immunotherapy. A total of 142 (84.5%) patients had a low MELD-Na score, defined as a score of 6–18, whereas 26 (15.5%) patients had a MELD-Na score ranging between 19 and 40, and were therefore classified as high-MELD. MELD-Na score stratified patients based on survival, with low-MELD patients living longer than high-MELD patients (Figure 1C, median OS 14 vs 1 months; p < 0.0001). Based on these data, liver function is highly predictive of survival on immunotherapy, and using the MELD-Na score may specifically identify patients with very poor survival, and, by allowing for specific exclusion of those high-risk patients, allow treatment of a larger number of patients with ICI than does the CP score on clinical trials in a diverse real-world population with advanced hepatocellular carcinoma.
Alpha-Fetoprotein (AFP) Levels and ICI Efficacy
AFP has been previously reported to be a predictor of ICI efficacy and, therefore, AFP level and trends were tested as surrogate biomarkers for survival. Pre-treatment AFP values were available for 180 patients. A total of 102 (52.6%) patients were reported to have a baseline serum AFP value ≤400 while 78 (40.2%) had an AFP level >400. Patients with a baseline serum AFP level ≥400ng/mL trended towards reduced survival compared to those with AFP <400 ng/mL, but this did not reach a statistical significance (Figure 2A, median OS 15 vs 9 months; p = 0.162). In 86 patients who had a baseline elevation in AFP as well as a subsequent AFP measurement, a drop by 15% or more in serum level at a 3-month re-assessment was predictive of longer survival compared to AFP non-responders (Figure 2B, mOS 10.0 months vs NR; p = 0.0012). Eighty-two percent of patients with a drop in serum AFP were alive at last follow-up.
Correlation of HCV Treatment with Survival
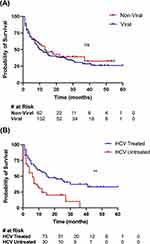
We sought to determine whether the etiology of underlying chronic liver disease has an effect on clinical outcomes. The etiology of chronic liver disease (CLD) was identified as viral if the patient was diagnosed with chronic HBV or HCV, regardless of the presence or absence of concomitant alcoholic or nonalcoholic fatty liver disease. CLD that was not caused by chronic hepatitis was considered nonviral. Survival rates were not significantly different for patients with viral vs nonviral CLD (Figure 3A, p = 0.44).
One concern that commonly arises in the Bronx is that patients with HCV may not have been treated for HCV infection. For a subset of patients with chronic hepatitis C and hepatocellular carcinoma, those who were treated with direct-acting antiviral medications and achieved sustained viral response (SVR) had a higher likelihood of survival than those who were not treated and had persistently elevated viral loads (Figure 3B, 12.0 vs 6.0 months; p = 0.0017).
Treatment-Related Immune Toxicity
Among the 194 patients analyzed, 54 patients (27.8%) experienced immune-related adverse events (irAEs), with 8 patients (4.1%) developing grade ≥3 irAEs. Toxicity data are shown in Table 3. Notably, one patient died due to perforated immune colitis. Dermatologic toxicities were the most common, with 24 skin-related AEs reported, including pruritus (10), rash (9), bullous pemphigoid (1), lichen planus (1), oral mucositis (1), vitiligo (1), and eczema (1). Thirteen patients reported fatigue, while seven thyroid-related AEs were reported, including hypothyroidism (6) and hyperthyroidism (1). Colitis was diagnosed in five patients who reported diarrhea, while four patients experienced liver toxicity, including elevated ALT/AST (3) and isolated hyperbilirubinemia (1). One patient was diagnosed with vasculitis attributed to ICI.
 |
Table 3 Presentation of Immune-Related Adverse Events and High-Grade irAEs |
Impact of Low Screening Rates in the Bronx
Given that immunotherapy can take weeks or months to induce a tumor response, we considered the impact of the frequency of screening on survival in our Bronx population. Within the 194 patients with HCC treated with ICI, screening rates for HCC were low. Chart review showed that 20 (10%) had been receiving regular biannual screening for HCC and 78 (40%) had an imaging study done for any reason in the 24 months preceding diagnosis. Among 194 patients, 38 (14.4%) were diagnosed with HCC based on routine screening, while the remainder had laboratory value abnormalities and/or were already experiencing symptoms at diagnosis. Patients diagnosed based on screening had prolonged OS, starting from the time of diagnosis, relative to those presenting with active symptomatology (Supplemental Figure 1, 58.0 vs 16.0 months; p = 0.0037). Furthermore, patients diagnosed based on screening were significantly less likely to die within 3 or 6 months of diagnosis (Supplemental Table 2, p = 0.042 and p = 0.004, respectively). The data show that screening rates are low in the Bronx and that many patients are diagnosed with advanced disease and have lower survival rates at 3–6 months. These statistics demonstrate that the Bronx has low screening rates, and that many patients in this population are given advanced disease diagnoses and have lower survival rates at 3–6 months.
Effect of Race and Ethnicity on Survival
In light of the Bronx’s broad racial and ethnic composition, we subsequently examined survival rates with respect to race and ethnicity. While there were no discernible racial disparities of significance, patients with Hispanic ethnicity lived longer than non-Hispanics (Figure 4, mOS 52 vs 23 months; p = 0.007). In order to determine whether other prognostic features accounted for increased survival in Hispanics, a multivariate analysis was performed. Non-Hispanic ethnicity was found to be an independent risk factor for decreased OS compared to Hispanic ethnicity (HR 0.6; 95% CI 0.4–0.88; p = 0.01), when age, gender, and Child-Pugh class are taken into account (Table 4). These results show that patients identifying as Hispanic survive longer than non-Hispanics when treated with ICI for HCC.
 |
Table 4 Predictors of Overall Survival in Multivariate Analysis |
 |
Figure 4 Kaplan–Meier analysis of survival with patients stratified by ethnicity. **p-value<0.01. |
Discussion
This report presents our experience at a single center treating patients with advanced hepatocellular carcinoma (HCC) using immunotherapy in a real-world setting. Our patient population has unique features, including a high proportion of Hispanic and Black patients, difficulty accessing healthcare due to socioeconomic barriers, and a high prevalence of chronic hepatitis C and alcohol abuse, which contribute to the development of chronic liver disease. Another distinctive feature of our population is that it comprises treated individuals who have compromised liver function, specifically those classified as Child-Pugh B and C (47.4% of all patients), often excluded from trials. Despite these differences compared to populations enrolled in pivotal trials, we found that treatment efficacy, as measured by mOS, was similar to that reported in seminal randomized control trials.21–25 Disease control rate, which was assessed through imaging three months after the start of therapy, corresponded with what has been reported in previous studies. Treatment with immunotherapy was generally well tolerated, with a low incidence of grade 3 or higher immune-related adverse events (irAEs). Overall, our real-life experience suggests that immunotherapy is relatively safe and active in a subset of patients with HCC.
This research confirms several factors associated with poorer survival rates in patients with advanced hepatocellular carcinoma (HCC) undergoing immunotherapy treatment. MELD-Na score greater than or equal to 19, and an increasing Child-Pugh class, were all found to be predictors of worse outcomes. Our work suggests that MELD-Na score at the time of treatment initiation could be a better stratification tool to select patients for treatment than the Child-Pugh score. Using a MELD cutoff of 19 rather than a Child-Pugh class of A as eligibility criterion for treatment could potentially increase the number of treated patients and broaden the label of immunotherapy in HCC. This hypothesis warrants further exploration in a prospective fashion. Similarly, a reduction of more than 15% in serum alpha-fetoprotein (AFP) levels at the three-month mark after starting therapy may be a reliable indicator of extended survival, but more research is needed to confirm this finding. This relationship has been confirmed in a past study including populations distinct from that of the Bronx.31 Remarkably, among our patients, over 80% of patients with a decline in AFP >15% were alive at last follow-up.
The etiology of chronic liver disease did not have a significant effect on survival outcomes when patients were treated with immune checkpoint inhibitors. Patients with both viral and non-viral diseases experienced similar rates of survival. This finding is in line with current data such as the meta-analysis by Won Jin Ho et al as well as the subgroup analysis of the HIMALAYA trial which studied dual immunotherapy, namely durvalumab with tremelimumab, in advanced HCC.22,31 In both settings, there was no significant difference in response rate or survival between viral and nonviral hepatocellular carcinoma. It is also worth highlighting that some of our patients could have mixed underlying etiologies for their chronic liver disease, in particular the combination of alcohol consumption and chronic hepatitis C, and which could impact the tumor microenvironment and therefore the immune response, in a difficult-to-predict way.
Our patient population was enriched by patients diagnosed with HCC, and we found that patients with chronic hepatitis C who receive treatment with direct-acting antivirals (DAA) and achieve sustained virologic response (SVR) prior to ICI therapy have longer survival times. Conversely, Singal et al demonstrated in 2019 that DAA therapy, after complete HCC response, and helping achieve SVR, is associated with improved overall survival.10 Our finding in the pre-cancer treatment setting could, however, be confounded by a selection bias favoring treatment of patients, perceived to be in better shape and with better liver function, with DAA while forgoing hepatitis C treatment in those thought to have limited benefit from viral eradication. Such preferential treatment, supported by AASLD guidance, which states that patients with limited life expectancy within 12 months are unlikely to benefit from HCV eradication32 and based on subjective perception of overall prognosis, could explain the elucidated difference in survival. Practice patterns still vary widely in the advanced disease setting and more data are required to guide adequate use of resources.
Hispanic and Latin populations residing in the Bronx face greater socioeconomic challenges than non-Hispanic populations. However, after adjusting for factors such as age, gender, and Child-Pugh class, we observed that these populations tended to exhibit longer lifespans. This unexpected result may be explained by the Hispanic paradox, a phenomenon that has been previously documented in medical literature. Kumar et al demonstrated in 2021 that Hispanics with non-small cell lung cancer had better survival outcomes than non-Hispanic Whites.33 Oppositely, Pinheiro et al found no Hispanic advantage in cancer survival.34 Further investigation, both in terms of behavioral, environmental, and genetic studies, is encouraged to fully understand the underlying mechanisms behind these phenomena.
Moreover, our study demonstrated that patients who were diagnosed with hepatocellular carcinoma (HCC) through routine screening achieved better overall survival rates than those who were diagnosed at a symptomatic stage. This highlights the critical significance of following screening guidelines meticulously and implementing structured programs that are designed to target underserved populations. By doing so, the incidence and impact of HCC, which is notoriously difficult to treat in advanced stages and with declining liver function, can hopefully be reduced.
In summary, our research offers a valuable contribution to the existing literature by demonstrating the efficacy and safety of immunotherapy in minority populations that have not been extensively studied in large-scale phase three trials. The observed survival and disease control rate was consistent with those reported previously, and no significant increase in toxicity was observed. Our findings emphasize the importance of diagnosing hepatocellular carcinoma (HCC) at an early, preferably asymptomatic stage while liver function is still intact, as these factors seem to positively influence treatment response and survival outcomes. Further, our data further support the hypothesis that many patients, usually excluded from clinical trials of ICC in HCC, stand to achieve substantial benefit from ICI therapy. However, it is important to acknowledge the limitations of our study, such as its retrospective design and inherent biases, as well as the relatively small sample size. Additionally, administered immunotherapies included diverse regimens and were not always in the first-line setting. Nonetheless, our data highlight the benefit of immunotherapy across racial and ethnic groups as well as the need to better study factors impacting prognosis within Hispanic populations. This is particularly true since Hispanic populations are themselves diverse as far as geographical origin and relative admixture with African and European populations.
Future research endeavors with larger sample sizes and prospective study designs may help to better understand the potential implications of our findings. Our real-world experience can serve as a foundation for the development of programs and interventions aimed at addressing the limited access and heightened hesitancy of minority populations to participate in clinical trials. Raising awareness about chronic liver disease, hepatocellular carcinoma, and implementing widespread screening campaigns for chronic viral hepatitides and alcohol abuse are critical steps towards reducing the significant burden of HCC among disadvantaged communities. By implementing such initiatives, there is the potential to improve early detection rates, reduce health disparities, and ultimately improve the overall health outcomes of underserved populations.
Abbreviations
HCC, Hepatocellular cancer; IHI, Immune Checkpoint Inhibitors; DCR, Disease Control Rate; mOS, Median Overall Survival; irAE, Immune Related Adverse Event; HCV, Chronic Hepatitis C Virus;; HBV, Chronic Hepatitis B Virus; NAFLD, Nonalcoholic Fatty Liver Disease; MASLD, Metabolic dysfunction-Associated Steatotic Liver Disease; NYC, New York City; OR, Odds Ratio; CI, Confidence Interval; SES, Socio-economic Status; aOR, Adjusted Odds Ratio; ECOG, Eastern Cooperative Oncology Group; CPC, Child-Pugh Class; EMR, Electronic Medical Record; mRECIST, Modified Response Evaluation Criteria in Solid Tumors; AFP, Alpha-Fetoprotein; MELD, Model for End-stage Liver Disease; MELD-Na, Model for End-stage Liver Disease – Sodium; MECCC, Montefiore Einstein Comprehensive Cancer Center; OS, Overall Survival; CLD, Chronic Liver Disease; CR, Complete Response; IQR, Inter-Quartile Range; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; DAA, Direct-Acting Antivirals; SVR, Sustained Virologic Response; AASLD, American Association for the Study of Liver Disease.
Data Sharing Statement
Data will be available to editors, reviewers, and readers upon reasonable request from the corresponding author Yvonne Saenger.
Statement of Ethics
All research was conducted in accordance with the World Medical Association Declaration of Helsinki. This study protocol was reviewed and approved by the Albert Einstein College of Medicine IRB, protocol #2021-13514. A waiver of informed consent was granted due to the retrospective nature of the work and the absence of direct impact on individual living patients.
Acknowledgment
We would like to acknowledge the Epidemiology Informatics and Study Management Unit directed by Mindy Ginsberg for their invaluable assistance in reviewing the EMR to define patient cohorts.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work is funded through pilot funding from the Montefiore Einstein Comprehensive Cancer Center and NCI grant R01CA260375.
Disclosure
Yvonne Saenger has received research funding from NextPoint Therapeutics and Regeneron and reports personal fees from Celldex, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Ca a Cancer J Clinicians. 2021;71(3):209–249. doi:10.3322/caac.21660
2. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1(Suppl1):4–13. doi:10.1002/hep.31288
3. Dongmo CM. Liver Cancer Incidence and Mortality by Year, Bronx County, 1976-2020. State University of New York at Albany; 2022.
4. Petrick JL, Florio AA, Znaor A, et al. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer. 2020;147(2):317–330. doi:10.1002/ijc.32723
5. Kamath GR, Taioli E, Egorova N. Liver Cancer Disparities in New York City: a Neighborhood View of Risk and Harm Reduction Factors. Front Oncol. 2018;8.
6. Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ. 2020;371:m3544. doi:10.1136/bmj.m3544
7. Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(8):525–543. doi:10.1038/s41575-021-00438-0
8. Frenette CT, Isaacson AJ, Bargellini I, Saab S, Singal AG. A Practical Guideline for Hepatocellular Carcinoma Screening in Patients at Risk. Mayo Clinic Proceed Innovat Qual Outcomes. 2019;3(3):302–310. doi:10.1016/j.mayocpiqo.2019.04.005
9. Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122(11):1757–1765. doi:10.1002/cncr.29971
10. Singal AG, Yopp A, Skinner S. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861–867. doi:10.1007/s11606-011-1952-x
11. Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Internal Med. 2011;154(2):85–93. doi:10.7326/0003-4819-154-2-201101180-00006
12. Flores YN, Datta GD, Yang L, et al. Disparities in Hepatocellular Carcinoma Incidence, Stage, and Survival: a Large Population-Based Study. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1193–1199. doi:10.1158/1055-9965.EPI-20-1088
13. Yu JC, Neugut AI, Wang S, et al. Racial and insurance disparities in the receipt of transplant among patients with hepatocellular carcinoma. Cancer. 2010;116(7):1801–1809. doi:10.1002/cncr.24936
14. Dakhoul L, Gawrieh S, Jones KR, et al. Racial Disparities in Liver Transplantation for Hepatocellular Carcinoma Are Not Explained by Differences in Comorbidities, Liver Disease Severity, or Tumor Burden. Hepatol Commun. 2019;3(1):52–62. doi:10.1002/hep4.1277
15. Ha J, Yan M, Aguilar M, et al. Race/Ethnicity-specific Disparities in Hepatocellular Carcinoma Stage at Diagnosis and its Impact on Receipt of Curative Therapies. J Clin Gastroenterol. 2016;50(5):423–430. doi:10.1097/MCG.0000000000000448
16. Mathur AK, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010;145(12):1158–1163. doi:10.1001/archsurg.2010.272
17. Sobotka LA, Hinton A, Conteh LF. African Americans are less likely to receive curative treatment for hepatocellular carcinoma. World J Hepatol. 2018;10(11):849–855. doi:10.4254/wjh.v10.i11.849
18. Sonnenday CJ, Dimick JB, Schulick RD, Choti MA. Racial and geographic disparities in the utilization of surgical therapy for hepatocellular carcinoma. J Gastrointest Surg. 2007;11(12):1636–1646. doi:10.1007/s11605-007-0315-8
19. Tan D, Yopp A, Beg MS, Gopal P, Singal AG. Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther. 2013;38(7):703–712. doi:10.1111/apt.12450
20. Ahn JC, Lauzon M, Luu M, et al. Racial and ethnic disparities in early treatment with immunotherapy for advanced HCC in the United States. Hepatology. 2022;76(6):1649–1659. doi:10.1002/hep.32527
21. Cheng A-L, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi:10.1016/j.jhep.2021.11.030
22. Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evidence. 2022;1(8). doi:10.1056/EVIDoa2100070
23. Finn RS, Ryoo B-Y, Merle P, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: a Randomized, Double-Blind, Phase III Trial. J Clin Oncol off J Am Soc Clin Oncol. 2020;38(3):193–202. doi:10.1200/JCO.19.01307
24. Yau T, Park J-W, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, Phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi:10.1016/S1470-2045(21)00604-5
25. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi:10.1016/S1470-2045(18)30937-9
26. De Wilde N, Vonghia L, Francque S, et al. Real-life multi-center retrospective analysis on nivolumab in difficult-to-treat patients with advanced hepatocellular carcinoma. World J Hepatol. 2022;14(8):1608–1620. doi:10.4254/wjh.v14.i8.1608
27. Himmelsbach V, Pinter M, Scheiner B, et al. Efficacy and Safety of Atezolizumab and Bevacizumab in the Real-World Treatment of Advanced Hepatocellular Carcinoma: experience from Four Tertiary Centers. Cancers. 2022;14(7):1722. doi:10.3390/cancers14071722
28. Saeed A, Hildebrand H, Park R, et al. Immune Checkpoint Inhibitors versus VEGF Targeted Therapy as Second Line Regimen in Advanced Hepatocellular Carcinoma (HCC): a Retrospective Study. J Clin Med. 2020;9(9):2682. doi:10.3390/jcm9092682
29. Uhlig J, Stein S, Kim HS. PD-1 targeted immunotherapy for advanced hepatocellular carcinoma: current utilization and outcomes in the USA. Future Oncol. 2022;18(14):1691–1703. doi:10.2217/fon-2021-1487
30. Bien MD, Cedric H, Patel VV, Blackstock OJ, Felsen UR. Reaching Key Populations: prEP Uptake in an Urban Health Care System in the Bronx, New York. AIDS & Behav. 2017;21(5):1309–1314. doi:10.1007/s10461-016-1663-8
31. Ho WJ, Danilova L, Lim SJ, et al. Viral status, immune microenvironment and immunological response to checkpoint inhibitors in hepatocellular carcinoma. J ImmunoTherapy Cancer. 2020;8(1):e000394. doi:10.1136/jitc-2019-000394
32. American Association for the Study of Liver Diseases, Infectious Diseases Society of America. AASLD/IDSA HCV Guidance: recommendations for Testing, Managing, and Treating Hepatitis C. Clin Liver Dis. 2018;12(5):117. doi:10.1002/cld.791
33. Kumar R, Castillero F, Bhandari S, Malapati S, Kloecker G. The Hispanic paradox in non-small cell lung cancer. Hematology/Oncol Stem Cell Therapy. 2021:9727. doi:10.1016/j.hemonc.2021.02.004
34. Pinheiro PS, Williams M, Miller EA, Easterday S, Moonie S, Trapido EJ. Cancer survival among Latinos and the Hispanic Paradox. Cancer Causes Control. 2011;22(4):553–561. doi:10.1007/s10552-011-9727-6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.