Back to Journals » Patient Preference and Adherence » Volume 18
Eliciting Depression Patients’ Preferences for Medication Management: A Protocol for Discrete Choice Experiment
Authors Xie P, Li HQ , Peng WL, Yang H
Received 15 October 2023
Accepted for publication 24 January 2024
Published 2 February 2024 Volume 2024:18 Pages 289—300
DOI https://doi.org/10.2147/PPA.S444800
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Peng Xie,1,* Hui-Qin Li,1,* Wan-Lin Peng,2 Hao Yang3
1People’s Hospital of Deyang City, Deyang City, Sichuan, 618000, People’s Republic of China; 2School of Nursing, Guangxi University of Chinese Medicine, Nanning, Guangxi, 530004, People’s Republic of China; 3West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, Chengdu, Sichuan, 610041, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui-Qin Li, People’s Hospital of Deyang City, Deyang City, Sichuan, 618000, People’s Republic of China, Tel + 86-0838-2208091, Email [email protected]
Introduction: Depression threatens people’s lives and imposes huge economic burden. Antidepressant therapy is the first-line treatment for depression, and patient adherence to medication is the key to successful treatment. Depression patients have poor medication adherence, which leads to failure of depression management and significantly poorer clinical outcomes. Incorporating patient preferences into clinical decisions can improve uptake rates, optimize treatment adherence. A discrete choice experiment (DCE) can elicit and quantify individual preferences. Previous DCE studies were conducted in developed countries and ignored the influences of factors other than the medication. This paper outlines an ongoing DCE that aims to (1) explore medication-management-related characteristics that may affect depression patients’ adherence to antidepressant, (2) elicit how depression patients consider the trade-offs among different medication managements.
Methods: The six attributes and their levels were developed through a literature review, semi-structured interviews and experts and focus group discussions. A fractional factorial design in the software Ngene 1.2 version was used to generate 36 choice sets, and they were divided into 3 blocks. A mixed logit model will be used to explore the patients’ preferences, willingness to pay and uptake rate of depression patients for medication management attributes.
Results: The final questionnaire consists of three parts. The first is the introduction, which introduces the purpose of the study and the requirements of completing the questionnaire. This was followed by a general information questionnaire, which included sociodemographic characteristics. The last part is DCE tasks, which include 13 DCE choice sets, and each choice set include two alternative and one “opt-out” option. The pilot-test results showed the questionnaire was easy to understand and could be used in formal surveys.
Conclusion: Our study shows how the development process of the study can be conducted and reported systematically and rigorously according to the theoretical foundation and design principles in DCE.
Keywords: depressive disorder, patient preferences, adherence, discrete choice experiment, protocol
Introduction
Depression is one of the most common mental disorders, with 350 million people worldwide1 and approximately 95 million people suffering from depression in China.2 Depression threatens people’s lives. It is the main risk factor for suicidal behavior3,4 and the main disease of patients with attempted suicide.5 At the same time, depression is one of the most expensive diseases6,7 and imposes a huge economic burden on society.8,9 According to the World Health Organization, depression will be the first in the global burden of disease in 2030, resulting in 12 billion days of lost productivity globally each year at an estimated cost of $925 billion.10 Compared with adults without common mental disorders, 4–15 more days out of role per year were recorded due to depression, and for depression, additional time lost per year due to presenteeism was 11–25 partial disability days for depression.11–13 Thus, the treatment of depression has become an urgent global problem.
Antidepressant therapy is still the first-line treatment for depression,14 and patient adherence to medication is the key to successful treatment for depression.10 Premature discontinuation of medication leads to significantly poorer clinical outcomes such as higher levels of depression,15 increases emergency department (ED) visits and hospitalizations,16,17 and impacts the overall health care utilization rate.18 Unfortunately, patients with depression have poor medication adherence, with 50%-70% of patients not being compliant,16,19,20 which is one of the main reasons for the limited treatment of depression and the failure of depression management.21 Therefore, it is necessary to develop and improve medication management strategies to improve medication adherence in patients with depression.
Incorporating patient preferences into clinical decisions can improve uptake rates, optimize treatment adherence, and reduce withdrawal during treatment.22–25 A discrete choice experiment (DCE) is an innovative approach to elicit and quantify individual preferences. Although some DCE studies26–28 have investigated the preferences of depression patients for pharmacotherapy, these studies were conducted in developed countries, and there is a lack of data on the preferences of depression patients in developing countries. According to the WHO report, there is a huge gap in the treatment of severe mental disorders between developed and developing countries.29 Due to the improvement of the drug subsidy system, differences in financial burden for individuals are minor in developed countries.30 In low and middle income countries, most patients with mental disorders do not receive cost-effective treatment.31 Therefore, the findings of these studies are not applicable in developing countries. In addition, these studies focused on the outcome properties of the medication and ignored the influences of factors other than the medication, such as follow-up strategies and health guidance, on patients.
Therefore, we are conducting a DCE in China to explore the preferences of patients with depression for oral medication, which could help clinicians and policymakers in developing countries develop distinctive medication management and improve medication adherence in patients with depression. This paper outlines an ongoing DCE that aims to (1) explore medication-management-related characteristics that may affect depression patients’ adherence to antidepressant, (2) elicit how depression patients consider the trade-offs among different medication managements.
Methods
Study Setting
This study will mainly collect data from four hospitals in Sichuan Province, all of which have well-established depression treatment systems, such as outpatient and inpatient departments.
Study Design
This study follows the design principle of DCE to explore the preferences of patients with depression for medication management, and the development process of the DCE is as follows: identifying and defining attributes and levels, generating choice sets and designing questionnaire, collecting the survey data and analyzing and explaining the results. The main stages of a DCE are shown in Figure 1.
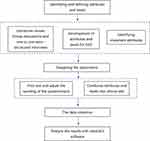 |
Figure 1 The development process of the DCE. |
Identifying and Defining Attributes and Levels
Literature Review
Identifying potential attributes and outline interviews based on a literature review. The literature related to the adherence of patients with depression was reviewed in the Web of Science, PubMed, Embase, Wanfang database and CNKI. Our retrieval strategy was as follows: (“Depress*” OR “Depressive Disorder”[MESH] OR “Dysthymic Disorder[MESH]” OR “MDD”) AND (“Treatment Adherence and Compliance”[MESH] OR “Medication Adherence”[MESH] OR “Patient Compliance”[MESH] OR “Patient Satisfaction”[Mesh] OR “Patient Preference”[Mesh]). In order to obtain more comprehensive information, based on the snowball method, we reviewed the reference lists of the articles obtained from the literature search one by one, and recorded the relevant literatures, and obtained 19 literatures.32–50
After screening the search results, we focused on drug response, drug properties, drug access and storage convenience, medication follow-up, medication instructors and other factors that affect the medication compliance of patients with depression or other mental disorders. Considering that China currently does not include antidepressants in free medical care, the cost attribute is an institutional factor that must be considered. Finally, we developed a list of potential attributes and levels (see Supplement 1) and explored them in more detail in the following focus group discussions (FGDs) and one-on-one interviews.
FGDs and One-on-One Interviews
FGDs and one-on-one interviews were conducted to further explore the potential attributes obtained from the literature, and new contextual attributes were obtained from the perspective of experts and patients with depression. To ensure sufficient heterogeneity, objective sampling was conducted for depression patients who met the inclusion and exclusion criteria based on hospital, age, severity of depression, recurrence, education level and gender (see Sampling and Recruitment for inclusion and exclusion criteria). The topics of focus group discussion and one-on-one interviews mainly included the following: (1) the accessibility of medical services, (2) the attitudes and views of patients with depression on treatment, (3) the availability of emotional or financial resources, (4) factors affecting the medication of patients with depression, and (5) available drug management strategies for patients with depression.
A researcher conducted one-on-one interviews with patients, and the data reached saturation after 15 interviews. After that, four focus group discussions (FGDs) were held, with three depression patients in each group, who did not participate in one-on-one interviews. Finally, in order to obtain more attributes and determine the suitability of potential attributes in current medical and health care, another researcher conducted one-to-one consultation with four experts (a psychiatric nurse, a pharmacist, a psychiatrist and a health economist) who have worked for more than ten years. Information from all FGDs and interviews was recorded and transcribed verbatim by an experienced researcher.
Qualitative Analysis of FGDs and One-on-One Interviews
The two authors analyzed qualitative data using thematic analysis. They independently read and analyzed the transcripts to define and compare all major and minor themes, which are the potential attributes and levels that can be extracted. After summarizing in text and tables, explanations and discussions with coinvestigators to obtain coinvestigator input form a broader list of attributes.
New attributes are revealed in the FGDs and interviews. Home management of medication, especially follow-up by a professional, is critical to whether a depressed person seeks or receives antidepressant treatment. At the same time, they pay special attention to adverse drug reactions. Participants emphasized that easier access to medication may promote acceptance and adherence to antidepressants. Diversified follow-up methods can respond to the changing medication management expectations of patients with depression, which is consistent with the opinions of experts. Cost seems to be a potential barrier to drug adherence in patients with depression. At present, China’s medical insurance system does not include the cost of drug treatment for patients with depression. Therefore, patients must pay for antidepressants at their own expense. Experts pointed out that the recurrence of depressive symptoms in patients is a potential factor affecting their adherence to medication, and relapsed patients may have more difficulty adhering to medication than first-time patients.
Development of Attributes and Levels for DCE
Through a literature review, FGDs, and one-on-one interviews, 10 key characteristics associated with taking medication in patients with depression were identified as follows: “adverse reactions”, “duration of efficacy”, “storage method”, “dosage form”, “cost”, “medication guidance provider”, “convenience of purchase”, “guidance continuity”, “follow-up method”, “follow-up frequency”, “medication reminder”, and “oral method”. Each DCE uses fewer than 10 attributes, which can reduce the cognitive burden on respondents.51 Referring to other DCEs, the importance of each attribute is divided into the following three levels: most, somewhat and least, and patients are required to vote on the importance of each attribute. Then, according to the number of “most” votes, the attributes are sorted, and the priority of the attributes is determined.
Some researchers believe that the best number of attributes in a DCE is 6.52 In our study, according to the ranking results, the six attributes of “Adverse Reactions”, “Provider”, “Follow-up Frequency”, “Cost”, “Follow-up Methods”, and “Convenience of Purchase” were finally included. These attributes form the basis of the final DCE design of this study. It is worth noting that “adverse reactions” rank first, which is the focus of the vast majority of patients with depression.
Each attribute is assigned levels based on potential levels indicated in the literature, participants’ descriptions of attributes, and expert recommendations applicable to the current health system, with the final attributes and their levels detailed in Table 1.
 |
Table 1 List of Attribute and Levels |
Generating Choice Sets
An unlabeled design was used to construct choice sets to avoid reducing respondents’ attention to target attributes.53 A full fractional design that includes all possible combinations of attribute levels is considered the best. In our study, the attribute levels can be combined into 23,570 choice sets by using a full fractional design ((35×2) × (35×2-1)=23,570). However, too many choice sets will not only lead to a high cognitive burden of respondents but also consume too much time and economic resources. Ngene allows researchers to force designs to remain orthogonal while optimizing efficiency.54 Therefore, after assuming the preferences for the attribute level based on qualitative interviews, 36 choice sets were generated by using the fractional factorial design developed by Burgess and Street in Ngene software55 and randomly divided into 3 blocks.56 To test the consistency of respondents’ selections, the second choice set for each block was repeated as the thirteenth choice set. In addition, an “opt-out” option was included for each choice set to determine whether depressed patients were likely to participate in antidepressant therapy.57
Questionnaire Design and Pilot Testing
The questionnaire consists of three parts. The first is the introduction, which introduces the purpose of the study and the requirements of completing the questionnaire. This was followed by a general information questionnaire, which included sociodemographic characteristics such as age, gender, education level, relapse of depression and severity of depression. The last part is the DCE selection task. It is worth noting that before a choice task, each attribute and its level will be described in detail to help participants understand. An example of a choice set is shown in Figure 2.
 |
Figure 2 An example of choice set. |
Based on the sample size of the pilot test in other DCEs (N=6 - 24),58–60 20 patients with depression were invited to participate in the pilot test. First, the 20 patients were asked to fill out the DCE questionnaire, and the number of projects they completed and the completion time were recorded. At the same time, all patients were asked to “think aloud” during the completion of each choice set.61 After that, personal cognitive interviews were conducted face-to-face or over the phone to improve the wording and understandability of DCE.62 All 20 patients with depression completed the questionnaire, with an average completion time of 6.2 minutes. Their preferences were consistent with the previous assumptions about attribute levels. Of the 20 patients, 16 thought the length of the questionnaire was acceptable and the wording of the questionnaire was clear and easy to understand, while 4 patients thought that an example of choice set should be provided. Therefore, based on feedback from the pilot test, we added an example of a choice set to demonstrate how to fill out the questionnaire, and combined pictures and words to explain more clearly the attributes.
Sampling and Recruitment
The subjects were patients with depression, including depressive relapse patients, and patients with any severity of depression were not excluded. The inclusion criteria are as follows: (1) patients have taken antidepressant drugs, and (2) have a certain ability to read and comprehend text and fill in the questionnaire independently. Patients with other psychiatric disorders were excluded.
The sample size for DCE is influenced by many factors, such as the heterogeneity of the target population, the complexity of the choice tasks, the desired precision of the results, and the need for subgroup analysis.63 Consistent with previous studies, we estimate sample size based on the empirical method proposed by Johnson and Orme.64–66 The calculation formula of the minimum sample size is as follows:
n > 500 c/(t × a)
In this equation, a is the number of alternatives in each choice set (excluding the “opt-out” option), t is the number of choice sets faced by each respondent (excluding the repeatedly included choice set), and c is equal to any attribute’s maximum number of levels. The minimum sample size required for each version of the questionnaire is 63 (t=12, a=2, C=3). There are three versions of the questionnaire for this study, and considering the 20% invalid questionnaire, we needed to recruit at least 228 depression patients to obtain a broad representation. Participants meeting the inclusion and exclusion criteria will be recruited from the outpatient and inpatient departments of four hospitals in Sichuan and will be asked to fill in questionnaires, and the questionnaires will be collected on site. In addition, emails or WeChat of patients who meet the inclusion and exclusion criteria will be obtained from the admission registries of these hospitals, and questionnaires will be provided to them via WeChat or email. The recruitment of participants for questionnaire survey in this study is planned to start in February 2024 and complete in February 2025.
Patient and Public Involvement
Patients and/or the public were not involved in the design, conduct or reporting of this research, but they will be invited in dissemination plans of this research.
Analysis Plan
Multinomial logit (MNL) models with low sample requirements and error rates will be used to preliminarily explore trade-offs between drug management characteristics included in the choice tasks,67 which will contribute to the overall optimization of the model, such as finding more explanatory variables to make factor levels more reasonable.68
However, the MNL model ignores individual heterogeneity and cannot handle differences in random preferences. The mixed logit model allows regression of each parameter interacting with each sociodemographic characteristic to explore differences in preferences between different groups, which compensates for MNL’s shortcomings.69 In the mixed logit model, the cost attribute will be modeled as a continuous variable to estimate the respondents’ willingness to pay (WTP), that is, how much the respondents are willing to pay for the improvement of the attribute level. It is assumed that all coefficients in the model are normally distributed. The statistical significance of the coefficient β indicates whether the attribute level influenced participants’ choices, while the value of the coefficient β indicates the relative importance of an attribute level to the referenced attribute level in a medication management plan. The nlcom command will be used to simulate the uptake rate, that is, the change in the probability that participants will accept a medication management plan when the level of one or more attributes changes compared to the baseline medication management programme.
Ethics and Dissemination
This study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of People’s Hospital of Deyang City [Ethics Number: 2023-04-070-K01] and Biomedical Ethics Committee of Sichuan University [Ethics Number: 20221122]. The recruitment of participants for questionnaire survey in this study is planned to start in February 2024 and complete in February 2025. All information relating to participants will be anonymous, and participants will have the right to withdraw from the study at any time. Prior to FGDs, one-on-one interviews and questionnaire surveys, the nature of the study, objectives and possible risks will be explained, and written informed consent will be obtained.
The DCE analysis will comprehensively cover the preferences of patients with depression on medication management characteristics. Our findings will be used to optimize the current medication management strategies for patients with depression by the following: (1) emphasizing priority intervention areas based on the preferences of patients with depression; for example, if patients with depression have a strong preference for medication guidance provided by nurses, a consistent nursing team can be developed to provide medication guidance to patients with depression; and (2) disseminating knowledge about the relative importance of medication adherence among people with depression and promoting awareness of potential differences among depression patients with different sociodemographic characteristics. The findings of our research will be shared through peer-reviewed journal articles, policy briefs, seminars and online blogs.
Discussion
Previous studies have confirmed that 50% to 70% of patients with depression are not adherent to their medication,70 which leads to worse clinical outcomes.16,19,20 Incorporating patient preferences into clinical decisions can improve adherence. A study explored the barriers and facilitators of adherence to antidepressants in depression patients, but did not weigh the relative importance of these factors, nor did it survey the preferences of depression patients.48 This provides limited reference for future intervention research and the development of medication management strategies. DCE can elicit and quantify individual preferences, and weigh the relative importance of influence factors. Given the poor adherence of patients with depression to taking antidepressants, DCE is used to explore key factors that may affect patient preferences and weigh the relative importance of these factors, allowing for possible strategy improvements or adjustments with a focus on medication management. This study attempts to explore the preferences of depression patients for medication management and comprehensively describe the development process of the study based on the theoretical basis and design principles in DCE research.
Previous studies have explored the treatment preferences of patients with depression, but they carried out in developed countries, and their results are not applicable for generalization to developing countries. Furthermore, these studies have mostly focused on the outcome attributes of treatment, such as treatment duration, side effects, or specifically, the preference characteristics of these treatment services are usually immutable.26,71,72 The aim of this study is to explore the preferences of depression patients in developing countries for medication management, and the results will provide preference data for depression patients in developing countries to provide reference for developing and optimizing medication management strategies. Moreover, our study focuses on the overall management of antidepressants and believes that the preferences of depression patients for antidepressants are related to more general attributes during the medication process, such as follow-up frequency. According to China’s medical policy, doctors can only prescribe approximately two weeks of antidepressants per visit, and many medications are not fully covered by medical insurance. When patients run out of antidepressants, they can continue to seek medical advice from doctors at the hospital or purchase them at community pharmacies. Therefore, cost and convenience of purchase are particularly important, which has been verified in qualitative interviews. In this study, both depression patients and experts believe that the cost attribute is particularly important, and this attribute will provide objective data for medical decision-makers to expand the proportion of medical insurance reimbursement in the future. To our knowledge, there is currently no standardized medication management strategy, which may be related to limited medical resources and cost-effectiveness. This study provides some insights into the preferences and compliance of medication management in patients with depression, and identifies key attributes that affect medication compliance from the patient’s perspective.
It is impractical to include all possible attributes and levels in DCE, which will reduce the statistical power of DCE evaluation results, so it is necessary to reduce the number of attributes to manageable.73 Scholars have confirmed that using simple ranking exercises is sufficient to achieve this purpose.74 In this protocol, we determine potential attributes and levels based on the method recommended,75,76 and use ranking to reduce the number of attributes to manageable levels, which to some extent ensures the importance and objectivity of attributes and ensures the accuracy of DCE evaluation results.
Although we strive to reduce the number of attributes in DCE to manageable, these attribute levels still generate many choice sets, which can impose cognitive burden on respondents. Therefore, a partial factorial design was used to control the number of choice sets and randomly divide the choice sets into four blocks, which can help to reduce the cognitive burden of the respondents. Mangham and his colleagues put forward, in low - or middle-income countries to carry out a study, the addition of figures help to improve the understanding of respondents.77 In this study, we added visual elements, such as combining figures and texts to explain attributes, which not only helps respondents understand the attributes levels, but also improves their participation by reducing possible boredom.
Our study has some strengths. Firstly, it is shown how the development process of the study can be conducted and reported systematically and rigorously according to the theoretical foundation and design principles in DCE research, which improves the transparency of the study. Secondly, attributes and levels largely determine the validity of assessment results of DCE. According to the recommended method, we use literature review and FGDs and one - on - one interviews to develop attributes and levels, these three methods complement each other, promoted the properties and level of deeper and broader understanding. Finally, China is a middle-income country, and its citizens have less education than the people of high-income countries. We combined figures and texts to explain the attributes, which will help respondents understand the questionnaire and improve participation.
This study has some limitations. First, this protocol included only patients with depression from Sichuan Province, China, which may not be fully representative of patients elsewhere in China. Fortunately, we included patients from four hospitals, which may have reduced sample bias to some extent. In addition, like other DCE studies, we did not include all possible attributes in the study, which may limit our discussion of the results in the future. In the future, it is necessary for scholars to include more comprehensive attributes and conduct multi-center studies to further confirm the information on the preferences of depression patients for medication management. Finally, although this manuscript systematically and rigorously conducts and reports on the development process of DCE research based on its theoretical foundation and design principles, the preference outcomes of patients with depression will only be provided in the future, which may lead to concerns from other researches. To reduce their concerns, we will strictly control the details during the stages of data collection and analysis to improve the quality of research.
Conclusion
This study showed how the development process of the study can be conducted and reported systematically and rigorously according to the theoretical foundation and design principles in DCE research, which improves the transparency of the study. The preference results from the study will provide references for developing and optimizing medication management strategies.
Abbreviations
ED, emergency department; DCE, discrete choice experiment; FGDs, focus group discussions; MNL, Multinomial logit; WTP, willingness to pay.
Data Sharing Statement
Details of data and materials can be obtained by contacting the corresponding author.
Ethics Approval and Consent to Participate
This study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of People’s Hospital of Deyang City [Ethics Number: 2023-04-070-K01] and Biomedical Ethics Committee of Sichuan University [Ethics Number: 20221122]. We introduced the study to all participants and obtained their consent.
Acknowledgments
Peng Xie and Hui-Qin Li are co-first authors for this study. We are grateful to all researchers for their efforts and all people who are willing to participate the study.
Author Contributions
Peng Xie: study design, execution and has drafted manuscript; Hui-Qin Li: conception, and has drafted and substantially revised manuscript; Wan-Lin Peng: execution, acquisition of data and analysis and interpretation; Hao Yang: acquisition of data and analysis and interpretation. All authors have agreed on the journal to which the article will be submitted, reviewed and agreed on all versions of the article and agree to take responsibility and be accountable for the contents of the article.
Funding
This work was supported by the funds of the Science and Technology Department of Sichuan Province [2022NSFSC0794].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zald D, McHugo M, Ray K, Glahn D, Eickhoff S, Laird A. Meta-analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cereb Cortex. 2014;24(1):232–248. doi:10.1093/cercor/bhs308
2. Huang Y, Wang Y, Wang H, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi:10.1016/S2215-0366(18)30511-X
3. Roca M, Vives M, López-Navarro E, García-Campayo J, Gili M. Cognitive impairments and depression: a critical review. Actas espanolas de psiquiatria. 2015;43(5):187–193.
4. Rock P, Roiser L, Riedel WJ, et al. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med London. 2014;44(10):2029–2040. doi:10.1017/S0033291713002535
5. Liu B, Qin P, Liu Y, Yuan L, Gu L, Jia C. Mental disorders and suicide attempt in rural China. Psychiatry Res. 2018;261:190–196. doi:10.1016/j.psychres.2017.12.087
6. Whiteford H, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet Oncol. 2013;382(9904):1575–1586. doi:10.1016/S0140-6736(13)61611-6
7. Graaf RD, Tuithof M, Dorsselaer SV, Have MT. Verzuim door psychische en somatische aandoeningen bij werkenden. Resultaten van de ‘Netherlands Mental Health Survey and Incidence Study-2’ (NEMESIS-2) [Absenteeism due to psychological or somatic disorders in workers. Results of the ‘Netherlands Mental H. Utrecht: Trimbos-Instituut; 2011.
8. Kazdin AE. Novel models for delivering mental health services and reducing the burdens of mental illness. Clin Psychol Sci. 2013;1(2):170–191. doi:10.1177/2167702612463566
9. World Bank Group and W. Out of the shadows: making mental health a global development priority organized by World Bank Group&WHO; 2016. Available from: http://www.worldbank.org/en/events/2016/03/09/out-of-the-shadows-making-mental-health-A-global-priority.
10. Chisholm D, Sweeny K, Sheehan P, et al. Scaling-up treatment of depression and anxiety: a global return on investment analysis. Lancet Psychiatry. 2016;3(5):415–424. doi:10.1016/S2215-0366(16)30024-4
11. Alonso J, Petukhova M, Vilagut G, et al. Days out of role due to common physical and mental conditions: results from the WHO World Mental Health surveys. Mol Psychiatry. 2011;16(12):1234–1246. doi:10.1038/mp.2010.101
12. Cardoso G, Xavier M, Vilagut G, et al. Days out of role due to common physical and mental conditions in Portugal: results from the WHO World Mental Health Survey. BJPsych Open. 2017;3(1):15–21. doi:10.1192/bjpo.bp.115.002402
13. Bruffaerts R, Vilagut G, Demyttenaere K, et al. Role of common mental and physical disorders in partial disability around the world. Br J Psychiatry. 2012;200(6):454–461. doi:10.1192/bjp.bp.111.097519
14. Ya-juan N. Interpretation of drug therapy of Chinese Guidelines for Prevention and Treatment of Depression. ClinMed J. 2018;16(5):6–8.
15. Bosworth H, Voils C, Potter G, Steffens D. The effects of antidepressant medication adherence as well as psychosocial and clinical factors on depression outcome among older adults. Inte j Geriatric Psychiatry. 2008;23(2):129–134. doi:10.1002/gps.1852
16. Sriharsha M, Treatment PA. Disease Related Factors Affecting Non-adherence among Patients on Long Term Therapy of Antidepressants. J Depression Anxiety. 2015;04:02. doi:10.4172/2167-1044.1000175
17. Liu X, Tepper P, Able S. Adherence and persistence with duloxetine and hospital utilization in patients with major depressive disorder. Int Clin Psychopharmacol. 2011;26(3):173–180. doi:10.1097/YIC.0b013e328343ba1e
18. Ho S, Chong H, Chaiyakunapruk N, Tangiisuran B, Jacob S. Clinical and economic impact of non-adherence to antidepressants in major depressive disorder: a systematic review. J Affective Disorders. 2016;193:1–10. doi:10.1016/j.jad.2015.12.029
19. Alekhya P, Sriharsha M, Ramudu RV, Shivanandh B, Reddy YH. Adherence to antidepressant therapy: sociodemographic factor wise distribution. Int J Pharm Clin Res. 2015;7(3):180–184.
20. Banerjee S, Varma RP. Factors Affecting Non-Adherence among Patients Diagnosed with Unipolar Depression in a Psychiatric Department of a Tertiary Hospital in Kolkata, India. Depress Res Treat. 2013;2013:809542. doi:10.1155/2013/809542
21. Jin-liang W, Chen-chen Y, Qi Y, Ping F, Yang X. Influencing factors of antidepressant polypharmacy in inpatients with depression. Chin J Hospital Pharm. 2019;39(20):2081–2085.
22. Whiteford HA, Degenhardt L, Rehm J. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586.
23. Swift JK, Callahan JL, Vollmer BM. Preferences. J Clin Psychol. 2011;67(2):155–165. doi:10.1002/jclp.20759
24. Lancsar E, Louviere J. Conducting Discrete Choice Experiments to Inform Healthcare Decision Making. PharmacoEconomics. 2008;26(8):661–677. doi:10.2165/00019053-200826080-00004
25. Say RE. The importance of patient preferences in treatment decisions--challenges for doctors. BMJ. 2003;327(1):542–545. doi:10.1136/bmj.327.7414.542
26. Ng-Mak D, Poon J, Roberts L, Kleinman L, Revicki D, Rajagopalan K. Patient preferences for important attributes of bipolar depression treatments: a discrete choice Experiment. Patient Preference Adherence. 2018;12:35–44. doi:10.2147/PPA.S151561
27. Fairchild AO. Patient preferences for ketamine-based antidepressant treatments in treatment-resistant depression: results from a clinical trial and panel. Neurol Psychiatry Brain Res. 2020;37:67–78. doi:10.1016/j.npbr.2020.05.003
28. Fifer S, Puig A, Sequeira V, et al. Understanding Treatment Preferences of Australian Patients Living with Treatment-Resistant Depression. Patient Preference Adherence. 2021;15:1621–1637. doi:10.2147/PPA.S311699
29. Wang PS, Aguilar-Gaxiola S, Alonso J, Angermeyer MC, Borges G. Use of mental health services for anxiety, mood, and substance disorders in 17 countries in the WHO world mental health surveys. Lancet Oncol. 2007;8(5):370. doi:10.1016/S1470-2045(07)70114-6
30. Beckman L, von Kobyletzki L, Svensson M. Economic costs of antidepressant use: a population-based study in Sweden. j Mental Health Policy Economics. 2019;22(4):125–130.
31. Patel V, Weiss H, Chowdhary N, et al. Effectiveness of an intervention led by lay health counsellors for depressive and anxiety disorders in primary care in Goa, India (MANAS): a cluster randomised controlled trial. Lancet Oncol. 2010;376(9758):2086–2095. doi:10.1016/S0140-6736(10)61508-5
32. Cartwright C, Gibson K, Read J, Cowan O, Dehar T. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Preference Adherence. 2016;10:1401–1407. doi:10.2147/PPA.S110632
33. Wiles N, Taylor A, Turner N, et al. Management of treatment-resistant depression in primary care: a mixed-methods study. Br J Gen Pract. 2018;68(675):e673–e681. doi:10.3399/bjgp18X699053
34. Desplenter F, Laekeman G, Simoens S. Differentiated information on antidepressants at hospital discharge: a hypothesis-generating study. Int j Pharmacy Practice. 2013;21(4):252–262. doi:10.1111/ijpp.12001
35. Interian A, Lewis-Fernández R, Gara M, Escobar J. A randomized-controlled trial of an intervention to improve antidepressant adherence among Latinos with depression. Depression Anxiety. 2013;30(7):688–696. doi:10.1002/da.22052
36. Al-Saffar N, Abdulkareem A, Abdulhakeem A, Salah A, Heba M. Depressed patients’ preferences for education about medications by pharmacists in Kuwait. Patient Educ Counseling. 2008;72(1):94–101. doi:10.1016/j.pec.2008.01.027
37. Klang S, Ben-Amnon Y, Cohen Y, Barak Y. Community pharmacists’ support improves antidepressant adherence in the community. Int Clin Psychopharmacol. 2015;30(6):316–319. doi:10.1097/YIC.0000000000000090
38. Richards D, Bower P, Chew-Graham C, et al. Clinical effectiveness and cost-effectiveness of collaborative care for depression in UK primary care (CADET): a cluster randomised controlled trial. Health Technol Assess. 2016;20(14):1–192. doi:10.3310/hta20140
39. Aljumah K, Hassali M. Impact of pharmacist intervention on adherence and measurable patient outcomes among depressed patients: a randomised controlled study. BMC Psychiatry. 2015;15(1):219. doi:10.1186/s12888-015-0605-8
40. Meglic M, Furlan M, Kuzmanic M, et al. Feasibility of an eHealth service to support collaborative depression care: results of a pilot study. J Med Int Res. 2010;12(5):e63. doi:10.2196/jmir.1510
41. Vannachavee U, Seeherunwong A, Yuttatri P, Chulakadabba S. The Effect of a Drug Adherence Enhancement Program on the Drug Adherence Behaviors of Patients With Major Depressive Disorder in Thailand: a Randomized Clinical Trial. Arch Psychiatric Nursing. 2016;30(3):322–328. doi:10.1016/j.apnu.2015.12.001
42. Wiles N, Thomas L, Turner N, et al. Long-term effectiveness and cost-effectiveness of cognitive behavioural therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care: follow-up of the CoBalT randomised controlled trial. Lancet Psychiatry. 2016;3(2):137–144. doi:10.1016/S2215-0366(15)00495-2
43. Yusuf H, Magaji MG, Maiha BB, Yakubu SI, Haruna WC, Mohammed S. Impact of pharmacist intervention on antidepressant medication adherence and disease severity in patients with major depressive disorder in fragile north-east Nigeria. J Pharm Heal Serv Res. 2021;12(3):410–416. doi:10.1093/jphsr/rmab030
44. Marasine NR, Sankhi S, Lamichhane R. Impact of pharmacist intervention on medication adherence and patient-reported outcomes among depressed patients in a private psychiatric hospital of Nepal: a randomised controlled trial. Hosp Pharm. 2020;57(1):26–31. doi:10.1177/0018578720970465
45. Capoccia K, Boudreau D, Blough D, et al. Randomized trial of pharmacist interventions to improve depression care and outcomes in primary care. Am J Health Syst Pharm. 2004;61(4):364–372. doi:10.1093/ajhp/61.4.364
46. Burton W, Chen C, Conti D, Schultz A, Edington D. The association of antidepressant medication adherence with employee disability absences. Am J Manag Care. 2007;13(2):105–112.
47. Shi J, Chen Y, Jiang Y, et al. Stigma and its associations with medication adherence in major depressive disorder. Psychiatry Res. 2024;331:115664. doi:10.1016/j.psychres.2023.115664
48. Ho S, Jacob S, Tangiisuran B. Barriers and facilitators of adherence to antidepressants among outpatients with major depressive disorder: a qualitative study. PLoS One. 2017;12(6):e0179290. doi:10.1371/journal.pone.0179290
49. Semahegn A, Torpey K, Manu A, Assefa N, Tesfaye G, Ankomah A. Psychotropic medication non-adherence and associated factors among adult patients with major psychiatric disorders: a protocol for a systematic review. Syst Rev. 2018;7(1):10. doi:10.1186/s13643-018-0676-y
50. Zhu Y, Wu Z, Sie O, et al. Causes of drug discontinuation in patients with major depressive disorder in China. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109755. doi:10.1016/j.pnpbp.2019.109755
51. DeShazo JR, Fermo G. Designing Choice Sets for Stated Preference Methods: the Effects of Complexity on Choice Consistency. J Environ Econ Manage. 2002;44(1):123–143. doi:10.1006/jeem.2001.1199
52. Qiu-chen W, Xiu-ying Z, Hui X, Hua Y, Yi P. Research progress in developing attributes and levels of discrete choice experiments. Mod Preventive Med. 2020;47(12):2199–2201+2210.
53. de Bekker-Grob E, Hol L, Donkers B, et al. Labeled versus unlabeled discrete choice experiments in health economics: an application to colorectal cancer screening. Value Health. 2010;13(2):315–323. doi:10.1111/j.1524-4733.2009.00670.x
54. Fields BE, Bell JF, Bigbee JL, Holly T, Joanne S. Registered Nurses’ Preferences for Rural and Urban Jobs: a Discrete Choice Experiment. Int j Nursing Studies. 2018;86:11. doi:10.1016/j.ijnurstu.2018.05.012
55. Burgess L, Street DJ. The optimal size of choice sets in choice experiments. Statistics. 2006;40(1):507–515. doi:10.1080/02331880601013841
56. Reed Johnson FP, Lancsar EP, Marshall DP, et al. Constructing Experimental Designs for Discrete-Choice Experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13. doi:10.1016/j.jval.2012.08.2223
57. Veldwijk J, Lambooij M, de Bekker-Grob E, Smit H, de Wit G. The effect of including an opt-out option in discrete choice experiments. PLoS One. 2014;9(11):e111805. doi:10.1371/journal.pone.0111805
58. Li H, Xue H, Yuan H, Wan G, Zhang X. Preferences of first-degree relatives of gastric cancer patients for gastric cancer screening: a discrete choice experiment. BMC Cancer. 2021;21(1):959. doi:10.1186/s12885-021-08677-9
59. Li H, Han J, Yuan H, Wan G, Xue H, Zhang X. Eliciting gastric cancer survivors’ preferences for follow-up services: a discrete choice experiment protocol. BMJ open. 2021;11(11):e049742. doi:10.1136/bmjopen-2021-049742
60. Bessen T, Chen G, Street J, et al. What sort of follow-up services would Australian breast cancer survivors prefer if we could no longer offer long-term specialist-based care? A discrete choice experiment. Br. J. Cancer. 2014;110(4):859–867.
61. JA W. R W, X G. PLoS One. 2014;9(1):e90635.
62. Lagarde M, Blaauw D. A review of the application and contribution of discrete choice experiments to inform human resources policy interventions. Human Resources for Health. 2009;7(1):62. doi:10.1186/1478-4491-7-62
63. Hensher DA, Swait JD, Adamowicz W, Louviere JJ. Stated Choice Methods: Analysis and Applications. Cambridge University Press; 2014.
64. R. J, B O. Getting the Most from CBC. Sequim: Sawtooth Research Paper Series, Sawtooth Software; 2003.
65. Orne B. Sample size issues for conjoint analysis. Getting Started with Conjoint Analysis: strategies for Product Design and Pricing Research. Int J Res. 2010:57–66.
66. Orme B. Getting Started with Conjoint Analysis: Strategies for Product Design and Pricing Research.
67. Ye F, Lord D. Comparing three commonly used crash severity models on sample size requirements: multinomial logit, ordered probit and mixed logit models. Anal Methods Accid Res. 2014;1:72–85. doi:10.1016/j.amar.2013.03.001
68. Hensher DA, Rose JM, Greene WH. Applied choice analysis: A primer. 2005.
69. Wang C, Wang D, Zhu W, Song S. Research progress of discrete choice model. Progress Geography. 2015;34:10.
70. Melfi C, Chawla A, Croghan T, Hanna M, Kennedy S, Sredl K. The effects of adherence to antidepressant treatment guidelines on relapse and recurrence of depression. Arch Gen Psychiatry. 1998;55(12):1128–1132. doi:10.1001/archpsyc.55.12.1128
71. Johnson FR. ??zdemir S, Manjunath R, Hauber AB, Burch SP, Thompson TR. Factors Affect Adherence Bipolar Disorder Treatments Medical Care. 2007;45(6):545–552.
72. Zimmermann TM, Clouth J, Elosge M, et al. Patient preferences for outcomes of depression treatment in Germany: a choice-based conjoint analysis study. Affect Disord. 2013;148(2–3):210–219. doi:10.1016/j.jad.2012.11.062
73. Lagarde M. Investigating attribute non-attendance and its consequences in choice experiments with latent class models. Health Economics Review. 2013;22(5):554–567. doi:10.1002/hec.2824
74. Hiligsmann M, van Durme C, Geusens P, et al. Nominal group technique to select attributes for discrete choice experiments: an example for drug treatment choice in osteoporosis. Patient Preference Adherence. 2013;7:133–139. doi:10.2147/PPA.S38408
75. Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. PharmacoEconomics. 2008;26(8):661–677.
76. Bridges JFPP, Hauber ABP, Marshall DP, et al. Conjoint Analysis Applications in Health—a Checklist: a Report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413. doi:10.1016/j.jval.2010.11.013
77. Mangham LJ, Kara H, Barbara MP. How to do (or not to do) … Designing a discrete choice experiment for application in a low-income country. Health Policy Plan. 2009;24(2):151–158. doi:10.1093/heapol/czn047
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
