Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Hormonal Changes in Women with Epilepsy
Authors Li Q , Zhang Z, Fang J
Received 6 December 2023
Accepted for publication 17 February 2024
Published 26 February 2024 Volume 2024:20 Pages 373—388
DOI https://doi.org/10.2147/NDT.S453532
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Qiwei Li,1 Zhiyun Zhang,1,2 Jiajia Fang1
1Department of Neurology, The Fourth Affiliated Hospital, International Institutes of Medicine, Zhejiang University School of Medicine, Yiwu, Zhejiang Province, 322000, People’s Republic of China; 2Department of Neurology, The Mianyang Central Hospital, Mianyang, Sichuan Province, 621000, People’s Republic of China
Correspondence: Jiajia Fang, The Fourth Affiliated Hospital, Zhejiang University School of Medicine, Yiwu, 322000, People’s Republic of China, Email [email protected]
Abstract: Epilepsy is a prevalent neurological disorder among women globally, often requiring long-term treatment. Hormonal fluctuations in women with epilepsy (WWE) can have reciprocal effects on epilepsy and antiseizure medications (ASMs), posing significant challenges for WWE. Notably, WWE commonly experience endocrine alterations such as thyroid dysfunctions, low bone metabolism, and reproductive hormone irregularities. On the one hand, the presence of hormones in women with epilepsy affects their susceptibility to epilepsy as well as the metabolism of antiseizure medications in various ways. On the other hand, epilepsy itself and the use of antiseizure medications impact the production, secretion, and metabolism of hormones, resulting in low fertility, increased risk of pregnancy complications, negative offspring outcomes, and so on. In order to develop more precise treatment strategies in the future, it is necessary to comprehend the explicit relationships between hormones, epilepsy, and antiseizure medications, as well as to elucidate the currently known mechanisms underlying these interactions.
Keywords: women with epilepsy, antiseizure medications, endocrine, hormonal changes
Introduction
Epilepsy is a prevalent chronic neurological disorder on a global scale, with an estimated lifetime prevalence of 7.60 cases per 1000 individuals.1 In the context of gender, the affliction affects approximately 6.85 cases per 1000 women,1 suggesting that fewer women endure the pain of illness compared to men. However, due to the dynamic nature of hormonal changes throughout the lifespan of women, coupled with the impact of epilepsy and the metabolism of antiseizure medications (ASMs), which presents a significant challenge arises for women, gynecologists, and neurologists. Research has indicated that women with epilepsy (WWE) have a higher propensity for developing metabolic or hormonal irregularities compared to their healthy counterparts of similar age,2 eg, the prevalence of polycystic ovary syndrome (PCOs) in women with endocrine disorders (WWE) is estimated to be around 10–25%, whereas in the general population, it varies between 8% and 13% due to variations in diagnostic criteria and ethnic disparities.3
In this review, we delve into the endocrine aberrations encompassing thyroid hormones, bone metabolism, and sex hormones, elucidating their enduring repercussions on women, their fetuses during gestation, and subsequent stages of life (Figure S1). This comprehensive analysis scrutinizes the available evidence pertaining to the matter of endocrine dysfunctions in WWE with endocrine disorders, while also highlighting the necessary actions to be undertaken in the forthcoming period.
Thyroid Hormones
Thyroid hormones (THs) are crucial for regulating energy metabolism and central nervous system (CNS) development.4 The synthesis and release of THs are controlled by the hypothalamic–pituitary–thyroid (HPT) axis, primarily through thyroid stimulating hormone (TSH) and thyrotropin-releasing hormone (TRH) negative feedback regulation. While the impact of thyroid hormone abnormalities in epilepsy patients has been extensively discussed, previous research has primarily focused on the relationship between ASMs and thyroid hormones. A comprehensive analysis comprising forty-seven studies investigating the relationship between epilepsy and thyroid hormone metabolism reveals that nearly one-third of patients with epilepsy (PWE) encountered fluctuations in thyroid hormone levels.5 The existing literature on the impact of thyroid hormones specifically on WWE remains scarce and inconclusive.
The Role of Thyroid Hormones in CNS and Epilepsy
Thyroid Hormones Play an Important Role in CNS Development and Stability
The maintenance of optimal thyroid hormone homeostasis is crucial for the proper development and stability of CNS starting from the early stages of fetal life.6 THs play a significant role in various processes such as neurogenesis, angiogenesis, and neuronal plasticity. Specifically, triiodothyronine (T3) regulates the activities of Cajal-Retzius and subplate cells, which are involved in axon routing, cell migration, and the stratification of the cerebral cortex.7 Furthermore, it has been observed that the regulation of extracellular matrix protein synthesis is directly THs, which in turn has been linked to a decrease in cortical thickness and a decline in basal progenitor cell proliferation.7,8 The study on transcranial magnetic stimulation revealed that both hypothyroidism and hyperthyroidism have an impact on cortical inhibitory circuits and cortical excitability.5 From a clinical perspective, hypothyroidism encompasses a range of manifestations, including developmental delay, hearing and speech impairments, strabismus, and impairment of voluntary motor activity (lower limb spasms, paraplegia and ataxia), with the most severe condition being historically known as cretinism.
There is speculation that THs may have a significant impact on the development of epilepsy. Su et al observed that two female patients diagnosed with Graves’ disease were unable to effectively manage their epilepsy even with the administration of ASMs during periods of elevated serum thyroid hormone levels. In contrast, both clinical seizures and electroencephalogram (EEG) abnormalities disappeared despite sleep deprivation and discontinuation of ASM once antithyroid drug treatment was prescribed.9 Broutian et al reported an additional clinical case involving comorbidities of Graves’ disease, which exhibited a significant prevalence of generalized 3-Hz spike-and-wave discharges, a characteristic EEG feature of idiopathic generalized epilepsy. Furthermore, the case also demonstrated bitemporal independent lateralized 3-Hz spike-and-wave activity.10 The observed EEG alterations in individuals with hyperthyroidism, such as diffuse, bilateral, or regional slowing, heightened fast activity, elevated alpha-rhythm frequency, and modified responsiveness to photic stimulation, suggest a potential interaction between thyroid dysfunction and thalamocortical network oscillations in idiopathic generalized epilepsy. This interaction may ultimately lead to a decreased seizure threshold and the initiation of epileptic episodes.
Putative Mechanism of Thyroid Hormone in Epilepsy
The elucidation of the mechanism by which THs influence susceptibility to seizures in the brain remains necessary. It has been confirmed that elevated levels of THs can modify the function of sodium-potassium adenosine triphosphatase, thereby leading to changes in neuronal sodium concentrations,11 ultimately resulting in a reduction of the seizure threshold. In contrast, the administration of TRH directly into the hippocampus demonstrated anticonvulsant properties in rats with amygdala-kindled seizures, as evidenced by a reduction in after-discharge duration and seizure duration.12 Consequently, an elevated thyroxin level diminishes the seizure threshold by decreasing the levels of TRH or its precursors in different limbic brain regions.13
Despite the direct role of THs on ion imbalance and TRH on neuroprotection, their indirect effects on seizure account for quite a lot (Figure 1).
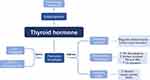 |
Figure 1 Possible mechanisms of THs on epilepsy pathogenesis. Notes: ↑: increase; ↓: decrease. |
The various impacts of THs on gamma-aminobutyric acid (GABA)ergic neurons, such as their influence on GABA circuit development and their regulation of enzymes involved in GABA synthesis and metabolism, ultimately result in epileptogenesis due to decreased TH levels. Additionally, both hyperthyroidism and hypothyroidism disrupt the equilibrium between the generation of oxidant agents and the antioxidant activity of defense systems. Since mitochondria DNA is susceptible to reactive oxygen species, which is the main reactant of oxidative stress, the development of epileptic seizures is speed up mitochondrial dysfunction.14,15 Moreover, thyroid hormones (THs) exert both non-genomic and genomic influences on the biogenesis and function of mitochondria. Specifically, T3 binds to a specific site within the mitochondria known as thyroid hormone response element-like sequences, which is facilitated by the thyroid hormone response-α gene called P43. This binding subsequently initiates transcription in a manner dependent on the presence of a ligand. THs are capable of binding to nuclear-localized thyroid hormone receptors and thyroid hormone response elements, thereby exerting control over gene expression. Furthermore, THs can generate co-activator and intermediate factors that have the potential to regulate a distinct set of target genes associated with THs.15
In addition to its impact on the general pathway of epileptic formation, TH dysfunction may also contribute to the development of specific types of epilepsy, such as focal cortical dysplasia. According to a study, the administration of goitrogen treatment to pregnant rats for a duration of five days during the perinatal period has been found to effectively induce a periventricular heterotopia in the offspring. This occurrence is attributed to abnormal cell adhesion, reduced expression of Sonic hedgehog, and modified morphology of radial glia near the ventricles, whose period is in accord with the mid-gestation of human pregnancy.16
In conclusion, THs’ impact on epilepsy mainly consists of the promotion of epilepsy and epileptogenesis. The former includes ion imbalance, GABAergic neurons, mitochondria biogenesis, and oxidative, while the latter may induce certain epilepsy, like FCD.
Impact of Epilepsy and ASMs on Thyroid Hormones
On the contrary, epilepsy itself and ASMs can also disrupt the homeostasis of THs, including their biosynthesis, release, transport, and metabolism.
Effects of Seizure on Thyroid Hormones Dysregulation
Epileptic seizures can promptly lead to a surge in hormonal activity, as evidenced by a temporary elevation in serum levels of T3, thyroxin (T4), and thyrotropin.17 Furthermore, a study conducted on animals revealed a notable alteration in the regional distribution of TRH within the central nervous system following seizures.18 However, the precise mechanisms responsible for these changes remain elusive. Another animal study demonstrated that the elevation of pituitary hormone levels subsequent to seizure events may be attributed to monoamine mechanisms.19 Clinically, the elevation of prolactin, follicle-stimulating hormone, growth hormone, and cortisol levels was observed after seizures compared to controls,20 while Han et al21 reported that serum TSH levels were significantly increased following a seizure, probably suggesting epileptic seizures can interfere the function of the HPT axis.
In addition to the immediate impacts of seizures on hormonal changes, various factors such as seizure type, duration, epileptiform discharge on EEG, and comorbidity have also been associated with alterations in THs. Given the close anatomical proximity and extensive interconnections between the hypothalamus and limbic systems, the hypothalamus plays a crucial role in the production and secretion of THs. Consequently, seizures involving the limbic system, such as epileptiform discharges, may render the HPT axis susceptible to modifications by influencing TRH secretion. Moreover, the disruption of the equilibrium between excitatory and inhibitory neurotransmitters may adversely affect the hormone balance within the HPT system.22 Nevertheless, conflicting results have been observed, as Eren et al23 found that levels of THs during the interictal period were comparable to those of the control group and did not exhibit any association with seizure type or activity.
In summary, discerning the independent factor proves challenging, as there exists an intricate interdependence relationship between epilepsy and alterations in thyroid hormones.
The Impact of ASMs on Thyroid Function
For PWE, almost one in three on ASMs develop thyroid hormonal disturbance and subclinical hypothyroidism,24 the incidence is even higher in WWE.5 The impacts of ASMs on THs have been recognized for more than 60 years though consistent results have not been obtained (Table 1).
 |
Table 1 Effects of ASMs on Thyroid Hormone Homeostasis |
In a meta-analysis, the authors25 found that patients receiving ASMs therapy had one or more of the following manifestations: a decrease in T4, free T4, T3, or total THs as well as an increase in TSH. The author further concluded that decreased T4 and T3 were most common in CBZ or phenytoin (PHT) -treated patients, while elevated TSH was in topiramate-treated. Decreased serum free T4 and free T3 and central hypothyroidism in Oxcarbazepine (OXC)-treated patients, while simply free T3 reduction in Phenobarbital (PB)-treated. Clinical hypothyroidism is often seen in topiramate-treated patients while subclinical hypothyroidism is seen in levetiracetam (LEV)-treated or VPA-treated patients. Conversely, Lamotrigine (LTG) appeared to make the mildest difference to thyroid hormones.
In a separate systematic review, Rochtus et al5 additionally documented a higher prevalence of comparable THs alterations in patients characterized by advanced age, female gender, intractable epilepsy, prolonged epilepsy duration, and treatment with multiple ASMs. The aforementioned conclusions are derived from observational studies, necessitating further substantiation through experimental studies to enhance their validity.
The good news is that thyroid dysfunction caused by ASMs seems to be reversible after cessation of the ASMs treatment.26,27
Multiple possible explanations for the effects of ASMs on THs levels are discussed (Figure 2). Enzyme-inducing antiseizure medications (EIASMs) leads to a reduction in concentrations of both free and bound THs28 as a result of hepatic cytochrome P450 isoenzymes. Consequently, the administration of EIASMs. Besides, PB, in particular, competes with THs for the binding of thyroxine-binding globulin, lowering T3 and T4 but having little effect on TSH.29 On the contrary, VPA, an enzyme inhibitor of ASM, also decreases the concentrations of THs by sharing the common pathway of glucuronide conjugation and oxidation. Other mechanisms that VPA could decrease TSH by the promoting effect of VPA on GABA, which decreases TSH through decreasing somatostatin,5 is still under discussion.
Furthermore, it has been observed that ASMs have an impact on the HPT axis, primarily through the inhibition of voltage-gated sodium and calcium channels, enhancement of GABAergic transmission, and antagonism of glutamate receptors. Consequently, it is plausible that comparable neurochemical mechanisms are implicated in the interaction between these medications and the synthesis of hypothalamic neurohormones such as gonadotropin-releasing hormone (GnRH), corticotropin-releasing hormone, and TRH.30,31
Influence of Thyroid Dysfunction on WWE
Hypothyroidism is commonly linked to depression, menorrhagia, infertility, and sexual dysfunction among women in the general population.32 Similarly, the presence of hyperthyroidism has been found to be associated with menstrual disorders, increased follicular atresia, and ovarian cysts.33 Furthermore, research has indicated potential connections between maternal hyperthyroidism/hypothyroidism and an elevated risk of attention deficit hyperactivity disorder, autism spectrum disorder, and epilepsy in offspring.34,35 The research findings indicated a significant association between isolated maternal hypothyroxinaemia and heightened risks of premature rupture of membranes, preterm birth, and fetal distress. However, no significant correlation was observed between isolated maternal hypothyroxinaemia and negative offspring outcomes such as low birth weight infants, fatal macrosomia, or offspring cognitive defects.36
Fetal THs completely rely on maternal THs till the start of fetal thyroid function near the second trimester.37 Experimental studies conducted on rats have demonstrated that maternal thyroid dysfunction can result in various biochemical disruptions within different regions of the offspring’s brain. These disturbances may ultimately contribute to the development of a pathophysiological condition.38 Furthermore, fetal hypothyroidism leads to cortical heterotopias as we mentioned above, which are associated with neurodevelopment disorders like epilepsy. A survey in the USA had shown that half of the pregnancies in WWE are unplanned39 so that THs deficiency caused by ASMs in WWE probably causes seizures to be passed from generation to generation and become a family tragedy.
Although few data have been reported on WWE with these symptoms due to thyroid dysfunction since most patients are asymptomatic, special attention still needs to pay to WWE due to their specific physiological characteristics.
Bone Health
The importance of bone health for women is becoming increasingly acknowledged. The prevalence of osteoporosis in premenopausal women varies significantly depending on the population studied, ranging from 0.5% to 50%.40 This risk is even more pronounced in WWE, as evidenced by a cross-sectional survey conducted by Beerhorst et al, which found that nearly 80% of PWE had low bone mineral density (BMD).40 Both men and women can be affected by certain factors, but women face a higher risk due to various factors, such as the loss of estrogen during menopause.41
The Role and Putative Mechanisms of Vitamin D on Epilepsy
Epidemiological studies have likely offered initial indications of potential links between Vitamin D and epilepsy. A study accessing the seasonal distribution of epileptic seizures found that most occurred during the winter while the least occurred in the summer.42 Less sun exposure, or less Vitamin D in other words, may contribute to an increase in the number of epileptics and the frequency of seizures. Conversely, Vitamin D supplements can improve seizure control in epileptics. Holló conducted a study wherein they assessed and addressed the deficiency of serum 25(OH)D by administering Vitamin D3 supplements to a cohort of 13 patients diagnosed with intractable epilepsy. The median decrease of seizures frequency was 40%, which significantly deviated from the anticipated placebo response.43 In Chinese pediatric patients, a comparable outcome of seizure control was observed following the administration of Vitamin D supplementation, with the proportion of individuals achieving seizure-free rising from an initial 49% to a final 76%.44
According to its molecular structure, bioactivation in the nervous system, and mechanism of action, Vitamin D3 can be classified as a neurosteroid hormone rather than a vitamin.45 The presence of the Vitamin D activating 1-α-hydroxylase enzyme and Vitamin D receptor (VDR) has been observed in various brain structures, including neuronal and glial cells.46 Conversely, in rats with VDR knockout genes, there was a notable increase in susceptibility to seizures.47
One of the primary mechanisms through which Vitamin D exerts its effects is via genomic actions. Acting as a neurosteroid, Vitamin D binds to the nuclear VDR and regulates the activity of calcium-binding proteins, such as Parvalbumin and calbindin, which are expressed in the nervous system. These calcium-binding proteins have the ability to modulate the concentration of calcium, thereby down-regulating the expression of L-type voltage-sensitive Calcium channels. Otherwise, overexpressing calcium48 would activate the nitric oxide synthase, form reactive oxygen species, and activates proteases and lipases, ultimately resulting in plasmic and mitochondrial membrane damage, which we mentioned above is characteristic of epilepsy.15
The second nongenomic strategy involves the activation of intracellular signaling cascades and the allosteric regulation of GABA(A) receptors.49 This process leads to the influx of chloride ions into cells, resulting in neuronal hyperpolarization, which effectively counteracts neuronal hyperexcitability and the occurrence of seizures.50
Furthermore, the potential inhibition of epilepsy by Vitamin D can be attributed to its anti-inflammatory properties.51 It is widely acknowledged that Vitamin D exerts inhibitory effects on inflammation and modulates the equilibrium between inhibitory and excitatory cytokines, favoring the former. Specifically, Vitamin D hinders the production of interferon-gamma and interleukin-2 while promoting the synthesis of interleukin-10.52,53 The active form of Vitamin D has been found to inhibit the production of tumor necrosis factor-α, interleukin-6, and NO in the EOC13 microglial cell line.54 Given the anti-inflammatory properties of Vitamin D and the presence of inflammation cytokines in seizures, further investigation is warranted to better understand the role of Vitamin D in epilepsy.
Impact of Epilepsy and ASMs on Bone Health
The Impact of ASMs on Bone Health
The low BMD observed in WWE can largely be attributed to the chronic utilization of ASMs and subsequent disruption of vitamin D metabolism. Despite the variability in findings regarding the impact of ASMs on bone health, which may be attributed to geographic variations, study design, or dietary habits among study populations, numerous reports have highlighted the detrimental effects of ASMs on BMD, particularly in patients taking multiple ASMs, though clinical data about newer ASMs are sparse (Table 2). Classic ASMs, particularly EI-ASMs and VPA, have been associated with decreased BMD and an increased risk of fractures. However, newer ASMs have shown improved tolerance among patients, although their effects on bone health remain a subject of debate.55 In a recent review on ASMs published in the JAMA, Kanner et al emphasize the importance of monitoring for osteopenia/ osteoporosis when using EI-ASMs or VPA. For PWE with comorbid osteopenia/osteoporosis, commonly prescribed ASMs such as LEV, LTG, and lacosamide are preferred.56
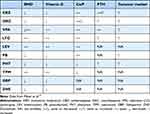 |
Table 2 Effects of ASMs on Bone Health |
Multiple mechanisms have been proposed to explain the impact of ASMs on bone health, with a particular focus on EI-ASMs: (1) ASMs that induce the CYP450 system stimulate the conversion of vitamin D into an inactive metabolite in liver microsomes, resulting in a decrease in bioavailable vitamin D.58 Consequently, this leads to reduced absorption of calcium in the intestines, increased mobilization of calcium from the bones, and a decrease in bone mineralization.59 (2) EIASMs have been found to significantly diminish the concentration of Vitamin K, a crucial factor in the synthesis of osteocalcin. This reduction in osteocalcin carboxylation results in decreased BMD and an elevated susceptibility to pathological fractures.60 (3) ASMs have been observed to interfere with the secretion of calcitonin, a hormone that plays a protective role by inhibiting PTH-induced bone resorption.61 (4) ASMs increased homocysteine level,62 thereby increasing osteoclastic activity and reducing osteoblastic activity, and reducing the number of trabeculae in bone;63 (5) ASMs could decrease the concentration of sex hormone binding globulin, thus increase bone metabolism indirectly, especially in WWE as estrogen play a protective role in bone health through enhancing bone formation,64 limiting bone resorption and avoiding immune dysfunction.65
Effects of Seizures on Bone Health
Determining the potential association between epilepsy and Vitamin D deficiency poses challenges in clinical settings, as patients diagnosed with epilepsy are promptly administered ASMs to mitigate potential risks. However, it was found that children with intractable epilepsy exhibited elevated levels of Vitamin D following a 3-month ketogenic diet in comparison to their initial levels, thereby indirectly validating the detrimental impact of seizures on epilepsy.66 Similar findings were observed in adult patients with intractable epilepsy who underwent a 12-week modified Atkins diet, as opposed to those who remained untreated.67 A population-based study has provided confirmation that treatment-naive patients with genetic generalized epilepsy exhibit a deficiency in Vitamin D. This deficiency is more frequently observed in patients with higher seizure frequency, longer disease duration, and younger age of onset.68 In rat models, Garip et al initially identified alterations in bone mineral and matrix components in rats with hereditary epilepsy.69 However, further validation of these findings is required through additional clinical and basic research.
The pathophysiological mechanisms that underlie this phenomenon remain poorly understood. It is hypothesized that the hypothalamus plays a role in regulating molecules associated with bone health, including neuropeptide-y, leptin, osteocalcin, calcitonin, and estrogen. Consequently, it is postulated that excessive epileptiform discharges may propagate to the hypothalamus, particularly affecting the limbic system, thereby disrupting bone health. However, the current body of limited evidence is insufficient to draw definitive conclusions.
Special Issues on Bone Health in WWE
Infertility
A decline in ovulation rates and endometrial receptivity has been documented during prolonged periods of darkness in winter, contrasting with the increased rates of conception and multiple pregnancies observed during the summer months, which align with the duration of daylight.70 Research has indicated a significant decrease in pregnancy rates among individuals with Vitamin D deficiency, as well as reduced success rates of assisted reproductive technology.71,72 The possible mechanisms of low fertility in the female with Vitamin D deficiency include: (1) Endometriosis: the presence of endometriosis has been found to have an inverse relationship with Vitamin D levels. Women with lower levels of 25(OH)D have been shown to have a 24% higher risk of developing endometriosis compared to those with higher levels.73 Furthermore, a significant prevalence of hypovitaminosis D has been observed in women with ovarian endometrioma.74 This suggests that Vitamin D deficiency in women with endometriosis may be attributed to its role in regulating the secretion of placental lactogen and human chorionic gonadotropin, as well as calcium transport in the placenta and decidualization of the endometrium.75 Consequently, WWE who exhibit Vitamin D deficiency are more susceptible to developing endometriosis. (2) PCOs: there is a growing body of evidence suggesting that Vitamin D can have an impact on both insulin secretion and metabolism, which are key factors in the pathogenesis of PCOs. PCOs is the most prevalent endocrine disorder and a leading cause of infertility among women in their reproductive years.76 A retrospective cohort study found that infertile women with PCOS who had higher levels of 25-hydroxyvitamin D (>30 ng/mL) were more likely to achieve ovulation compared to those without.77 (3) Others, factors such as gametogenesis, fertilization, the preimplantation stage, the final stage of organ development, and preeclampsia are influenced by the individual’s vitamin D status.71 In light of these aforementioned considerations, it can be inferred that individuals with low levels of vitamin D are more susceptible to experiencing infertility.
Pregnancy Complications
Pregnant women are commonly identified as being at a heightened risk for Vitamin D deficiency, despite receiving Vitamin D supplementation, particularly in relation to WWE during pregnancy. The presence of Vitamin D deficiency in pregnant women increases their vulnerability to various complications, including preeclampsia, insulin resistance, gestational diabetes mellitus, bacterial vaginosis, and the likelihood of undergoing a cesarean section delivery.78 Conversely, the administration of 4,000IU/d of Vitamin D supplementation during pregnancy has been shown to mitigate the risk of complications, such as maternal infections, cesarean section, and preterm delivery.79
Offspring Outcomes
Adequate maternal Vitamin D levels are also associated with offspring health to some extent. The concentration of Vitamin D in fetuses primarily depends on the concentration in mothers; therefore, low levels of Vitamin D in women of childbearing age may contribute to the likelihood of infantile rickets. Additionally, the weight of the offspring at birth and their growth in the years following birth are linked to the concentrations of Vitamin D in mothers.80 Furthermore, aside from its impact on neonatal outcomes, a deficiency in Vitamin D has been found to have detrimental implications for children in the long term. Research has demonstrated that insufficient levels of Vitamin D during intrauterine development can potentially affect lung development, subsequently leading to the onset of asthma and chronic obstructive pulmonary disease during childhood.81 Additionally, other adverse effects including impaired bone development, dental cavities, schizophrenia, and type I diabetes have been documented.79
However, there exist conflicting findings concerning the correlation between Vitamin D and offspring outcomes.82 Additionally, a randomized controlled trial conducted in Bangladesh, a region with prevalent Vitamin D deficiency, aimed to evaluate the impact of prenatal and/or postpartum Vitamin D supplementation on offspring outcomes and morbidity, but yielded no statistically significant differences among the groups.83
Sex Hormones
The sex steroid hormones, such as estrogen and progestin, exert significant influences on women’s health across their lifespan. The hypothalamic-pituitary-ovarian (HPO) axis regulates the secretion of these hormones. The interplay among sex steroid hormones, epilepsy, and ASMs is intricate and manifests diverse effects on women.
The Role of Sex Hormones in Epilepsy
The Effects of Sex Hormones on Epilepsy
As previously stated, estrogen serves as a protective factor against osteoporosis in women. Nevertheless, sex steroid hormones can have both positive and negative effects on women with catamenial epilepsy, a condition that affects approximately one-third of female.84 This condition is characterized by cyclic fluctuations in seizure frequency that align with the menstrual cycle. Furthermore, it is noteworthy that seizures frequently undergo alterations in their pattern, expression, or onset during periods of natural hormonal changes, including adolescence, pregnancy, perimenopause, and menopause.85 These fluctuations in seizure activity are primarily influenced by the predominantly proconvulsant effects of estrogen and the anticonvulsant effects of progesterone and its metabolites.
Putative Mechanisms of Reproductive Hormones on Epilepsy
Estradiol (E2), the predominant and biologically potent form of estrogen, has been demonstrated to function as a posttranscriptional modulator, exerting a positive regulatory influence on the density of spines and excitatory N-methyl-D-aspartate receptors, thereby promoting heightened neuronal excitability.86 Additionally, the potential involvement of genetic repression of inhibitory neurotransmitters in this process remains a topic of ongoing debate. Conversely, certain investigations have proposed that estrogen might exhibit anticonvulsant properties via the β-E2 subtype.87
Similarly, allopregnanolone (AP), a specific type of progestin, has been hypothesized to function within a genetic pathway to regulate the synthesis and release of neurotransmitters. Unlike E2, AP facilitates the enhancement of GABAergic neurons, resulting in its anticonvulsant properties in WWE.87 Furthermore, progestin can exert its effects through alternative mechanisms, such as binding to progesterone receptors88 and influencing glutamatergic (excitatory) transmission.89
Furthermore, it is important to consider the role of androgens and testosterone, despite their relatively lower levels compared to estrogen and progestin. These hormones function as bimodal modulators of seizure susceptibility, displaying both anticonvulsant and proconvulsant properties.80 On one hand, they have the ability to convert into estrogens, thereby increasing seizure susceptibility in both animal and human models. On the other hand, they can transform into neurosteroids and propanediol, which act as positive allosteric modulators of GABA(A) receptors, thus inhibiting seizures.90
Effects of Epilepsy and ASMs on Reproductive Hormones
Effects of Epilepsy on Productive Hormones
The other way around, the interictal and ictal discharge may disrupt the functioning of the HPO axis, thereby influencing the levels of sex steroid hormones. The hypothalamus, responsible for the regulation, production, and secretion of GnRH, receives direct connections from the cerebral hemisphere, particularly the temporolimbic structures (eg, hippocampus and amygdala) implicated in seizure generation.91 Consequently, the epileptic discharge affects hypothalamic regions such as the arcuate nucleus and paraventricular nucleus, leading to an increase in the frequency or amplitude of GnRH pulses.92
Consistent with the aforementioned thesis, high serum estradiol, which is the consequence of GnRH pulse acting on follicle-stimulating hormone (FSH), was observed in amygdaloid-kindled female rats,93,94 while increased excitability of GnRH neurons was observed in kainic acid-induced temporal lobe epilepsy (TLE) rat models.95
Moreover, it has been observed that there exist lateral biochemical and physiological distinctions between the left and right limbic systems as well as the hypothalamus.92 In a clinical context, it has been found that right unilateral TLE is more commonly linked to hypothalamic amenorrhea, which is characterized by reduced levels of luteinizing hormone (LH) and estradiol due to a lower frequency of GnRH pulses. Conversely, left unilateral TLE is more frequently associated with PCOS, which is characterized by elevated LH/ FSH ratios and increased serum testosterone levels as a result of a higher frequency of GnRH pulses.96 Furthermore, it is worth noting that different forms of seizures exhibit distinct associations with sexual, reproductive, and gonadal dysfunction. Specifically, focal epilepsy displays a stronger correlation with sexual anxiety, difficulties in intercourse, vaginismus, vaginal discharge, and lack of arousal. Primary generalized epilepsy exhibits a closer relationship with decreased libido, sexual dissatisfaction, menstrual irregularities, hyperandrogenism, anovulatory cycles, and elevated serum FSH levels.91
On the other hand, an imbalance of neurotransmitters, a contributing factor to the development of epilepsy, also plays a role in modulating the excitability of GnRH neurons. Specifically, GABA depolarizes GnRH neurons and promotes the secretion of GnRH through its interaction with A receptors. Additionally, Glutamine binds to its respective receptors to regulate the excitability of GnRH neurons. Conversely, dopamine inhibits the excitability of GnRH neurons, and the occurrence of epilepsy activity leads to a depletion of dopamine, thereby increasing the aforementioned excitability.92
To sum up, epilepsy plays a profound role in the regulation of reproductive hormones more than epileptiform discharge on the cerebral hemisphere.
The Effects of ASMs on Reproductive Hormones
The interaction between sex hormones and ASMs is a multifaceted pharmacokinetic phenomenon. Neurologists are increasingly acknowledging the impact of altered blood levels of active sex hormones on sexual and reproductive dysfunction (as indicated in Table 3). Both older generation (eg, CBZ, PHT, PB) and newer generation (eg, OXC, rufinamide, felbamate, eslicarbazepine, perampanel) ASMs have been found to decrease concentrations of reproductive hormones.97 Furthermore, it has been observed that VPA-treated patients exhibit elevated serum levels of prolactin, testosterone, androstenedione, and diminished luteal progesterone.92 In contrast, LTG does not impact estrogen metabolism or blood levels, and can even reverse reproductive abnormalities induced by VPA.98 Nevertheless, LTG does enhance progesterone metabolism, resulting in a reduction of estrogen blood levels by 10–19%.99 In contrast to the reversible impact of LTG on reproductive hormones, LEV does not exhibit any drug-specific sexual side effects in LEV-treated WWE.91 However, it has been observed that men on LEV experience elevated total testosterone levels due to the binding of LEV to the SV2A receptor present in the brain, as well as in gonadal and endocrine tissues.100
 |
Table 3 Effects of ASMs on Level of Sex Hormones or Contraceptive Steroids |
The classification of altered reproductive hormone mechanisms induced by ASMs can be delineated as follows: 1) EIASMs: including PHT, PB, and CBZ lead to a reduction in the bioactivity of E2 and testosterone due to an increased synthesis of sex hormone-binding globulin (SHBG) and/or stimulation of aromatase. 2) the causes of VPA are complex: it involves the inhibition of testosterone metabolism, which is regulated by enzymes such as cytochrome P450 isoenzymes 2C9 and 2C19, as well as aromatase epoxide hydrolase;100 the weight gain and insulin resistance induced by VPA would additionally promote testosterone secretion from the ovaries and reduce serum SHBG levels;27 ③ VPA may lead to inappropriate LH secretion as a result of gonadotropin pulsatility with simultaneous reduction of FSH and E2 synthesis, hence increases the levels of testosterone;91 ④ VPA has been observed to impact gene expression, such as NR0BI, which acts as an inhibitor for promoters of various genes involved in steroidogenesis.27 3) newer generation of ASMs-related reproductive dysfunction may be related to central nervous system neurotransmitters like GABA, serotonin, dopamine, and glutamate,91 but we will not discuss this here as changes in sex hormones due to them have not been reported explicitly.
Special Reproductive Problems in WWE
Menstrual Irregularity, Infertility, and Premature Menopause
As previously stated, both ASMs and epilepsy both alter the concentration of reproductive hormones, either through their influence on the HPO axis or through other mechanisms. It has been observed that PCOs is more prevalent in WWE compared to those unaffected.92 PCOs is characterized by elevated androgen levels and impaired ovulation, resulting in clinical manifestations such as menstrual irregularities and reduced fertility. However, it should be noted that the etiology of menstrual irregularities and reduced fertility in WWE extends beyond PCOs. Approximately 33% of WWE experience amenorrhea, oligomenorrhea, polymenorrhea, and menorrhagia, whereas the control group only exhibits a prevalence of 12%.101
Research conducted by Epilepsy Birth Control Registry revealed that the infertility risk for WWE is 9.2%. However, after adjusting for confounding factors, the estimated infertility risk for WWE is 10–12%, compared to a control group with a risk of 6.4%. Based on these results, the author concluded that most WWE are fertile but their infertility risk may be higher than controls.102
Menopause in WWE is a challenging phase that requires specific attention. Pre-menopause is distinguished by an anovulatory cycle and relative hyperestrogenism, whereas post-menopause is characterized by low estrogen levels. WWE are more susceptible to experiencing early perimenopause and menopause, with a significantly higher prevalence of 14% compared to 3.7% incidence in control group.103 Additionally, a higher lifetime seizure frequency among WWE is linked to a greater likelihood of earlier onset of menopause.84
In conclusion, it can be inferred that epilepsy has the potential to induce functional modifications in the HPO axis. Furthermore, the administration of ASMs may impact the liver and gonads, resulting in disruptions in sex hormone levels. Consequently, WWE experience a greater burden compared to general females.
Hormonal Contraceptive
Furthermore, it is imperative to consider the impact of exogenous sex hormones in addition to endogenous sex hormones. The management of contraception in WWE is of utmost importance due to the potential risks posed to both the mother and fetus in the event of contraceptive or seizure management failure. It is worth noting that these contraceptives may have detrimental effects on WWE as they can lower the seizure threshold.104 A study conducted by Herzog et al revealed that patients using HCs are more likely to experience an increase or decrease in seizures compared to those not using HCs, with an increase in seizures being more prevalent.105
Furthermore, it should be noted that HCs, particularly those containing estrogen, have the potential to stimulate enzymes and decrease the concentration of ASMs in the bloodstream. Notably, the levels of LTG can surpass those observed for older ASMs due to hepatic glucuronidation facilitated by UGT1A4.106 Consequently, WWE who have their condition well managed may encounter breakthrough seizures upon initiating oral contraceptive.107
However, the intricate pharmacokinetic interactions between HC and ASMs are disregarded in two-thirds of women who are prescribed EIASMs, which leads to ineffective seizure management and unintended pregnancies. The consequences of such unplanned pregnancies include fetal growth restriction108 or malformation,99 as a result of the failure to transition to a more suitable ASM beforehand, as well as alterations in seizure frequency due to changes in ASM metabolism109 and fluctuations in sex hormone levels.
In order to ensure the well-being of WWE who are prescribed HC and ASMs, it is imperative to closely monitor them for potential exacerbation of seizures or adverse reactions. If feasible, alternative non-HC treatments should be considered as a potential switch.
Female Sexual Dysfunction
Another problem in WWE is female sexual dysfunction. A self-rated questionnaire survey about sexual life in the past 4 weeks110 indicated that sexual dysfunction is more common in WWE compared to the controls based on the cut-off point 25 in the female sexual function index. Similarly, the estimated prevalence of hyposexuality is much higher in WWE compared with the general population (ranging from 18% to 60% and 22% to 45.5% respectively).91
There are multiple contributing factors to sexual dysfunction in WWE. One such factor is neuro-endocrinological, wherein seizures and interictal discharges have the potential to impact the hypothalamus, potentially resulting in sex hormone disorders. For instance, PRL, a polypeptide secreted by lactotrophic cells of the anterior pituitary gland and primarily regulated by the hypothalamic-pituitary-gonadal axis, has been found to exhibit significantly elevated levels in both generalized tonic-clonic seizures and partial seizures.111 An elevated concentration of PRL hampers the release of GnRH, thereby impacting the secretion of FSH and LH, resulting in diminished levels of gonadal hormones and the development of hypogonadism, ultimately leading to sexual dysfunction.110,112 The second is iatrogenic: the pharmacokinetic interactions of ASMs on hormones cannot be neglected either. Decreased estrogen levels due to ASMs resulted in insufficient genital blood flow and lubrication, leading to sexual pain and low libido, while high estrogen will enhance sexual motivation.113 Third is psychiatric/psychosocial: self-report rating scales suggest anxiety and depression are more common in PWE in contrast to healthy controls with 22.8% VS 11.2% and 17.4% VS 10.7%, respectively, which result in sexual dysfunction.114
Conclusions and Future Directions
The tridirectional relationship among epilepsy, ASMs, and various hormones, including thyroid hormones, Vitamin D, and reproductive hormones, is evident. This relationship implies that one disorder can potentially lead to the development of others. Hormonal fluctuations can influence the susceptibility of WWE to seizures and can also impact the metabolism of ASMs. Conversely, epilepsy and ASMs can disrupt the production, secretion, and metabolism of hormones, posing significant challenges for WWE. The prevalence of endocrine abnormalities is higher in WWE due to their unique physiological characteristics. Consequently, WWE encounters a significant prevalence of illness attributed to hormonal abnormalities, notably infertility and menstrual irregularity. Furthermore, these hormonal irregularities exert a substantial impact on the long-term health outcomes of the offspring, even persisting into adulthood.
However, numerous avenues for future research remain available. It is imperative to conduct extensive, prospective studies to enhance our comprehension of the prevalence and mechanisms underlying the potential correlations between ASMs and hormone abnormalities, particularly with regard to newer ASMs. Furthermore, further investigation is needed to elucidate the molecular mechanisms through which hormones influence the susceptibility to epilepsy, such as in the case of catamenial epilepsy, as this will facilitate the pursuit of a definitive treatment. The implementation of precision medicine, specifically hormone replacement therapy targeted towards addressing bone-related concerns and preventing osteoporosis in postmenopausal women, is an increasingly prominent and influential trend. Furthermore, in-depth mechanistic and genetic investigations into the origins of hormone dysfunction-associated congenital malformations and neurodevelopmental abnormalities in children will facilitate more informed pre-conceptual counseling. This, in turn, will contribute to a more equitable approach to marriage and childbirth for WWE with hormone-related conditions.
Abbreviations
ASMs, antiseizure medications; WWE, women with epilepsy; PCOs, polycystic ovary syndrome; THs, thyroid hormones; CNS, central nervous system; HPT, hypothalamic–pituitary–thyroid; TSH, thyroid stimulating hormone; TRH, thyrotropin-releasing hormone; PWE, patients with epilepsy; VPA, valproate; CBZ, carbamazepine; T3, triiodothyronine; EEG, electroencephalogram; GABA, Gamma-Amino-Butyric Acid; T4, thyroxin; PHT, phenytoin; OXC, oxcarbazepine; PB, phenobarbital; LEV, levetiracetam; LTG, lamotrigine; EIASMs, Enzyme-inducing antiseizure medications; GnRH, gonadotropin-releasing hormone; BMD, bone mineral density; VDR, Vitamin D receptor; HPO, hypothalamic-pituitary-ovarian; E2, Estradiol; AP, allopregnanolone; FSH, follicle-stimulating hormone; TLE, temporal lobe epilepsy; LH, luteinizing hormone; HC, hormonal contraceptive; PRL, prolactin.
Funding
This study was funded by Major science and technology projects of Zhejiang province (2023C03080), and Key projects of major health science and technology plan of Zhejiang Province (WKJ-ZJ-2129).
Disclosure
The authors declare no competing interests in this work.
References
1. Fiest KM, Sauro KM, Wiebe S, Patten SB. Prevalence and incidence of epilepsy. Neurology. 2017;88(3):296–303. doi:10.1212/WNL.0000000000003509
2. Morrell MJ. Reproductive and metabolic disorders in women with epilepsy. Epilepsia. 2003;44(s4):11–20. doi:10.1046/j.1528-1157.44.s4.2.x
3. Li S, Zhang L, Wei N, Tai Z, Yu C, Xu Z. Research progress on the effect of epilepsy and antiseizure medications on PCOS Through HPO Axis. Front Endocrinol. 2021;12:787854. doi:10.3389/fendo.2021.787854
4. Sawicka-Gutaj N, Gruszczyński D, Zawalna N, et al. Microbiota Alterations in Patients with Autoimmune Thyroid Diseases: a Systematic Review. Int J Mol Sci. 2022;23(21):13450. doi:10.3390/ijms232113450
5. Rochtus AM, Herijgers D, Jansen K, Decallonne B. Antiseizure medications and thyroid hormone homeostasis: literature review and practical recommendations. Epilepsia. 2022;63(2):259–270. doi:10.1111/epi.17117
6. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550–1562. doi:10.1016/S0140-6736(17)30703-1
7. Bernal J. Thyroid hormone regulated genes in cerebral cortex development. J Endocrinol. 2017;232(2):R83–R97. doi:10.1530/JOE-16-0424
8. Prezioso G, Giannini C, Chiarelli F. Effect of thyroid hormones on neurons and neurodevelopment. Horm Res Paediatr. 2018;90(2):73–81. doi:10.1159/000492129
9. Su Y, Izumi T, Kitsu M, Fukuyama Y. Seizure threshold in juvenile myoclonic epilepsy with graves disease. Epilepsia. 1993;34(3):488–492. doi:10.1111/j.1528-1157.1993.tb02589.x
10. Broutian A, Dolgova S, Belyakova-Bodina A, Abramova A, Lukianova A, Noskova T. Bitemporal independent 3-Hz spike-and-waves in adult patient with idiopathic generalized epilepsy and Graves disease. Clin Neurophysiol Pract. 2020;5:206–208. doi:10.1016/j.cnp.2020.09.001
11. Sundaram SM, Marx R, Lesslich HM, Dietzel ID. Deficiency of thyroid hormone reduces voltage-gated Na+ currents as well as expression of Na+/K+-ATPase in the mouse hippocampus. Int J Mol Sci. 2022;23(8):4133. doi:10.3390/ijms23084133
12. Wan RQ, Noguera EC, Weiss SR. Anticonvulsant effects of intra-hippocampal injection of TRH in amygdala kindled rats. NeuroReport. 1998;9(4):677–682. doi:10.1097/00001756-199803090-00021
13. Pekary AE, Sattin A. Regulation of TRH and TRH-related peptides in rat brain by thyroid and steroid hormones. Peptides. 2001;22(7):1161–1173. doi:10.1016/S0196-9781(01)00429-6
14. Wiens SC, Trudeau VL. Thyroid hormone and γ-aminobutyric acid (GABA) interactions in neuroendocrine systems. Comp Biochem Physiol a Mol Integr Physiol. 2006;144(3):332–344. doi:10.1016/j.cbpa.2006.01.033
15. Tamijani SMS, Karimi B, Amini E, et al. Thyroid hormones: possible roles in epilepsy pathology. Seizure. 2015;31:155–164. doi:10.1016/j.seizure.2015.07.021
16. O’Shaughnessy KL, Thomas SE, Spring SR, Ford JL, Ford RL, Gilbert ME. A transient window of hypothyroidism alters neural progenitor cells and results in abnormal brain development. Sci Rep. 2019;9(1):4662. doi:10.1038/s41598-019-40249-7
17. Motta E. Epilepsy and hormones. Neurol Neurochir Pol. 2000;34(1):31–36.
18. Shapiro S, Kubek M, Sanders S, Durbin S, Goodwin S, Javed T. Regional changes in central nervous system thyrotropin-releasing hormone after pentylenetetrazol-induced seizures in dogs. Neurosurgery. 1992;31(5):935–939. doi:10.1227/00006123-199211000-00017
19. Grahame-Smith DG, Green AR, Costain DW. MECHANISM OF THE ANTIDEPRESSANT ACTION OF ELECTROCONVULSIVE THERAPY. Lancet. 1978;311:8058):254–257. doi:10.1016/S0140-6736(78)90495-6
20. Luef G. Hormonal alterations following seizures. Epilepsy Behav. 2010;19(2):131–133. doi:10.1016/j.yebeh.2010.06.026
21. Han JY, Lee IG, Shin S, Park J. Seizure duration may increase thyroid-stimulating hormone levels in children experiencing a seizure. J Int Med Res. 2020;48(5):030006051988840. doi:10.1177/0300060519888401
22. Spergel DJ. Modulation of gonadotropin-releasing hormone neuron activity and secretion in mice by non-peptide neurotransmitters, gasotransmitters, and gliotransmitters. Front Endocrinol. 2019;10:329. doi:10.3389/fendo.2019.00329
23. Eren F, Özgüncü C, Ekmekci AH. The relationship between pituitary gland dimensions, thyroid functions, and seizure activity in patients with epilepsy. Arch Epilepsy. 2022;28(1):39–42. doi:10.54614/ArchEpilepsy.2022.93585
24. Hamed SA. The effect of antiepileptic drugs on thyroid hormonal function: causes and implications. Expert Rev Clin Pharmacol. 2015;8(6):741–750. doi:10.1586/17512433.2015.1091302
25. Han Y, Yang J, Zhong R, Guo X, Cai M, Lin W. Side effects of long-term oral anti-seizure drugs on thyroid hormones in patients with epilepsy: a systematic review and network meta-analysis. Neurol Sci. 2022;43(9):5217–5227. doi:10.1007/s10072-022-06120-w
26. Verrotti A, Laus M, Scardapane A, Franzoni E, Chiarelli F. Thyroid hormones in children with epilepsy during long-term administration of carbamazepine and valproate. Eur J Endocrinol. 2009;160(1):81–86. doi:10.1530/EJE-08-0325
27. Svalheim S, Sveberg L, Mochol M, Taubøll E. Interactions between antiepileptic drugs and hormones. Seizure. 2015;28:12–17. doi:10.1016/j.seizure.2015.02.022
28. Zhang YX, Shen CH, Lai QL, et al. Effects of antiepileptic drug on thyroid hormones in patients with epilepsy: a meta-analysis. Seizure. 2016;35:72–79. doi:10.1016/j.seizure.2016.01.010
29. Vainionpää LK, Mikkonen K, Rättyä J, et al. Thyroid function in girls with epilepsy with carbamazepine, oxcarbazepine, or valproate monotherapy and after withdrawal of medication. Epilepsia. 2004;45(3):197–203. doi:10.1111/j.0013-9580.2004.26003.x
30. Miller J, Carney P. Central hypothyroidism with oxcarbazepine therapy. Pediatr Neurol. 2006;34(3):242–244. doi:10.1016/j.pediatrneurol.2005.08.032
31. Valproic acid and CEBPalpha-mediated regulation of adipokine gene expression in hypothalamic neurons and 3T3-L1 adipocytes. Available from: https://pubmed.ncbi.nlm.nih.gov/18212493/.
32. Dunn D, Turner C. Hypothyroidism in Women. Nurs Womens Health. 2016;20(1):93–98. doi:10.1016/j.nwh.2015.12.002
33. Silva JF, Ocarino NM, Serakides R. Thyroid hormones and female reproduction. Biol Reprod. 2018;99(5):907–921. doi:10.1093/biolre/ioy115
34. Andersen SL, Andersen S, Vestergaard P, Olsen J. Maternal thyroid function in early pregnancy and child neurodevelopmental disorders: a Danish nationwide case-cohort study. Thyroid. 2018;28(4):537–546. doi:10.1089/thy.2017.0425
35. Ge GM, Leung MTY, Man KKC, et al. Maternal thyroid dysfunction during pregnancy and the risk of adverse outcomes in the offspring: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2020;105(12):3821–3841. doi:10.1210/clinem/dgaa555
36. Zhuo L, Wang Z, Yang Y, Liu Z, Wang S, Song Y. Obstetric and offspring outcomes in isolated maternal hypothyroxinaemia: a systematic review and meta-analysis. J Endocrinol Invest. 2022;46(6):1087–1101. doi:10.1007/s40618-022-01967-4
37. Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2017;342:68–100. doi:10.1016/j.neuroscience.2015.09.070
38. Ahmed OM, El‐Tawab SM A, Ahmed RG. Effects of experimentally induced maternal hypothyroidism and hyperthyroidism on the development of rat offspring: i. The development of the thyroid hormones–neurotransmitters and adenosinergic system interactions. Int J Dev Neurosci. 2010;28(6):437–454. doi:10.1016/j.ijdevneu.2010.06.007
39. Johnson EL, Burke AE, Wang A, Pennell PB. Unintended pregnancy, prenatal care, newborn outcomes, and breastfeeding in women with epilepsy. Neurology. 2018;91(11):e1031–e1039. doi:10.1212/WNL.0000000000006173
40. Pepe J, Body JJ, Hadji P, et al. Osteoporosis in premenopausal women: a clinical narrative review by the ECTS and the IOF. J Clin Endocrinol Metab. 2020;105(8):2487–2506. doi:10.1210/clinem/dgaa306
41. Beerhorst K, Tan IY, De Krom M, Verschuure P, Aldenkamp AP. Antiepileptic drugs and high prevalence of low bone mineral density in a group of inpatients with chronic epilepsy. Acta Neurol Scand. 2013;128(4):273–280. doi:10.1111/ane.12118
42. Baxendale S. Seeing the light? Seizures and sunlight. Epilepsy Res. 2009;84(1):72–76. doi:10.1016/j.eplepsyres.2008.11.015
43. Holló A, Clemens Z, Kamondi A, Lakatos P, Szűcs A. Correction of vitamin D deficiency improves seizure control in epilepsy: a pilot study. Epilepsy Behav. 2012;24(1):131–133. doi:10.1016/j.yebeh.2012.03.011
44. Dong N, Guo HL, Hu YH, et al. Association between serum vitamin D status and the anti-seizure treatment in Chinese children with epilepsy. Front Nutr. 2022;9:968868. doi:10.3389/fnut.2022.968868
45. McGrath J, Feron F, Eyles D, Mackay-Sim A. Vitamin D: the neglected neurosteroid? Trends Neurosci. 2001;24(10):570–571. doi:10.1016/S0166-2236(00)01949-4
46. Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the Vitamin D receptor and 1α-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi:10.1016/j.jchemneu.2004.08.006
47. Kalueff AV, Minasyan A, Keisala T, Kuuslahti M, Miettinen S, Tuohimaa P. Increased severity of chemically induced seizures in mice with partially deleted Vitamin D receptor gene. Neurosci Lett. 2006. doi:10.1016/j.neulet.2005.10.007
48. xia DX, Wang Y, hong QZ. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009;30(4):379–387. doi:10.1038/aps.2009.24
49. Kalueff AV, Minasyan A, Keisala T, Kuuslahti M, Miettinen S, Tuohimaa P. The Vitamin D neuroendocrine system as a target for novel neurotropic drugs. CNS Neurolog Disord Drug Targ. 2006;5(3):363–371.
50. Sarto-Jackson I, Sieghart W. Assembly of GABA A receptors (Review). Mol Member Biol. 2008;25(4):302–310. doi:10.1080/09687680801914516
51. Miratashi Yazdi SA, Abbasi M, Miratashi Yazdi SM. Epilepsy and vitamin D: a comprehensive review of current knowledge. Rev Neurosci. 2017;28(2):185–201. doi:10.1515/revneuro-2016-0044
52. Bartels LE, Jørgensen SP, Agnholt J, Kelsen J, Hvas CL, Dahlerup JF. 1,25-dihydroxyvitamin D3 and dexamethasone increase interleukin-10 production in CD4+ T cells from patients with Crohn’s disease. Int Immunopharmacol. 2007;7:1755–1764. doi:10.1016/j.intimp.2007.09.016
53. Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88(2):491S–499S. doi:10.1093/ajcn/88.2.491S
54. Lefebvre D, Hellencourt C, Montero‐Menei CN, Bernard R, Couez D. Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J Neurosci Res. 2003;71(4):575–582. doi:10.1002/jnr.10491
55. Miziak B, Chrościńska-Krawczyk M, Czuczwar SJ. An update on the problem of osteoporosis in people with epilepsy taking antiepileptic drugs. Expert Opin Drug Saf. 2019;18(8):679–689. doi:10.1080/14740338.2019.1625887
56. Kanner AM, Bicchi MM. Antiseizure medications for adults with epilepsy: a review. JAMA. 2022;327(13):1269–1281. doi:10.1001/jama.2022.3880
57. Meier C, Kraenzlin ME. Antiepileptics and bone health. Ther Adv Musculoskelet Dis. 2011;3(5):235–243. doi:10.1177/1759720X11410769
58. Fan HC, Lee HS, Chang KP, et al. The Impact of Anti-Epileptic Drugs on Growth and Bone Metabolism. Int J Mol Sci. 2016;17(8):1242. doi:10.3390/ijms17081242
59. Pack A. Bone health in people with epilepsy: is it impaired and what are the risk factors? Seizure. 2008;17(2):181–186. doi:10.1016/j.seizure.2007.11.020
60. Onodera K, Takahashi A, Sakurada S, Okano Y. Effects of phenytoin and/or vitamin K2 (menatetrenone) on bone mineral density in the tibiae of growing rats. Life Sci. 2002;70(13):1533–1542. doi:10.1016/S0024-3205(01)01522-3
61. Arora E, Singh H, Gupta Y. Impact of antiepileptic drugs on bone health: need for monitoring, treatment, and prevention strategies. J Fam Med Prim Care. 2016;5(2):248. doi:10.4103/2249-4863.192338
62. Kim DW, Lee SY, Shon YM, Kim JH. Effects of new antiepileptic drugs on circulatory markers for vascular risk in patients with newly diagnosed epilepsy. Epilepsia. 2013;54(10):e146–9. doi:10.1111/epi.12338
63. Behera J, Bala J, Nuru M, Tyagi SC, Tyagi N. Homocysteine as a Pathological Biomarker for Bone Disease. J Cell Physiol. 2017;232(10):2704–2709. doi:10.1002/jcp.25693
64. Börjesson AE, Lagerquist MK, Windahl SH, Ohlsson C. The role of estrogen receptor α in the regulation of bone and growth plate cartilage. Cell Mol Life Sci. 2013;70(21):4023–4037. doi:10.1007/s00018-013-1317-1
65. Fischer V, Haffner-Luntzer M. Interaction between bone and immune cells: implications for postmenopausal osteoporosis. Semin Cell Dev Biol. 2022;123:14–21. doi:10.1016/j.semcdb.2021.05.014
66. Bergqvist AGC, Schall JI, Stallings VA. Vitamin D status in children with intractable epilepsy, and impact of the ketogenic diet. Epilepsia. 2007;48(1):66–71. doi:10.1111/j.1528-1167.2006.00803.x
67. Molteberg E, Taubøll E, Kverneland M, et al. Substantial early changes in bone and calcium metabolism among adult pharmacoresistant epilepsy patients on a modified Atkins diet. Epilepsia. 2022;63(4):880–891. doi:10.1111/epi.17169
68. Elmazny A, Amer H, Rashed L, Khalil S, Magdy R. Vitamin D status of untreated children and adolescent Egyptian patients with genetic generalized epilepsy: a case–control study. Epilepsy Behav. 2020;103:106840. doi:10.1016/j.yebeh.2019.106840
69. Garip S, Sahin D, Severcan F. Epileptic seizure-induced structural and functional changes in rat femur and tibia bone tissues: a Fourier transform infrared imaging study. J Biomed Opt. 2013;18(11):111409. doi:10.1117/1.JBO.18.11.111409
70. Rojansky N, Brzezinski A, Schenker JG. Seasonally in human reproduction: an update. Hum Reprod. 1992;7(6):735–745. doi:10.1093/oxfordjournals.humrep.a137729
71. Berridge MJ. Vitamin D deficiency: infertility and neurodevelopmental diseases (attention deficit hyperactivity disorder, autism, and schizophrenia). Am J Physiol-Cell Physiol. 2018;314(2):C135–C151. doi:10.1152/ajpcell.00188.2017
72. Ozkan S, Jindal S, Greenseid K, et al. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. 2010;94(4):1314–1319. doi:10.1016/j.fertnstert.2009.05.019
73. Harris HR, Chavarro JE, Malspeis S, Willett WC, Dairy-Food MSA. Calcium, Magnesium, and Vitamin D intake and endometriosis: a prospective cohort study. Am J Epidemiol. 2013;177(5):420–430. doi:10.1093/aje/kws247
74. Ciavattini A, Serri M, Delli Carpini G, Morini S, Clemente N. Ovarian endometriosis and vitamin D serum levels. Gynecol Endocrinol. 2017;33(2):164–167. doi:10.1080/09513590.2016.1239254
75. Anagnostis P, Karras S, Goulis DG. Vitamin D in human reproduction: a narrative review: vitamin D and reproduction. Int J Clin Pract. 2013;67(3):225–235. doi:10.1111/ijcp.12031
76. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–231. doi:10.1038/nrendo.2010.217
77. Pal L, Zhang H, Williams J, et al. Vitamin D status relates to reproductive outcome in women with polycystic ovary syndrome: secondary analysis of a multicenter randomized controlled trial. J Clin Endocrinol Metab. 2016;101(8):3027–3035. doi:10.1210/jc.2015-4352
78. Kaushal M, Magon N. Vitamin D in pregnancy: a metabolic outlook. Indian J Endocrinol Metab. 2013;17(1):76. doi:10.4103/2230-8210.107862
79. Wagner CL, McNeil R, Hamilton SA, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol. 2013;208(2):137.e1–137.e13. doi:10.1016/j.ajog.2012.10.888
80. Elsori DH, Hammoud MS. Vitamin D deficiency in mothers, neonates and children. J Steroid Biochem Mol Biol. 2018;175:195–199. doi:10.1016/j.jsbmb.2017.01.023
81. Duijts L, Reiss IK, Brusselle G, De Jongste JC. Early origins of chronic obstructive lung diseases across the life course. Eur J Epidemiol. 2014;29(12):871–885. doi:10.1007/s10654-014-9981-5
82. Von Websky K, Hasan AA, Reichetzeder C, Tsuprykov O, Hocher B. Impact of vitamin D on pregnancy-related disorders and on offspring outcome. J Steroid Biochem Mol Biol. 2018;180:51–64. doi:10.1016/j.jsbmb.2017.11.008
83. Roth DE, Morris SK, Zlotkin S, et al. Vitamin D supplementation in pregnancy and lactation and infant growth. N Engl J Med. 2018;379(6):535–546. doi:10.1056/NEJMoa1800927
84. Vélez-Ruiz NJ, Pennell PB. Issues for women with epilepsy. Neurol Clin. 2016;34(2):411–425. doi:10.1016/j.ncl.2015.11.009
85. Velíšková J, DeSantis KA. Sex and hormonal influences on seizures and epilepsy. Horm Behav. 2013;63(2):267–277. doi:10.1016/j.yhbeh.2012.03.018
86. Reddy DS, Thompson W, Calderara G. Molecular mechanisms of sex differences in epilepsy and seizure susceptibility in chemical, genetic and acquired epileptogenesis. Neurosci Lett. 2021;750:135753. doi:10.1016/j.neulet.2021.135753
87. Pottoo FH, Javed MN, Barkat MA, et al. Estrogen and serotonin: complexity of interactions and implications for epileptic seizures and epileptogenesis. Curr Neuropharmacol. 2019;17(3):214–231. doi:10.2174/1570159X16666180628164432
88. Del Río JP, Alliende MI, Molina N, Serrano FG, Molina S, Vigil P. Steroid hormones and their action in women’s brains: the importance of hormonal balance. Front Public Health. 2018;6:141. doi:10.3389/fpubh.2018.00141
89. Motta E, Golba A, Ostrowska Z, et al. Progesterone therapy in women with epilepsy. Pharmacol Rep PR. 2013;65(1):89–98. doi:10.1016/s1734-1140(13)70967-8
90. Reddy DS, Jian K. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABA A receptors. J Pharmacol Exp Ther. 2010;334(3):1031–1041. doi:10.1124/jpet.110.169854
91. Hamed SA. The effect of epilepsy and antiepileptic drugs on sexual, reproductive and gonadal health of adults with epilepsy. Expert Rev Clin Pharmacol. 2016;9(6):807–819. doi:10.1586/17512433.2016.1160777
92. Amini L, Hematian M, Montazeri A, Gharegozli K. Comparing the frequency of polycystic ovary syndrome in women with and without epilepsy. J Fam Med Prim Care. 2018;7(1):16. doi:10.4103/jfmpc.jfmpc_115_17
93. Edwards HE, Burnham WM, Ng MM, Asa S, MacLusky NJ. Limbic seizures alter reproductive function in the female rat. Epilepsia. 1999;40(10):1370–1377. doi:10.1111/j.1528-1157.1999.tb02007.x
94. Hum KM, Megna S, Burnham WM. The effects of right and left amygdala kindling on the female reproductive system in rats. Epilepsia. 2009;50(4):880–886. doi:10.1111/j.1528-1167.2008.01982.x
95. Li J, Robare JA, Gao L, et al. Dynamic and sex-specific changes in gonadotropin-releasing hormone neuron activity and excitability in a mouse model of temporal lobe epilepsy. eneuro. 2018;5:5. doi:10.1523/ENEURO.0273-18.2018
96. Herzog AG, Coleman AE, Jacobs AR, et al. Interictal EEG discharges, reproductive hormones, and menstrual disorders in epilepsy. Ann Neurol. 2003;54(5):625–637. doi:10.1002/ana.10732
97. Gooneratne IK, Wimalaratna S. Update on management of epilepsy in women for the non-neurologist. Postgrad Med J. 2016;92(1091):554–559. doi:10.1136/postgradmedj-2016-134191
98. Sidhu HS, Srinivasa R, Sadhotra A. Evaluate the effects of antiepileptic drugs on reproductive endocrine system in newly diagnosed female epileptic patients receiving either Valproate or Lamotrigine monotherapy: a prospective study. Epilepsy Res. 2018;139:20–27. doi:10.1016/j.eplepsyres.2017.10.016
99. Ganju BL, India TP. Management of epilepsy in pregnancy: a report from the International league against epilepsy task force on women and pregnancy. Epileptic Disord. 2019;21(6).
100. Burakgazi Dalkilic E. Effects of antiepileptic drugs on hormones. Neurosci Lett. 2021;754:135800. doi:10.1016/j.neulet.2021.135800
101. Herzog AG, Friedman MN. Menstrual cycle interval and ovulation in women with localization-related epilepsy. Neurology. 2001;57(11):2133–2135. doi:10.1212/WNL.57.11.2133
102. MacEachern DB, Mandle HB, Herzog AG. Infertility, impaired fecundity, and live birth/pregnancy ratio in women with epilepsy in the USA: findings of the epilepsy birth control registry. Epilepsia. 2019;60(9):1993–1998. doi:10.1111/epi.16312
103. Klein P, Serje A, Pezzullo JC. Premature ovarian failure in women with epilepsy. Epilepsia. 2001;42(12):1584–1589. doi:10.1046/j.1528-1157.2001.13701r.x
104. Pack AM, Davis AR, Kritzer J, Yoon A, Camus A. Antiepileptic drugs: are women aware of interactions with oral contraceptives and potential teratogenicity? Epilepsy Behav. 2009;14(4):640–644. doi:10.1016/j.yebeh.2009.01.024
105. Herzog AG, Mandle HB, Cahill KE, Fowler KM, Hauser WA, Davis AR. Contraceptive practices of women with epilepsy: findings of the epilepsy birth control registry. Epilepsia. 2016;57(4):630–637. doi:10.1111/epi.13320
106. Pennell PB. Antiepileptic drug pharmacokinetics during pregnancy and lactation. Neurology. 2003;61(6 Suppl 2):S35–42. doi:10.1212/wnl.61.6_suppl_2.s35
107. Gaffield ME, Culwell KR, Lee CR. The use of hormonal contraception among women taking anticonvulsant therapy. Contraception. 2011;83(1):16–29. doi:10.1016/j.contraception.2010.06.013
108. Viale L, Allotey J, Cheong-See F, et al. Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet. 2015;386(10006):1845–1852. doi:10.1016/S0140-6736(15)00045-8
109. Stephen LJ, Harden C, Tomson T, Brodie MJ. Management of epilepsy in women. Lancet Neurol. 2019;18(5):481–491. doi:10.1016/S1474-4422(18)30495-2
110. Tao L, Duan Z, Liu Y, Hou H, Zhang X. Correlation of sexual dysfunction with sex hormone and estrogen receptor gene polymorphism in Chinese Han women with epilepsy. Epilepsy Res. 2021;169:106527. doi:10.1016/j.eplepsyres.2020.106527
111. Bauer J. Epilepsy and prolactin in adults: a clinical review. Epilepsy Res. 1996;24(1):1–7. doi:10.1016/0920-1211(96)00009-5
112. Balercia G, Boscaro M, Lombardo F, Carosa E, Lenzi A, Jannini EA. Sexual symptoms in endocrine diseases: psychosomatic perspectives. Psychother Psychosom. 2007;76(3):134–140. doi:10.1159/000099840
113. Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke. Stroke. 2016;47(7):1734–1741. doi:10.1161/STROKEAHA.116.013052
114. Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15(1):106–115. doi:10.1016/S1474-4422(15)00225-2
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.