Back to Journals » Journal of Experimental Pharmacology » Volume 15
Efficacy and Anti-Inflammatory Activity of Ashwagandha Sustained-Release Formulation on Depression and Anxiety Induced by Chronic Unpredictable Stress: in vivo and in vitro Studies
Authors KrishnaRaju AV, Somepalli V, Thanawala S , Shah R
Received 19 April 2023
Accepted for publication 21 June 2023
Published 25 July 2023 Volume 2023:15 Pages 291—305
DOI https://doi.org/10.2147/JEP.S407906
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Alluri Venkata KrishnaRaju,1 Venkateswarlu Somepalli,2 Shefali Thanawala,3 Rajat Shah3
1Department of Pharmacology and Clinical Research, Laila Nutraceuticals, Vijayawada, Andhra Pradesh, India; 2Department of Research and Development, Laila Nutraceuticals, Vijayawada, Andhra Pradesh, India; 3Medical Affairs, Nutriventia Limited, Mumbai, Maharashtra, India
Correspondence: Shefali Thanawala, Nutriventia Limited, Mumbai, India, Tel +912267163000, Email [email protected]
Background: Stress is the psychological, physiological, and behavioral response of an individual’s body when they perceive a lack of equilibrium between the demands placed upon them and their ability to meet those demands. Adaptogens are herbs that help with stress management, and Ashwagandha is one such safe and effective adaptogen.
Objective: We evaluated the anti-neuroinflammatory potential of Ashwagandha sustained-release formulation (AshwaSR) by estimating the in vitro expression of pro-inflammatory cytokines, and its efficacy on anxiety and depression in an in vivo study.
Methods: Our in vitro study investigated the anti-inflammatory potential of AshwaSR by estimating the expression of tumour necrosis factor [TNF]-α and interleukin [IL]-1β levels in LPS-induced THP-1 human monocytes, and the antioxidant effects by its potential to inhibit the superoxide [SO] generation in PMA-induced HL-60 human monocytic cells. The in vivo study assessed the efficacy of AshwaSR on chronic unpredictable stress (CUS)-induced comorbid anxiety and depression in Sprague Dawley rats. Antidepressant and anxiolytic effects of AshwaSR were evaluated by open field test (OFT), elevated plus maze (EPM), forced swim test (FST), and Morris water maze (MWM) test.
Results: AshwaSR inhibited TNF-α, IL-1β and superoxide production in a dose-dependent manner in the in vitro study. The in vivo CUS model induced depression-like and anxiety-like behaviour. Treatments with AshwaSR and escitalopram showed improvement in the EPM and MWM models compared to the CUS-group.
Conclusion: In vitro study demonstrated that AshwaSR inhibits expressions of pro-inflammatory cytokines, IL-1β and TNF-α, and superoxide production. Further, the in vivo study confirmed its anxiolytic and stress-relieving effects in the CUS model that confirmed AshwaSR’s potential in managing stress and stress-related symptoms.
Keywords: Withania somnifera, antidepressant, anxiolytic stress, CUS model, anti-inflammatory, antioxidant
Introduction
Any change that causes physical, emotional, or psychological burden can be termed as stress. While stress can be both positive and pro-adaptive and forms a part of everyone’s life, chronic stress is often associated with unfavorable outcomes.1 Long-term exposure to stressful events or surroundings is detrimental to mental health, and it poses as a risk factor for many neuropsychiatric diseases such as depression, mood swings, and anxiety.2–4 Large population-based studies have revealed that approximately 35% of people in the general population experience anxiety-related symptoms in their lifetime.5,6 Similarly, developing depression is worrisome, and the World Health Organization (WHO) identified depression to be the leading cause of disability.7 Notably, stress-related disorders can significantly affect the quality of life of people across all age groups.8
Evidence from research studies shows that the central nervous system (CNS) and the immune system respond to physiological and psychological stressors through multiple pathways which result in by result in accentuated expression of the inflammatory mediators, ie, the pro-inflammatory cytokines. The immune system further responds to these stressors and communicates with the CNS that results in elevated pro-inflammatory cytokine levels.9,10
The primary management of stress-related disorders includes pharmacotherapies and psychological interventions, which are expensive and have a narrow therapeutic margin. In contrast, natural products are more affordable and less likely to cause side effects when administered at the recommended doses. Current global trend for the widespread use of complementary and alternative medicine is observed.11 In fact, several studies have identified a considerable number of individuals who prefer to use these therapies to manage their symptoms of depression and anxiety.12
Ashwagandha or Withania somnifera (L.) Dunal, a small, wood-like xerophytic plant, is primarily grown in the Asian regions including India, Afghanistan, Baluchistan, Sind, as well as in the Mediterranean regions. Ashwagandha has been used for more than 3000 years in Ayurvedic medicine as a “Rasayana” or as an adaptogen. It is mentioned to exert diverse pharmacological actions such as antioxidant, neuroprotective, anticancer, cardioprotective, thyroid modulating, immunomodulating, antimicrobial, and anti-inflammatory, activities.13 Research has identified some bioactive constituents which include withanolides, sitoindosides, glycowithanolides, and other phytochemicals which contribute to therapeutic activities of the herb.14 Their therapeutic implications for neurodegenerative diseases have also been documented.15–17 Considering its tremendous physical and mental health benefits, the WHO monographs have included Ashwagandha on their list of selected medicinal plants.18
Recent studies have shown the potential of Ashwagandha against neuroinflammation by estimating the expression of pro-inflammatory cytokines (tumor necrosis factor [TNF]-α, interleukin [IL]-1β, and IL-6).19,20 Additionally, various in vitro and in vivo studies as well as clinical studies have explored the efficacy of Ashwagandha in alleviating chronic stress symptoms.21–26 However, most of these studies have reported the use of twice-daily doses of standard immediate-release ashwagandha formulations.
We developed a sustained-release (SR) formulation of Ashwagandha, ProlanzaTM (Ashwagandha root extract SR capsules 300 mg containing 15 mg withanolides; AshwaSR; Nutriventia Limited, Mumbai, Maharashtra, India, and Laila nutraceuticals, Vijayawada, Andhra Pradesh, India) that intended to provide superior bioavailability of its active constituents – viz., withanolides, thereby maximizing its therapeutic effect for longer periods after administering a single daily dose. Thus, the SR formulation offers the advantage of single daily dosing that ultimately results in better compliance.27 For this study, our botanist Dr. K. N. Reddy identified the plant (Withania Somnifera (L) Dunal), and deposited a voucher specimen in the Laila Nutraceuticals Herbarium bearing the specimen number 6852. We designed an in vitro study to evaluate the anti-inflammatory activity of AshwaSR on [TNF]-α, [IL]-1β and SO. Based on the positive results from the in vitro study, we developed an in vivo study to investigate efficacy of AshwaSR in chronic unpredictable stress-induced comorbid depression and anxiety using various behavioral assays. No additional approvals were required to conduct preclinical research with plant material, in accordance with institutional/local regulations.
Materials and Methods
Preclinical Pharmacokinetic Data of AshwaSR
Our unpublished data from preclinical pharmacokinetic study evaluated pharmacokinetics of withanolides (12-deoxy withastromonolide, withanolide A and total withanolides) in AshwaSR with two commercially marketed immediate-release (IR) formulations in male Sprague Dawley rats who were administered the formulations at dose equivalent to 50mg/kg of total withanolides once daily. AshwaSR contains brown to dark brown colour of Ashwagandha Root Extract granules SR (total withanolides concentration: Not Less Than 4% by HPLC modified USP method). Blood samples per specified time points were collected from pre dose till 24 hr. We found that relative bioavailability of withanolides was higher for AshwaSR in terms of exposure (Area Under Curve), peak plasma concentration (Cmax), and time to reach maximum concentration (Tmax) compared to IR formulations. Among the withanolides evaluated, 12 deoxy withastromonolide showed the highest exposure in all formulations. The improved PK profile of withanolides in our new formulation, AshwaSR, is probably due to improved dispersibility and solubility as compared to the commercial IR formulations. As the PK behavior of withanolides can be markedly altered by changes in formulation, we chose SR formulation as it provided better bioavailability. For the present investigation, we used AshwaSR pellets, which were encapsulated based on the animals’ body weights. The calculated quantity of pellets (100 mg/kg body weight) was encapsulated and dosed using mini-capsule applicator (9el; Torpac, USA) and gavaged to the rats.
In vitro Experimental Study
Inhibition of IL-1β Production
For in vitro studies, known quantity of test product samples were dissolved in DMSO, diluted serially and applied to the test system to achieve different concentrations. The final concentration of dimethyl sulfoxide (DMSO) in the test system was 0.2%. The inhibition of IL-1β production by AshwaSR was determined by lipopolysaccharide (LPS)-induced THP-1 (ATCC, Cat# TIB-202, Manassas, VA, USA) human monocytes. Briefly, THP-1 cells were harvested on RPMI (Sigma Cat# R8005-10X1L) supplemented with 10% Fetal Bovine Serum (FBS; ATCC Cat# 30–2020 and 10mM Sodium Pyruvate, Sigma Cat# P3662-25G). We added equal number of THP-1 cells (125000 cells in 200µL) to every well of the 96-well flat bottom culture plate. Differentiation was initiated by treating the cells with 20 nM of phorbol 12-myristate 13-acetate (PMA; Sigma Cat# 79346, St. Louis, MO, USA), and the plate was incubated for an additional 48 h at 37°C in a CO2 incubator. The cells were washed with Dulbecco’s Modified Eagle medium (DMEM, Sigma Cat# D5648-10X1L, St. Louis, MO, USA) twice which was then supplemented with 10% newborn calf serum (Gibco, Cat#16010159, Waltham, MA, USA), and pretreated with various concentrations (2.5, 5, 10, 25, 50 µg/mL) of AshwaSR (ARE; LOTNO# N03622000) or positive control: Z-VAD (OMe)-FMK (either 1 or 10 µM), Calbiochem (Cat# 627610 Rahway, NJ, USA) for 1 h. The cells were then treated with an inducer, 100 ng/mL LPS (Sigma Cat# L6529-1MG, St. Louis, MO, USA) except for the vehicle control (cells+0.2% DMSO) and were incubated further for 4 h at 37°C in a CO2 incubator. After 4 h, the culture plate was centrifuged at 270×g for 5 min, and the cell-free supernatant (125 µL) was collected and stored at −80°C. These were then analyzed for IL-1β by ELISA (Human IL-1β Duoset ELISA; R&D Systems, Cat# DY201 MN, USA).
Inhibition of TNF-α Production
The THP-1 cells (ATCC, Cat# TIB-202, Manassas, VA, USA; 125,000 cells/well) were plated in every well of the 96-well flat bottom culture plate using the DMEM medium without phenol red (Sigma Cat# D5648-10X1L, St. Louis, MO, USA), supplemented with 1% FBS. Cells were pretreated with varying concentrations (1, 2.5, 7.5 and 10 µg/mL) of the test compounds for 2 h (incubated at 37°C in a CO2 incubator [pretreatment]). Independently, the cells were also treated with different concentrations (1, 5 or 10µM) of U0126 (Sigma Cat# 19–147, St. Louis, MO, USA) that was referenced as the positive control for TNF-α inhibition. Thereafter, all cells except for the vehicle control (cells+0.2%DMSO) were treated with an inducer, LPS (100 ng/mL), and incubated in a CO2 incubator for 4 h at 37°C. The culture plate was then centrifuged (270×g) for 10 min and cell-free supernatants (125 µL) were collected and stored at −80°C. We then analyzed the supernatants for TNF-α by ELISA (Human TNF-α Duoset ELISA; R&D Systems, Cat # DY210, MN, USA).
Inhibition of Superoxide Generation
The inhibition of superoxide production by AshwaSR was evaluated in phorbol 12- myristate 13- acetate (PMA)-induced HL-60 (ATCC, Cat# CCL-240, Manassas, VA, USA) human monocytic cells. These HL-60 cells were washed with Hank’s Balanced Salt Solution (HBSS) once and were then taken into 50 mL falcon tubes. Lucigenin (working concentration 200μM) was added to the falcon tube and 50000 cells/well were added in a 96-well white plate (Corning, Cat# 3917, Somerville, MA, USA). The cells were immediately stimulated with 100nM PMA, either in the absence or presence of varying concentrations (1, 2.5, 5, 7.5, and 10 µg/mL) of AshwaSR or 1, 2.5, 5, 10 and 20 µg/mL of the positive control (green tea extract, obtained from the internal phytochemistry department). A spectrophotometer (Enspire Multimode Reader, Perkin Elmer) was set in the kinetic mode and the relative luminescence was measured up to 1 hour.
In vivo Experimental Procedure
Based on the findings of in vitro studies, in vivo study was designed with the aim to evaluate the efficacy of AshwaSR on chronic unpredictable stress (CUS)-induced comorbid depression and anxiety using various behavioral assays in Sprague Dawley rats.
Study Design
All animals were acclimatized for six days prior to enrollment in the study and were examined for clinical signs. They were reported to be free from physiological and clinical abnormalities during this period.
We selected 28 healthy rats from an acclimatized colony of 32 and randomized them based on their body weight into four groups of seven animals as follows: control animals (CL-group), CUS-induced animals (CUS-group), AshwaSR treated CUS-induced animals (AshwaSR-group), and escitalopram treated CUS-induced animals (ESC-group). Individual animal body weights were recorded on the day of dosing and dose volume was calculated based on the body weight of each rat.
A full dose of Ashwagandha for humans would be between 1000 and 1500 milligrams per day of extract. By translating the human dose to the rat dose, we arrived at the 100 mg dose of AshwaSR (containing NLT 5% withanolides). Animals in the CL-group and CUS-group were administered 0.5% CMC-Na orally. The AshwaSR pellets were filled into mini-capsules by using capsule filling equipment and orally administered to the animals. AshwaSR was encapsulated in Torpac’s size 9 gelatin capsules and administered orally using TORPAC9el dosing syringe. Torpac’s capsules of size 9 provide a convenient method for orally dosing laboratory rats. Such dosing is similar to hard gelatin capsules in clinical setting.28 Animals in the ESC-group received escitalopram orally at a dose of 20mg/kg/body weight. All animals were dosed with either vehicle, test item, or reference standard once daily until day 35. All animals except those in the CL-group underwent various predefined paradigms of chronic unpredictable stress as shown in Table 1.
 |
Table 1 Experimental Schedule for the Chronic Unpredictable Stress Procedure |
All the animals were evaluated for the following parameters:
Body Weight
The body weight of animals was recorded prior initiating treatment and was followed on a weekly basis (on days 1, 8, 15, 22, 29, and 35), and on the termination day of the experiment (day 36) for organ weight compilation.
Open Field Test (OFT)
This test intends to estimate the locomotor activity and willingness to explore majorly in rodents later explored in various other animals.29 This test also readily helps to investigate different pharmacological compounds for anxiolytic or anxiogenic effects.30 The open field test (OFT) apparatus (100 X 100×37 cm) used in this study was a square platform made of polypropylene, with black lines dividing the platform’s bottom into 12 equal squares. The animals were left alone in the Open Field’s center for 10 minutes to explore it freely.31 Resting time, moving time, distance travelled, fecal pellet count and additional number of entries in the center zone were recorded for 10 min on day 29.
Elevated Plus Maze (EPM)
The elevated plus maze (EPM) is a broadly used behavioral assay for rodents. It is validated to assess any anxiolytic activity of pharmacological agents, and to define the underlying mechanisms of the anxiety-related behavior of animals. In this test, each animal is put inside a plus sign-shaped device that had two uniformly sized one open and two closed arms and was raised 50 centimeters from the floor.31 We can simultaneously determine the anti-anxiety behavior (increased open arm time and/or open arm entries) by measuring the spontaneous motor activity (total and/or closed arm entries).32 The number of times any animal entered with open and closed arms with all four paws, and the time spent in open arms were recorded for 5 min on day 29.
Forced Swim Test (FST)
The forced swim test (FST) is a rodent behavioral test. It helps evaluate the antidepressant efficacy of new compounds, and the experimental manipulations which render or prevent depressive-like states.33 The FST exposes animals to stress that plays an important role in the tendency of developing major depression.34 For this study, each animal was placed for 6 min in a transparent, inescapable cylindrical tank (50 cm depth × 20 cm height) filled with water.31 We recorded the activity of each rat using the SMART® video tracking system (version 2.5). The durations of mobility (moving time) and immobility (resting time) in seconds were scored on day 35.
Morris Water Maze (MWM)
The Morris water maze (MWM) assesses spatial or place learning for rodents. It is a robust and reliable test which strongly correlates with N-methyl-D-aspartic acid (NMDA) receptor function and hippocampal synaptic plasticity.35 This test utilizes a rat’s natural ability to swim without undergoing any major distress despite the fact that this task induces a significant stress response.36 Accordingly, in this study, the rats were placed in a circular pool (170 cm diameter), which was filled with water in a dimly lit room. We placed spatial cues in the walls around the pool (square, cross, triangle, and in stripes). We divided the pool into four imaginary quadrants, and in one of these quadrants, we placed a hidden transparent platform.37 We collected the data using a fixed camera that was placed on the ceiling and connected to the SMART® video tracking system on days 30 to 34.
Acquisition Trial
In this experiment, rats were exposed to acquisition trials for 4 days (day 30 to 33), followed by one probe trial on day 5 (day 34, ie, the day after the acquisition trial was complete). The rats were then lowered gently, feet first into the water and were then allowed to swim for 1 minute (60 seconds) to find the hidden platform. If they could successfully find the platform under 1 minute (60 seconds), the trial was stopped and the rat was allowed to stay on the platform for 30 seconds before being removed from the maze. Each rat was subjected to 4 trials from four different starting points in a day. The maze contains 8 starting points. On days 1 and 3 of the experiment (days 30 and 32), the rats were placed on 1st, 3rd, 5th and 7th starting points, and on days 2 and 4 (days 31 and 33), they were placed from 2nd, 4th, 6th and 8th starting points. Target latency (time to reach the escape platform in ms), and the distance travelled were measured in acquisition trials for 4 days (days 30 to 33).
Probe Trial
We assessed memory retention on day 5. For assessing the retention of memory of animals, we removed the hidden platform from the pool and each animal received a single 2-minutes probe trial. We measured the target latency (time to reach the escape platform location in ms), number of crossings in the target quadrant, and the time spent in the target quadrant on day 5 (day 34).
Serum Corticosterone Estimation
Blood samples were collected on day 35 via retro-orbital puncture under mild isoflurane anesthesia using a capillary tube. Serum was separated by centrifuging the blood at 1500 xg for 10 minutes at 4°C and was stored at −80°C, and Corticosterone (DRG International, Cat# EIA-4164) was estimated as per the manufacturer’s instructions.
Organ Index
On the day of the study termination (day 36), all rats were euthanized by CO2 asphyxiation in a euthanasia chamber and their brains, thymus, and adrenals were excised, weighed and stored at −80°C until further use.
Statistical Analysis
Descriptive statistics was used, and we expressed the data as mean±SD/SEM. We analyzed the data using one-way ANOVA followed by Dunnett’s test way or two-way ANOVA followed by the Bonferroni test to compare all treatment groups with the CUS control. A p value of <0.05 was considered to be statistically significant. We computed the data using GraphPad Prism v5.01 (GraphPad Software, Inc., CA; USA).
Results
In vitro Studies
AshwaSR showed dose-dependent inhibition of IL-1β and TNF-α production from the LPS-induced THP-1 human monocytes at the tested treatment concentrations (Figures 1 and 2). The IC50 concentration required for 50% inhibition of TNF-α production was found to be 8.98±0.03 μg/mL for AshwaSR. Similarly, AshwaSR also inhibited superoxide generation in a dose-dependent manner in PMA-induced HL-60 human monocytic cells (Figure 3).
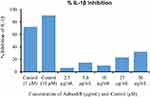 |
Figure 1 % IL-1β inhibition by AshwaSR (Prolanza) and control. |
 |
Figure 2 % TNF-α inhibition by AshwaSR (Prolanza) and control. |
 |
Figure 3 % Superoxide inhibition by AshwaSR (Prolanza) and control. |
In vivo Study
Body Weight and Organ Index
We did not observe any statistically significant difference across the groups compared to the CL-group. However, a consistent upward trend in body weight was observed in the CUS-group animals compared to CL-group animals on day 15, 22 and 29 (Supplementary Figure 1). No significant difference was observed in the collected organ index, ie, brain, thymus and adrenal indices across the groups when compared with CL-group (Supplementary Table 1).
Open Field Test
We observed a decrease in resting time and an increase in the moving time in the CUS-group compared to the CL-group. Compared to the CUS-group, we observed a 27.74% increase in the resting time, 10.46% decrease in the moving time in the AshwaSR-group, and 36.35% increase in the resting time and 13.71% reduction in moving time in the ESC-group. Statistically non-significant effects were noted in the treatment-group. Similarly, no significant differences were observed in distance travelled (cm), fecal pellet counts and central zone entries in the AshwaSR-group or the ESC-group when compared with the CL-group and CUS-groups. Interestingly, there was a significant increase in the moving speed of AshwaSR-group (30.85±9.45 cm/sec; p < 0.05) and ESC-group (30.23±6.68 cm/sec; p < 0.05) animals compared to the CUS-group (21.02±4.58 cm/sec); but no significant difference was observed in the moving speed between the CUS-group and CL-group (Supplementary Figure 2).
Elevated Plus Maze Test
Significant increase in entries and time spent in open arm was observed in CUS-group compared to CL-group that indicated a restlessness due to CUS (open arm entries: 5.00±2.89 vs 0.29±0.49; p < 0.001; time spent: 46.71±47.13 sec vs 2.86±5.67 sec; p < 0.01). Significant reduction in entries and the time spent in open arm were observed in AshwaSR-group (open arm entries: 0.57±1.13; p < 0.001; time spent: 5.86±10.04 sec; p < 0.01) and in the ESC-group (open arm entries: 0.57±0.79; p < 0.001; time spent: 5.71±7.45 sec; p < 0.01) compared to the CUS-group (open arm entries: 5.00±2.89; time spent: 46.71±47.13 sec). However, closed-arm entries in the treatment groups were statistically insignificant compared with the CUS-group animals. The results are depicted in Figures 4A–C.
Forced Swim Test (FST)
This test was conducted on day 35. No significant differences in resting time, moving time, and distance travelled, and speed was noted across the treatment group animals (AshwaSR-group or ESC-group) compared to CUS-group. The changes observed in the percent resting time and percent moving time were also in line with the differences observed in resting time and moving time. Interestingly, the AshwaSR-group exhibited a moderate increase in the moving time and distance travelled and a decrease in resting time compared to the CUS-group. However, none of these changes were statistically significant (Supplementary Figure 3).
Morris Water Maze Test (MWM)
In the MWM test, acquisition trial was conducted from days 30 to 33 and target latency (ms) and distance travelled (cm) were recorded. On days 32 and 33, a significant reduction in target latency and in the distance travelled was noted in both the AshwaSR-group and ESC-group compared to the CUS-group (Figures 5A and B).
In a probe trial conducted on day 34, target crossings, time spent in the target quadrant (sec) and target latency were measured. There was a trend that showed a prominent decrease in target latency time (AshwaSR-group and ESC-group) and an increase in target crossings and time spent in the target quadrant only in the AshwaSR-group compared to the CUS-group. However, these changes were not statistically significant (Supplementary Table 2).
Serum Corticosterone
Induction of CUS elevated corticosterone levels in the CUS-group (462.47±282.68 ng/mL) compared to the CL-group (397.67±133.56 ng/mL). Compared to the CUS-group, serum corticosterone levels decreased by 14.54% in the AshwaSR-group and 47.99% in the ESC-group.
Discussion
Continuous exposure to stress and stressful life events is a known risk factor for developing many psychological disorders in humans such as anxiety, depression, cognitive impairment, etc. Historical data also support the induction of immunological and behavioral changes typical of the chronic stress response, as well as in rats when exposed to various unpredictable stressors for several weeks.38 Onset of anxiety-like behavior and cognitive deficit as a result of exposure to CUS is well documented in rats.39 The root extract of Ashwagandha (Withania somnifera [L.] Dunal) is an important Ayurvedic medicinal ingredient which acts as a “Rasayana” or “adaptogen”. It possesses anti-stress, antioxidant, immunomodulatory, rejuvenating, anti-inflammatory, anti-arthritic, and anticancer properties in addition to boosting memory and cognition. Ayurveda, the traditional Indian medicinal system, supports use of Withania somnifera roots for medicinal and internal administration purposes. Withanolide A, withanolide B, 12-deoxy-withastramonolide, and withanosides are major active phytochemical components of roots. While health promoting effect of these withanolides is well established, the basic pharmacokinetic data show their quick elimination,40,41 requiring multiple daily dosage in order to gain optimum health benefits.
Thus, after confirming the sustained-release profile of AshwaSR, we evaluated its anti-depressant and anxiolytic effects at a dose of 100mg/kg in rats exposed to various unpredictable stress events.22 We observed that some studies proved that Ashwagandha acted as a mood stabilizer in clinical conditions of anxiety and depression, and showed its prominent anti-oxidant properties. Another study by Bhatnagar et al stated that Ashwagandha root extract could be developed as a potential preventive or therapeutic drug for stress induced neurological disorders. In line with this, a study by Jain et al Treatment with W. somnifera root powder extract significantly reduced (80%) the number of degenerating cells in hippocampal cell bodies.42–44
We used escitalopram (20 mg/kg; P.O.) as a reference product in our study. During the study period, the rats that received the test items were free from any clinical signs and confirming that both AshwaSR and escitalopram were well tolerated at the tested doses.
In the FST, no significant differences were observed in resting time, moving time, distance travelled and speed across the treatment groups compared to the CUS-group. Interestingly, the AshwaSR group exhibited a moderate increase in moving time and distance travelled, and a decrease in the duration of immobility compared to the CUS-group.
In EPM, the test findings confirmed anxiolytic activity of AshwaSR based on the significant reduction observed in the number of entries and the time spent in open arm when compared with the CUS-induced animals. In this study, we observed an increase in open arm entries in the CUS-group. This could be due to the CUS methodology, which induces irritability and aggressive behavior such as rattling, attacking, etc., in rodents.45 Additionally, as is widely known, exposure to novel environments such as OFT immediately before EPM is shown to increase motor activity and leads to higher probability of the animals entering the open arms of the maze.32 This could be due to the hyperactive hypothalamic-pituitary-adrenal (HPA) axis, and is a feature of maladaptive response to chronic stress.38 Similar behavior has been reported in earlier studies where increased open arm entries of CUS-group animals in the EPM (65%, and 27% increase) have been reported when OFT was performed before EPM.46,47
In this study, AshwaSR exerted an antistress effect on irritability and anxiety induced by CUS. Therefore, the increased open arm entries were reduced significantly in AshwaSR and ESC groups.
In the MWM test, CUS induced cognitive abnormalities were evident with increased duration of target latency and distance travelled during acquisition trails (day 3 and day 4). These abnormalities were significantly ameliorated by AshwaSR and ESC (p < 0.001).
Furthermore, the beneficial effects of AshwaSR on higher retention of memory were evident from the significant reduction in target latency time and in the distance travelled during acquisition trails in the MWM test. Notably, there was no change in serum corticosterone levels as AshwaSR and ESC both prevented any rise in serum corticosterone levels in treated rats. No change was noted in the body weight in the collected organ indices due to CUS.
Many studies have documented the anxiolytic effect of Ashwagandha in preclinical and clinical studies.21,22,48 An experimental study by Bhattacharya et al showed equivalent anxiolytic effects of Ashwagandha extract to lorazepam in the EPM test.22 A Bayesian network meta-analysis that aimed to determine the efficacy of 12 medicinal plants for treating anxiety demonstrated that Ashwagandha (mean difference: −4.90, 95% credible interval: −9.70 to −0.17) exhibited anxiolytic effects which were measured by the Hamilton Anxiety Scale scores.48 Similar to these studies, we found comparable anxiolytic effects of AshwaSR with ESC in EPM evaluation. Of note, there was no significant difference in any OFT parameters such as moving time, resting time, distance, speed, fecal count, and central zone entries following exposure to various stressors.
The body’s response to stress is mediated via HPA axis activation, which leads to a surge in circulating corticosterone that affects microglia, especially in the hippocampus and the prefrontal cortex. Further, the sympathetic release of noradrenaline caused by stress exerts direct effects on microglial activity and is evident by increase in the expression of pro-inflammatory cytokine (IL-1β) within the CNS. Notably, neuroinflammation is connected to systemic inflammation which ultimately leads to the development of neurodegenerative diseases.49,50 The breakdown of the blood brain barrier during systemic inflammatory pathogenesis leads to passage of peripheral inflammatory material (leukocytes activation, migration, and transport of circulatory inflammatory mediators) within the CNS which ultimately activates microglia. These chronically activated microglial cells get accumulated which results in neuronal damage by secretion of various inflammatory and neurotoxic mediators like PGE2, monocyte chemoattractant protein-1 (MCP-1), interleukins such as IL-1β, IL-6, TNF-α, nitric oxide, superoxides and fatty acid metabolites.51–53 We noticed that the results of our study were in accordance with the previous studies that reported inhibition of the release of various pro-inflammatory cytokines such as, IL-1β, IL-6 and TNF-α levels in the, hippocampus, hypothalamus and pyriform cortex at transcriptional as well as translational levels.20 We similarly observed the dose-dependent inhibitory effects of AshwaSR on IL-1β, TNF-α production from LPS-induced THP-1 human monocytes at tested treatment concentrations. Similarly, AshwaSR also exhibited dose-dependent inhibition of superoxide generation in PMA-induced HL-60 human monocytic cells. This inhibition of the specific inflammatory cytokines and superoxide point towards reduction in neuroinflammation, which may explain the anxiolytic effects of AshwaSR. Cognitive flexibility measures the ability to rapidly adapt to varying and complex set of directions and/or to manipulate this information. Chronic stress is also associated with impaired cognition. A study showed that Ashwagandha significantly increased the neurotransmitter levels in rats, and proved to be an effective therapeutic agent.20 Many studies have reported that Ashwagandha significantly increased antioxidant levels, and that the pharmacological effects of its root extracts are rich in withanolides which inhibit neutrophil infiltration and cytokine secretion. In one study, administering Ashwagandha prevented post-traumatic stress disorder-induced memory impairment as it preserved changes in the antioxidant mechanisms of the hippocampus.54–58 Evidence has shown that Ashwagandha helps in improving cognitive performance in animals. Shah et al demonstrated the antidepressant effects of Ashwagandha alone or in combination with imipramine or fluoxetine using the forced-swimming model. Gupta et al showed both anxiolytic and antidepressant effect of Ashwagandha dunal root extract in social isolation reared animals.59 Studies have supported the beneficial effect of Ashwagandha in stabilizing mood in clinical depression and anxiety.22
Limitations
In this study, exposure to various stressors did not yield significant changes in the parameters of FST, the most used test to measure the antidepressant efficacy of a product in CUS-induced animals.
Conclusion
The in vitro study showed significant inhibitory effects of AshwaSR on pro-inflammatory cytokines, ie, TNF-α and IL-1β and antioxidant action through the inhibition of superoxide generation which could be responsible for its beneficial effects on CUS-induced anxiety. The in vivo study which was designed based on these results further demonstrated that AshwaSR modulated the stress-induced anxiety by correcting CUS-induced alterations in the animals.
Furthermore, AshwaSR also improved cognitive functions and memory in this CUS model study. Taken together, these results point towards possibility of using AshwaSR as an anti-stress and anxiolytic agent in single daily dose owing to its better solubility and dispersibility.
Abbreviations
AshwaSR, Ashwagandha sustained-release formulation; CL, control; CNS, central nervous system; CUS, chronic unpredictable stress; EPM, elevated plus maze; ESC, Escitalopram; FBS, Fetal Bovine Serum; FST, forced swim test; HBSS, Hank’s Balanced Salt Solution; HPA, hypothalamic-pituitary-adrenal; IAEC, Institutional Animal Ethics Committee; IL, interleukin; LPS, lipopolysaccharide; MWM, Morris water maze; OFT, open field test; PMA, phorbol 12-myristate 13-acetate; SR, sustained-release; TNF, tumor necrosis factor; WHO, World Health Organization.
Data Sharing Statement
We will not be sharing the raw data.
Animals and Ethics
We considered 32 male rats, Rattus norvegicus (Sprague Dawley), aged 8–12 weeks and weighed between 214.4 to 275.8 g. 3–4 animals per cage were housed at 22±3°C temperature and 30–70% relative humidity and 12 h light–dark cycle except on the day of inversion of the light/dark cycle for induction of CUS. All the animals were housed in the solid-bottom autoclaved polypropylene cages with a stainless-steel top grill with separate provisions for animal feed. They received rodent feed pellets (Lab meal-M®, VRK nutritional solutions Pvt. Ltd., Pune) regularly, and mineral water for drinking in polycarbonate bottle ad libitum. Corncob was used as bedding material which was changed twice a week.
The study plan was reviewed and approved by the Institutional Animal Ethics Committee (IAEC) of the Laila Impex R&D center and was documented in the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India (CPCSEA registration No. of the Test Facility: 204/PO/Rc/S/2000/CPCSEA) specified “Form-B” plan (IAEC number:LI/IAEC/LI190106). This study was conducted with the strict adherence to the recommendations of animal welfare guidelines regarding animal care, and all animals were handled humanely with due regard for their welfare in compliance with CPCSEA regulations. All the procedures were implemented in this study were in match with the accepted practices to minimize/avoid risk of causing pain, distress or discomfort to the animals.
Consent for Publication
All authors confirm that the details of images, videos, recordings, etc can be published, and that all authors provide their consent for the same.
Acknowledgments
The study was sponsored by Nutriventia Limited, and Laila Nutraceuticals, India. The authors would like to acknowledge Ms. Arohi Sarang, Ms. Neelanjana Bhattacharyya and Ms. Komal Dobariya from the CBCC Global Research team for their writing and editorial support in the development of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Nutriventia Limited and Laila Nutraceuticals.
Disclosure
Venkateswarlu Somepalli and AV KrishnaRaju are employees of Laila Nutraceuticals, India. Rajat Shah and Shefali Thanawala are employees of Nutriventia Limited, India. Rajat Shah has a patent pending, International (PCT) application WO 2023/021434.
References
1. Micioni Di Bonaventura E, Botticelli L, Del Bello F, et al. Investigating the role of the central melanocortin system in stress and stress-related disorders. Pharmacol Res. 2022;185:106521. doi:10.1016/j.phrs.2022.106521
2. Peng Z, Peng S, Lin K, et al. Chronic stress-induced depression requires the recruitment of peripheral Th17 cells into the brain. J Neuroinflammation. 2022;19(1):186. doi:10.1186/s12974-022-02543-6
3. Woo JM, Postolache TT. The impact of work environment on mood disorders and suicide: evidence and implications. Int J Disabil Hum Dev. 2008;7(2):185–200. doi:10.1515/IJDHD.2008.7.2.185
4. Tafet GE, Nemeroff CB. The links between stress and depression: psychoneuroendocrinological, genetic, and environmental interactions. J Neuropsychiatry Clin Neurosci. 2016;28(2):77–88. doi:10.1176/appi.neuropsych.15030053
5. Kessler RC, Aguilar-Gaxiola S, Alonso J, et al. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2009;18(1):23–33. doi:10.1017/S1121189X00001421
6. Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015;17(3):327–335. doi:10.31887/DCNS.2015.17.3/bbandelow
7. World Health Organization. 2016 Fact Sheet on Depression. Genevas: World Health Organization; 2016.
8. Kyrou I, Christou A, Panagiotakos D, et al. Effects of a hops (Humulus lupulus L.) dry extract supplement on self-reported depression, anxiety and stress levels in apparently healthy young adults: a randomized, placebo-controlled, double-blind, crossover pilot study. Hormones. 2017;16(2):171–180. doi:10.14310/horm.2002.1738
9. Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology. 2016;233(9):1637–1650. doi:10.1007/s00213-016-4218-9
10. Grippo AJ, Scotti MA. Stress and neuroinflammation. Mod Trends Pharmacopsychiatry. 2013;28:20–32.
11. Harris PE, Cooper KL, Relton C, Thomas KJ. Prevalence of complementary and alternative medicine (CAM) use by the general population: a systematic review and update. Int J Clin Pract. 2012;66(10):924–939. doi:10.1111/j.1742-1241.2012.02945.x
12. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1–23.
13. Verma S. Therapeutic uses of Withania somnifera (Ashwagandha) with a note on withanolides and its pharmacological actions. Asian J Pharm Clin Res. 2011;4:1.
14. Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: a Rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med. 2011;8(5 Suppl):208–213. doi:10.4314/ajtcam.v8i5S.9
15. Jahanbakhsh SP, Manteghi AA, Emami SA, et al. Evaluation of the efficacy of Withania somnifera (Ashwagandha) root extract in patients with obsessive-compulsive disorder: a randomized double-blind placebo-controlled trial. Complement Ther Med. 2016;27:25–29. doi:10.1016/j.ctim.2016.03.018
16. Chengappa KN, Bowie CR, Schlicht PJ, Fleet D, Brar JS, Jindal R. Randomized placebo-controlled adjunctive study of an extract of withania somnifera for cognitive dysfunction in bipolar disorder. J Clin Psychiatry. 2013;74(11):1076–1083. doi:10.4088/JCP.13m08413
17. Chengappa KNR, Brar JS, Gannon JM, Schlicht PJ. Adjunctive use of a standardized extract of withania somnifera (Ashwagandha) to treat symptom exacerbation in schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2018;79(5). doi:10.4088/JCP.17m11826
18. Mirjalili MH, Fakhr-Tabatabaei SM, Alizadeh H, Ghassempour A, Mirzajani F. Genetic and withaferin A analysis of Iranian natural populations of Withania somnifera and W. coagulans by RAPD and HPTLC. Nat Prod Commun. 2009;4(3):337–346.
19. Gupta M, Kaur G. Aqueous extract from the Withania somnifera leaves as a potential anti-neuroinflammatory agent: a mechanistic study. J Neuroinflammation. 2016;13(1):193. doi:10.1186/s12974-016-0650-3
20. Gupta M, Kaur G. Withania somnifera as a potential anxiolytic and anti-inflammatory candidate against systemic lipopolysaccharide-induced neuroinflammation. Neuromolecular Med. 2018;20(3):343–362. doi:10.1007/s12017-018-8497-7
21. Kaur T, Singh H, Mishra R, et al. Withania somnifera as a potential anxiolytic and immunomodulatory agent in acute sleep deprived female Wistar rats. Mol Cell Biochem. 2017;427(1–2):91–101. doi:10.1007/s11010-016-2900-1
22. Bhattacharya SK, Bhattacharya A, Sairam K, Ghosal S. Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: an experimental study. Phytomedicine. 2000;7(6):463–469. doi:10.1016/S0944-7113(00)80030-6
23. Pratte MA, Nanavati KB, Young V, Morley CP. An alternative treatment for anxiety: a systematic review of human trial results reported for the Ayurvedic herb ashwagandha (Withania somnifera). J Altern Complement Med. 2014;20(12):901–908. doi:10.1089/acm.2014.0177
24. Remenapp A, Coyle K, Orange T, et al. Efficacy of Withania somnifera supplementation on adult’s cognition and mood. J Ayurveda Integr Med. 2022;13(2):100510. doi:10.1016/j.jaim.2021.08.003
25. Auddy B, Hazra J, Mitra A, Abedon B, Ghosal S. A standardized withania somnifera extract significantly reduces stress-related parameters in chronically stressed humans: a double-blind, randomized, placebo-controlled study. J Am Nutraceut Ass. 2008;11:50–56.
26. Chandrasekhar K, Kapoor J, Anishetty S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J Psychol Med. 2012;34(3):255–262. doi:10.4103/0253-7176.106022
27. Laufs U, Rettig-Ewen V, Böhm M. Strategies to improve drug adherence. Eur Heart J. 2011;32(3):264–268. doi:10.1093/eurheartj/ehq297
28. Li H, Zhang M, Xiong L, Feng W, Williams RO. Bioavailability improvement of carbamazepine via oral administration of modified-release amorphous solid dispersions in rats. Pharmaceutics. 2020;12(11):1023. doi:10.3390/pharmaceutics12111023
29. Perals Bertomeu D, Griffin A, Bartomeus I, Sol D. Revisiting the open-field test: what does it really tell us about animal personality? Anim Behav. 2017;2017:123.
30. Seibenhener ML, Wooten MC, Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015;96:e52434. doi:10.3791/52434
31. da Silva Marques JG, Antunes FTT, da Silva Brum LF, et al. Adaptogenic effects of curcumin on depression induced by moderate and unpredictable chronic stress in mice. Behav Brain Res. 2021;399:113002. doi:10.1016/j.bbr.2020.113002
32. Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. doi:10.1038/nprot.2007.44
33. Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD, The mouse forced swim test. J Vis Exp. 2012;59:e3638. doi:10.3791/3638
34. Yankelevitch-Yahav R, Franko M, Huly A, Doron R. The forced swim test as a model of depressive-like behavior. J Vis Exp. 2015;(97). doi:10.3791/52587
35. Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. doi:10.1038/nprot.2006.116
36. Venkataramaiah C, Gunduluru S, Rajendra W. Morris water maze – a benchmark test for learning and memory disorders in animal models: a review. Asian J Pharm Clin Res. 2018;11:25. doi:10.22159/ajpcr.2018.v11i5.24292
37. Zhang L, Fang Y, Xu Y, et al. Curcumin Improves Amyloid β-Peptide (1-42) induced spatial memory deficits through BDNF-ERK signaling pathway. PLoS One. 2015;10(6):e0131525. doi:10.1371/journal.pone.0131525
38. Monteiro S, Roque S, de Sá-calçada D, Sousa N, Correia-Neves M, Cerqueira JJ. An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front Psychiatry. 2015;6:6. doi:10.3389/fpsyt.2015.00006
39. Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33(2):320–331. doi:10.1038/sj.npp.1301410
40. Patil D, Gautam M, Mishra S, et al. Determination of withaferin A and withanolide A in mice plasma using high-performance liquid chromatography-tandem mass spectrometry: application to pharmacokinetics after oral administration of Withania somnifera aqueous extract. J Pharm Biomed Anal. 2013;80:203–212. doi:10.1016/j.jpba.2013.03.001
41. Dahikar PRK, Sahni Y. Pharmacokinetics of Withania somnifera (Ashwagandha) in healthy Buffalo calves. Buffalo Bull. 2012;31(4):219–223.
42. Dey A, Chatterjee SS, Kumar V. Triethylene glycol-like effects of Ashwagandha (Withania somnifera (L.) Dunal) root extract devoid of withanolides in stressed mice. Ayu. 2018;39(4):230–238. doi:10.4103/ayu.AYU_219_16
43. Alzoubi KH, Al Hilo AS, Al-Balas QA, El-Salem K, El-Elimat T, Alali FQ. Withania somnifera root powder protects against post-traumatic stress disorder-induced memory impairment. Mol Biol Rep. 2019;46(5):4709–4715. doi:10.1007/s11033-019-04915-3
44. Jain S, Shukla SD, Sharma K, Bhatnagar M. Neuroprotective effects of Withania somnifera Dunn. in hippocampal sub-regions of female albino rat. Phytother Res. 2001;15(6):544–548. doi:10.1002/ptr.802
45. Mineur YS, Prasol DJ, Belzung C, Crusio WE. Agonistic behavior and unpredictable chronic mild stress in mice. Behav Genet. 2003;33(5):513–519. doi:10.1023/A:1025770616068
46. Dubey VK, Ansari F, Vohora D, Khanam R. Possible involvement of corticosterone and serotonin in antidepressant and antianxiety effects of chromium picolinate in chronic unpredictable mild stress induced depression and anxiety in rats. J Trace Elem Med Biol. 2015;29:222–226. doi:10.1016/j.jtemb.2014.06.014
47. Karson A, Demirtaş T, Bayramgürler D, Balci F, Utkan T. Chronic administration of infliximab (TNF-α inhibitor) decreases depression and anxiety-like behaviour in rat model of chronic mild stress. Basic Clin Pharmacol Toxicol. 2013;112(5):335–340. doi:10.1111/bcpt.12037
48. Zhang W, Yan Y, Wu Y, et al. Medicinal herbs for the treatment of anxiety: a systematic review and network meta-analysis. Pharmacol Res. 2022;179:106204. doi:10.1016/j.phrs.2022.106204
49. Herrera AJ, Espinosa-Oliva AM, Oliva-Martin MJ, Carrillo-Jimenez A, Venero JL, de Pablos RM. Collateral damage: contribution of peripheral inflammation to neurodegenerative diseases. Curr Top Med Chem. 2015;15(21):2193–2210. doi:10.2174/1568026615666150610142027
50. Zubair Alam M, Alam Q, Amjad Kamal M, et al. Infectious agents and neurodegenerative diseases: exploring the links. Curr Top Med Chem. 2017;17(12):1390–1399. doi:10.2174/1568026617666170103164040
51. Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi:10.1038/nrn2038
52. Lynch MA. The multifaceted profile of activated microglia. Mol Neurobiol. 2009;40(2):139–156. doi:10.1007/s12035-009-8077-9
53. Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7(4):354–365. doi:10.1016/j.nurt.2010.05.014
54. Suganya K, Kayalvizhi E, Yuvaraj R, Chandrasekar M, Kavitha U, Konakanchi Suresh K. Effect of Withania Somnifera on the antioxidant and neurotransmitter status in sleep deprivation induced Wistar rats. Bioinformation. 2020;16(8):631–637. doi:10.6026/97320630016631
55. Balkrishna A, Solleti SK, Singh H, Sharma N, Varshney A. Withanolides from withania somnifera ameliorate neutrophil infiltration in endotoxin-induced peritonitis by regulating oxidative stress and inflammatory cytokines. Planta Med. 2022;88(6):466–478. doi:10.1055/a-1438-2816
56. Rasool M, Varalakshmi P. Immunomodulatory role of Withania somnifera root powder on experimental induced inflammation: an in vivo and in vitro study. Vascul Pharmacol. 2006;44(6):406–410. doi:10.1016/j.vph.2006.01.015
57. Grunz-Borgmann E, Mossine V, Fritsche K, Parrish AR. Ashwagandha attenuates TNF-α- and LPS-induced NF-κB activation and CCL2 and CCL5 gene expression in NRK-52E cells. BMC Complement Altern Med. 2015;15:434. doi:10.1186/s12906-015-0958-z
58. Trivedi MK, Mondal SC, Gangwar M, Jana S. Effect of a novel ashwagandha-based herbomineral formulation on pro-inflammatory cytokines expression in mouse splenocyte cells: a potential immunomodulator. Pharmacogn Mag. 2017;13(Suppl 1):S90–s94. doi:10.4103/0973-1296.197709
59. Gupta GL, Rana AC. Protective effect of Withania somnifera dunal root extract against protracted social isolation induced behavior in rats. Indian J Physiol Pharmacol. 2007;51(4):345–353.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


