Back to Journals » Journal of Inflammation Research » Volume 17
CSF Findings in Chinese Patients with NMDAR, LGI1 and GABABR Antibody-Associated Encephalitis
Authors Qiao S, Li H, Cui C, Zhang C, Wang A, Jiang W, Zhang S
Received 12 October 2023
Accepted for publication 1 February 2024
Published 18 March 2024 Volume 2024:17 Pages 1765—1776
DOI https://doi.org/10.2147/JIR.S383161
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Shan Qiao,1,2,* Haiyun Li,3,* Caisan Cui,4 Chong Zhang,1 Aihua Wang,1 Wenjing Jiang,3 Shanchao Zhang1,5
1Department of Neurology, the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong First Medical University, Jinan, People’s Republic of China; 2Department of Medical Genetics, School of Basic Medical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, People’s Republic of China; 3Department of Geriatric Medicine, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, People’s Republic of China; 4Department of Neurology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, People’s Republic of China; 5School of Medicine, Cheeloo College of Medicine, Shandong University, Jinan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shanchao Zhang, Email [email protected]; Wenjing Jiang, Email [email protected]
Purpose: CSF inflammation in subtypes of antibody-defined autoimmune encephalitis (AE) ranges in intensity from moderate to severe. In a retrospective, cross-sectional study, we characterized CSF findings in Chinese patients with anti-N-methyl-D-aspartate receptor encephalitis (NMDAR-E), anti-leucine-rich glioma-inactivated 1 encephalitis (LGI1-E), and anti-gamma aminobutyric acid-B receptor encephalitis (GABABR-E).
Patients and Methods: The AE cases, including 102 NMDAR-E, 68 LGI1-E and 15 GABABR-E, were included. CSF inflammatory parameters consisted primarily of CSF leukocytes, oligoclonal bands (OCBs), and CSF/serum albumin ratios (QAlb). Ten serum cytokines were evaluated in order to classify AE subtypes.
Results: 88% of NMDAR-E, 80% of GABABR-E, and 51% of LGI1-E patients had aberrant CSF features. In NMDAR-E, the CSF leukocyte count, CSF protein concentration, and age-adjusted QAlb were significantly higher than in LGI1-E, but did not differ from GABABR-E. Blood-CSF barrier dysfunction was less common in NMDAR-E patients with > 40 years old. On admission, inflammatory CSF response was more prevalent in NMDAR-E patients with a higher CASE score. With age < 60 years, CSF inflammatory changes were less frequent in LGI1-E patients, but more common in GABABR-E patients. MCP-1, IL-10, IL-1β, and IL-4 were potential classifiers for NMDAR-E, LGI1-E, and GABABR-E, and correlated substantially with CSF leukocyte count and QAlb.
Conclusion: Subtype-specific patterns are formed by the various inflammatory CSF parameters in NMDAR-E, LGI1-E, and GABABR-E, and their correlation with disease severity, age, and disease duration. CSF inflammatory characteristics associated with MCP-1, IL-10, IL-1β, and IL-4 may be potential immunopathogeneses targeting markers for these AE subtypes.
Keywords: autoimmune encephalitis, cytokines, neuroinflammation, cerebrospinal fluid
Introduction
NMDA receptor encephalitis (NMDAR-E), leucine-rich glioma inactivated protein-1 encephalitis (LGI1-E), and gamma aminobutyric acid-B receptor encephalitis (GABABR-E) are the three common subtypes of autoimmune encephalitis (AE) in China.1 In terms of clinical features, MRI presentation, and cerebrospinal fluid (CSF) parameters, these subtypes differ significantly.2 NMDAR-E typically presents as a global encephalitic syndrome characterized by decreased consciousness, stereotypic movements, and vegetative dysfunction,3 whereas LGI1-E and GABABR-E are characterized by a typical limbic AE.1 On cranial MRI, patients with LGI1-E and GABABR-E frequently exhibit mesiotemporal T2-hyperintensities,4 whereas MRI findings in NMDAR-E are frequently normal, despite the presence of heterogeneous white matter lesions in approximately half of the patients.5 Previous systematic analysis of NMDAR-E, LGI1-E and GABABR-E have shown that LGI1-E typically exhibits minimal and infrequent CSF inflammation, while NMDAR-E and GABABR-E are associated with robust and frequent inflammation with regard to basic CSF parameters comprising leukocytes, total protein, and oligoclonal bands (OCBs).6,7 This analysis has not, however, been validated in a larger cohort of NMDAR-E, LGI1-E and GABABR-E cases in China. Therefore, we performed a retrospective analysis of the detailed inflammatory CSF findings in LGI1-E, NMDAR-E and GABABR-E patients. In addition, the interactions between various CSF parameters and their associations with disease duration, severity, age, and serum-specific cytokines were investigated.
Materials and Methods
Patients and Data Collection
Patients were retrospectively recruited from 2016 to February 2022, with all subjects meeting specific inclusion criteria. These criteria included a diagnosis of AE with CSF and/or serum anti-NMDAR antibody positivity, anti-LGI1 antibody positivity, or anti-GABABR antibody positivity, as defined in 2016 using the fixed cell-based assay (Euroimmun, Germany).8 A total of 185 patients were recruited from The First Affiliated Hospital of Shandong First Medical University and Qilu Hospital, including 102 with NMDAR-E, 68 with LGI1-E, and 15 with GABABR-E. Clinical data, such as demographic information, age at onset, prodromal symptoms, clinical manifestation, and CSF findings, were obtained through a retrospective review of medical records. Follow-up data were collected via clinical examinations conducted during return visits and telephone interviews. The modified Rankin scale (mRS) was used to evaluate each patient’s neurological status, and the Clinical Assessment Scale in Autoimmune Encephalitis (CASE) score was calculated for each patient with NMDAR-E.9 All data were omitted for the following reasons: (1) patients with laboratory evidence of infectious encephalitis (eg, viral, bacterial, mycobacterium tuberculosis, parasitic); (2) patients with incomplete data or with a history of other CNS disease (eg, multiple sclerosis, epilepsy, stroke, and related disease prior to the onset of encephalitis); and (3) patients with coexisting antibodies, such as myelin oligodendrocyte glycoprotein (MOG) antibody, aquaporin 4 (AQP4) antibody and other antibodies.
Sample Collection
The fasting blood samples of seven patients with NMDAR-E were collected on the day following their arrival, while they were still experiencing active disease symptoms, prior to the initiation of immunotherapy, as described in our previous reports.10,11 These samples were promptly frozen at a temperature of −80°C until they could be analyzed.
Human Inflammation Molecular Array
The Human Inflammation Array (RayBiotech) was used to identify ten different cytokines/chemokines in serum, including MCP1, IL10, IL-1β, IL4, IL6, IL13, IL1a, IL8, TNF-ɑ, and IFN-γ. The testing was carried out following the manufacturer’s instructions, as described in previous studies.10,11
Definitions
Pleocytosis was classified by >5 leukocytes/μL. Age-normalized QAlb (QAlb/Qlim) was calculated by dividing QAlb by the age dependent upper limit (Qlim; 4+age/15×10−3).12 Blood-CSF barrier dysfunction was defined as QAlb/Qlim >1. mRS score of ≤2 points were considered as good functional status, while poor functional status defined as mRS ≥3.
Statistics
Principal component analysis (PCA) was employed to classify patients with AE based on the expression of serum cytokines.13 In order to further identify the cytokine classifiers specific to NMDAR-E, LGI1-E, and GABABR-E, orthogonal partial least squares discriminant analysis (OPLS-DA) was utilized.13 The model was assessed using the goodness of prediction (q2) and R-square on the Y-axis (diagnosis, R2Y). A q2 value greater than 0.4 was considered significant and indicative of good prediction.14 Predictors were defined as the predictive components with a variable importance in projection (VIP) score exceeding 1.0, while those with a score greater than 1.5 were considered the most effective predictors.15 The OPLS-DA analysis was performed using SIMCA 17.0 software (Umetrics, Sweden).
The statistical analysis was conducted using SPSS V23.0 (SPSS, Chicago, IL, USA), while GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA) was utilized for creating the figures. Categorical variables were examined using either the Fisher exact test or the χ2 test. For ordinal and continuous variables, median and interquartile range (IQR) values were obtained. The Student’s t-test or Mann–Whitney U-test was employed for two groups, while the Kruskal–Wallis test followed by the Dunn multiple comparisons test was used for more than two groups. The Spearman rank correlation and the Pearson correlation coefficient were utilized to examine relationships between two continuous variables. Missing values were handled through multiple imputations by SPSS. According to a previous study,16 the NMDAR-E cohort was divided into three age groups (≤20 years, 21–40 years, and >40 years), while the LGI-E and GABABR-E cohort was divided into two age groups (≤60 years vs >60 years). The discriminating ability of the clinical parameters was evaluated using the area under the receiver operating characteristic curves (AUCs) and the corresponding 95% confidence intervals. Multiple logistic regression studies were conducted to study the effect of various parameters on categorical variables. A backward stepwise multivariate logistic regression with an elimination criteria of p <0.05 was employed to select variables. The results were presented as odds ratios (ORs) and 95% confidence intervals (CIs). SPSS imputed missing data to enhance statistical power and reduce the risk of bias.
Results
The demographics of the patients with NMDAR-E, LGI1-E and GABABR-E
The patient cohorts consisted of 102 cases diagnosed with NMDAR-E, 68 cases with LGI1-E, and 15 cases with GABABR-E. In the NMDAR-E group, female patients accounted for more than 50% of the cases, while in the LGI1-E and GABABR-E groups, male patients accounted for more than 50% of the cases. The median age at diagnosis was 18 years (10.50–33.50 years) for NMDAR-E, 59.50 years (52–67.75 years) for LGI1-E, and 64 years (54–69 years) for GABABR-E. All patients with GABABR-E and over 80% of patients with LGI1-E experienced seizure onset, whereas approximately 70% of patients with NMDAR-E did. Detailed demographic features of the patients with NMDAR-E, LGI1-E, and GABABR-E can be found in Table S1.
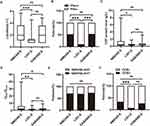
Higher CSF leukocyte count, CSF protein level and more frequent OCBs in NMDAR-E compared with LGI1-E
The leukocyte count in CSF of patients with NMDAR-E was substantially higher than that of patients with LGI1-E, but did not differ significantly from patients with GABABR-E (Figure 1A). Moreover, the proportion of NMDAR-E and GABABR-E patients with CSF leukocyte count above 5/μL was substantially higher than that of LGI1-E patients (Figure 1B). The patients with NMDAR-E had a higher CSF protein level than those with LGI1-E, but the difference was not statistically significant between NMDAR-E and GABABR-E (Figure 1C). Similarly, NMDAR-E patients were present with higher QAlb/QLim value compared with LGI-E and GABABR-E patients (Figure 1D). However, the proportion of patients with blood-CSF barrier dysfunction remained relatively constant across these three subtypes of encephalitis (Figure 1E). In addition, OCBs were detected in one-third of patients with NMDAR-E but in less than one-tenth of patients with LGI1-E (Figure 1F). Using univariate logistic regression analysis (Table S2), we were able to identify several factors associated with a decreased odds ratio (OR) in predicting the occurrence of LGI1-E, including CSF lymphocyte count, age-adjusted QAlb, and CSF-restricted OCBs, all of which were statistically significant at p <0.01. In addition, the multivariate logistic regression analysis confirmed that both CSF lymphocyte count and age-adjusted QAlb were independently associated with a lower OR for the occurrence of LGI1-E (p = 0.002 and 0.019, respectively). Comparing GABABR-E and NMDAR-E (Table S3), we found that CSF leukocyte count, QAlb/QLim, and CSF-restricted OCBs were not predictive of NMDAR-E diagnosis (p >0.05). However, in the GABABR-E versus LGI1-E model (Table S3), we determined that CSF leukocyte count alone was associated with a decreased OR for LGI1-E (p =0.001). Concerning blood-CSF barrier dysfunction, there was no significant difference between patients with positive and negative CSF OCB for NMDAR-E, LGI1-E and GABABR-E (Figure S1A). Similarly, there is no difference in CSF leukocyte count between AE patients with QAlb/QLim >1 and those with QAlb/QLim ≤1 for NMDAR-E, LGI1-E, and GABABR-E (Figure S1B). In addition, the difference in CSF leukocyte count between CSF OCB-positive and -negative patients remained statistically insignificant (Figure S1C).
Effect of disease duration on inflammatory CSF changes in NMDAR-E, LGI1-E and GABABR-E
To examine the effect of disease duration on CSF inflammatory abnormalities, all patients were categorized according to the duration of their illness (≤1 week, 2–3 weeks, ≥4 weeks) at the time of LP. GABABR-E patients had a significantly reduced CSF leukocyte count than NMDAR-E and LGI1-E patients by the fourth week (p <0.05; Figure S1D). The percentage of patients with QAlb/QLim >1 and CSF-restricted OCB positivity was unaffected by disease duration (Figure S1E and S1F). The relationship between CSF leukocyte count, the blood-CSF barrier, and CSF-restricted OCB was investigated further to determine if the timing of LP affected this relationship. In NMDAR-E and GABABR-E patients who underwent early LP (≤1 week and 2–3 weeks) and late LP (≥4 weeks), the CSF leukocyte count in the subgroup with QAlb/QLim >1 was comparable to that of the subgroup with QAlb/QLim ≤1 (Figure S1G and S1I). Within 2–3 weeks of clinical onset, more LGI1-E patients with QAlb/QLim >1 had an elevated CSF leukocyte count, but this trend was not statistically significant in LGI1-E patients who underwent LP ≥4 weeks after the onset of symptoms (Figure S1H). In the patients with NMDAR-E, LGI1-E, and GABABR-E who had early LP, there was no significant difference in CSF leukocyte count between those who tested positive and who tested negative for CSF OCB. This also held true for the patients who experienced LP later (Figure S1J-S1L). In these three classes of adverse events, the timing of LP had no effect on the proportion of the patients with CSF-restricted OCB positivity, irrespective of whether their QAlb/QLim was >1 or ≤1 (Figure S1M-S1O).
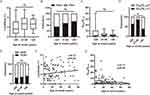
The blood-CSF barrier is more dysfunctional in younger patients with NMDAR-E
According to a previous study,16 the patients with NMDAR-E were divided into three groups: young (≤20 years, N=55), intermediate (21–40 years; N=29), and older (>40 years, N=18), and the LGI1-E and GABABR-E cohorts were dichotomized only (<60 years, N=34, ≥60 years, N=34; <60 years, N =4, ≥60 years, N=11) in our cohort. Even though older patients were more likely to be male, the subcategories for each AE subtype were very similar (Figure S2A-S2C). Age at onset was independently associated with an increased OR for LGI1-E incidence (p <0.001), as shown in Table S2. According to previous studies, the preponderance of LGI1-E patients are older males, whereas NMDAR-E patients are younger females.17 CSF leukocytes were not substantially different between younger and older patients (Figure 2A and B). NMDAR-E patients with age > 40 years had a decreased frequency of CSF-blood barrier disruption (p <0.05; Figure 2C and D). For OCBs, the youngest group did not differ from the older groups (Figure 2E), and there were no age-related differences in CSF leukocyte count or age-adjusted QAlb in NMDAR-E (Figure 2F and G). There was no significant difference between LGI1-E and GABABR-E in CSF leukocyte count (Figure S2D and S2E), age-adjusted QAlb (Figure S2F and S2G), or frequency of CSF-restricted OCBs (Figure S2H). Similar to NMDAR-E, no age-related differences were observed in CSF leukocytes or QAlb in LGI1-E or GABABR-E (Figure S2I, S2J and S2K, S2L).
Definitively inflammatory CSF can be most clearly defined as CSF with either pleocytosis or OCBs or both. The incidence of inflammatory CSF decreased in NMDAR-E aged >20 years (Figure S3A). In LGI1-E, the tendency toward decreased frequency of inflammatory CSF was more pronounced in the patients aged <60 years compared to those aged ≥60 years (Figure S3B). In contrast, GABABR-E patients with age ≥60 years exhibited a significant tendency toward less frequent CSF inflammation (Figure S3C). A subgroup analysis of patients with NMDAR-E, LGI1-E, and GABABR-E aged >40 years was conducted to rule out a significant age-related bias in CSF findings for NMDAR-E, LGI1-E, and GABABR-E. At admission, each cohort had the same age and mRS (Figure S3D and S3G). However, LGI1-E was associated with a lower proportion of female patients and a prolonged time between disease onset and LP (Figure S3E and S3F). NMDAR-E exhibited more pronounced inflammatory CSF response than LGI1-E in patients older than 40 years (Figure S3H-S3K). However, gender had no effect on CSF results in any subtype of AE (Figure S4).
Inflammatory CSF changes is associated with CASE score on admission in NMDAR-E
The patients with NMDAR-E and LGI1-E who had an mRS score of 0–2 on admission had no significant difference in inflammatory CSF changes when compared to those who had an mRS score of 3 or higher on admission (Figure S5A-S5D and S5E-S5H). In GABABR-E, the patients with mRS = 4, 5 were not present with more inflammatory CSF changes than those with mRS = 1–3 (Figure S5I-S5L). Following that, all of the recruited patients were classified as having mild, moderate, or severe functional impairment based on their mRS scores at the time of admission. The resulting subcohorts for both AE subtypes had similar age, gender distribution, and duration from onset to LP (Figure S6). The CSF leukocyte count remained no difference in severe NMDAR-E and LGI1-E patients compared to mild patients (Figure 3A and B). Furthermore, there was no significant difference in age-adjusted QAlb, CSF-restricted OCBs, or inflammatory CSF status between severe and mild (Figure 3C–F). mRS ≥3 on admission, on the other hand, was associated with a decreased OR of the occurrence of LGI1-E compared to NMDAR-E (p <0.001) (Table S2), implying that NMDAR-E patients were more likely to develop worse neurological impairment after disease onset. Because there were only two GABABR-E patients with mRS ≤2 on admission, statistical analysis in the GABABR-E cohort was not performed. In the GABABR-E vs NMDAR-E model (Table S3), mRS ≥3 on admission cannot differentiate NMDAR-E from GABABR-E (p =0.580), but mRS ≥3 on admission was more common in the GABABR-E vs LGI1-E model (p =0.003).
The CASE score was found to be appropriate for grading and monitoring the severity of AE symptoms.9 CASE score was calculated in the medical record of NMDAR-E patients alone in our patient cohort. Gender, age at onset, and LP duration after disease onset did not affect CASE score in NMDAR-E (Figure 4A-D). CSF lymphocyte count, age-adjusted QAlb, and CSF-restricted OCBs were not classified by CASE score at admission (Figure 4E-I), whereas inflammatory CSF became more common in NMDAR-E patients with higher CASE score at admission (Figure 4J).
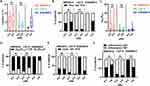
Distinct serum cytokines and the association of them with inflammatory CSF signatures in NMDAR-E, LGI1-E and GABABR-E
On the basis of the results of our previous studies,10,11 we investigated whether serum cytokines could serve as inflammatory biomarkers, separating the three AE categories and inferring their underlying pathology, as well as determining their association with CSF inflammatory parameters. To differentiate between NMDAR-E, LGI1-E, and GABABR-E cases, a representative PCA score plot was constructed using cytokine data (Figure S7A). Each point on the graph represented the ten cytokine parameters for a single patient; points that were closer together exhibited a greater degree of inflammation similarity. Although the PCA plot demonstrated that all AE patients were divided into three patient cohorts (NMDAR-E, LGI1-E, and GABABR-E), two LGI1-E patients were closely aggregated with four GABABR-E patients (Figure S7A, red dashed circle). OPLS-DA models using the same cytokine data as PCA had significantly greater discriminant power to cluster NMDAR-E, LGI1-E, and GABABR-E compared to PCA (Figure S7B). The PCA model for NMDAR-E versus LGI1-E underperformed the OPLS-DA model (q2=0.851 versus 0.948; Figure S7C and S8A). Similarly, the PCA model of NMDAR-E versus GABABR-E had a q2 of 0.362, which was significantly lower than the OPLS-DA model’s q2 of 0.895 (Figure S7D and S8B). The S-plot revealed distinct classifiers between NMDAR-E and LGI1-E (FigureS8C), and between NMDAR-E and GABABR-E (Figure S8D). Following this, the ranking of VIP scores revealed that MCP-1, IL-10, and IL-1β were the three most influential cytokines in the differentiation between NMDAR-E and LGI1-E (Figure S8E), with VIP scores of 1.87, 1.36, and 1.20, respectively. In addition, the four novel cytokines (MCP-1, IL-10, IL-1β, and IL-4) were more influential in differentiating NMDAR-E from GABABR-E (Figure S8F). The levels of these four novel cytokines were compared between NMDAR-E and LGI1-E and NMDAR-E and GABABR-E. IL-1β and IL-4 levels were significantly greater in LGI1-E patients than in NMDAR-E and GABABR-E patients (p<0.001, p<0.05; Figure 5A and 5C). Serum IL-10 levels were significantly higher in GABABR-E than in NMDAR-E and LGI1-E (all p <0.05) (Figure 5B). MCP-1 expression was higher in the serum of the NMDAR-E subgroup compared to the LGI1-E subgroup (p <0.001) and the GABABR-E subgroup (p <0.05) (Figure 5D). As shown in Tables S2 and S3, we examined the association between four selected cytokines (MCP-1, IL-10, IL-1β, and IL-4) and CSF lymphocyte count and age-adjusted QAlb in three subgroups of encephalitis. In our study, serum IL-1β expression was positively associated with CSF lymphocyte count in GABABR-E alone (r=0.67, p=0.03), not in NMDAR-E (r=0.04, p=0.92) and LGI1-E (r=0.25, p=0.28), and MCP1 level had increased tendency with higher CSF lymphocyte count in LGI1-E (r=0.47, p=0.04), not NMDAR-E (r=0.29, p=0.53) and GABABR-E (r=0.23, p=0.51). Across all subtypes of encephalitis, there was no correlation between serum IL-10, IL-4 and CSF lymphocyte count (Figure 5E) (all p>0.05). In addition, serum IL-1β was positively associated with age-adjusted QAlb in GABABR-E (r=0.66, p=0.04) but not in NMDAR-E and LGI1-E (all p>0.05). There was no correlation between serum IL-10 and age-adjusted QAlb of NMDAR-E, LGI1-E and GABABR-E (NMDAR-E, r=0.43, p=0.35; LGI1-E, r=−0.02, p=0.92; GABABR-E, r=0.56, p=0.10). Higher serum IL-4 concentrations were associated with higher age-adjusted QAlb in LGI1-E (r=0.71, p=0.02) and GABABR-E (r=0.55, p=0.01) but not in NMDAR-E (p>0.05). MCP1 level was correlated with age-adjusted QAlb in LGI1-E alone (Figure 5F), but not in NMDAR-E (r=0.42, p=0.32) or GABABR-E (r=0.44, p=0.20).
Discussion
NMDAR-E, LGI1-E, and GABABR-E are the three common AE subtypes in China, with NMDAR-E and GABABR-E for those with prominent CSF inflammation and LGI1-E for those with little or no CSF inflammation changes. Aside from the expected quantitative differences, our detailed analysis of CSF findings in acute NMDAR-E, LGI1-E, and GABABR-E shows that each of the three AE subtypes exhibits a distinct pattern of CSF changes. These are distinguished by differences in the interdependence of CSF parameters and their associations with age, disease duration, and disease severity.
On a quantitative level, NMDAR-E patients had higher CSF leukocyte counts than LGI-E patients. NMDAR-E had a higher rate of blood-CSF barrier dysfunction and CSF-restricted OCBs than LGI-E. These findings were consistent with previous ones.16,18 In terms of CSF leukocyte counts, blood-CSF barrier function, and CSF-restricted OCB frequency, NMDAR-E was found to be similar to GABABR-E as well.
The disease-specific mutual interactions of CSF parameters in NMDAR-E, LGI1-E and GABABR-E were not observed in CSF leukocytes, age-adjust QAlb and CSF-restricted OCBs in our study, which was inconsistent with the study of NMDAR-E and LGI1-E by Marc Durr and coworkers that CSF leukocytes were higher when OCBs were present in NMDAR-E, and strongly associated with blood-CSF barrier dysfunction in LGI1-E.16 It has been proposed that in LGI1-E, systemically synthesized anti-LGI1-IgG and complement from plasma enter the brain at sites of blood-barrier dysfunction, where both induce focal inflammation.19,20 This disparity could be due to patient heterogeneity, the size of the recruited patient cohort, sampling time, storage conditions, test method, and other differences between previous work and our current study.
Age, disease duration, and disease severity are patient-specific covariates that differentially affect the CSF findings for NMDAR-E, LGI1-E, and GABABR-E. In NMDAR-E and LGI1-E, the frequency of inflammatory CSF changes tended to be lower in senior patients, whereas in GABABR-E, younger patients were more susceptible to inflammatory CSF changes. NMDAR-E and GABABR-E had higher CSF leukocytes and more frequent OCB positivity than LGI1-E in all AE subtypes in patients older than 40 years. This was generally in line with previous research indicating that CSF leukocyte counts drop with age in NMDAR-E but not in LGI1-E.16 The CSF leukocyte count was independent of LP time points after disease onset in NMDAR-E, which contrasts with a previous report of higher CSF leukocyte counts in NMDAR-E at very early time points compared to later time points.21 However, our data also partially supported the previous finding that blood-CSF barrier dysfunction became more pronounced in older patients with NMDAR-E, but not in older patients with LGI1-E.16 For GABABR-E, there was no association between the frequency of blood-CSF barrier dysfunction and age at onset. A larger patient population and sample size were required to investigate the potential relationship between them. According to the study by Marc Durr et al,16 OCB was twice as infrequent in mild NMDAR-E (mRS ≤2) than in severe NMDAR-E (mRS ≥3). In our study, NMDAR-E with inflammatory CSF changes but not CSF-restricted OCB positivity, had a higher incidence of severe clinical symptoms as measured via the CASE score, as opposed to the mRS. The CASE score is a valuable assessment tool for capturing the big picture and assessing the severity and progression of symptoms.22 The CASE score enables the recording of symptoms in more detail than the mRS score.23 Intriguingly, our results did not necessarily substantiate the reported correlation between the CASE score and mRS being stronger in patients with a worse clinical condition.24 The significant dissociation of mRS and CASE score with CSF inflammatory parameters might be explained by differences in sample sizes and CSF sampling, symptom severity depending on the antibody present, and the varied sensitivity of the mRS and CASE scores in recording patients’ symptoms. In our study, the clinical severity of LGI1-E and GABABR-E was independent of the presence or absence of OCB. It has been reported that AE subtypes with NMDAR-E and LGI1-E typically exhibit less CSF inflammation with older age, possibly as a consequence of immune senescence.25 Even in the patients with NMDAR-E and LGI1-E, advanced age was more likely to reduce the likelihood of inflammatory CSF findings. However, the patients with GABABR-E who were younger than 50 years old exhibited an increased CSF inflammatory response, whereas CSF inflammatory changes became less severe with age ≥50 years old.
Our findings contradicted previous claims that OCBs are uncommon in early NMDAR-E and increase in frequency over time.21 In our cohort, nearly 25% of patients with NMDAR-E were positive for OCBs within the first week, compared to 10% at first LP in the previous study.21 In addition, positive OCBs did not become substantially more common at subsequent time points in NMDAR-E, LGI1-E, or GABABR-E. It is unclear for these discrepancies, which might be the consequence of distinct OCB detection techniques.
In addition, we examined whether serum cytokines/chemokines in NMDAR-E, LGI1-E, and GABABR-E could mediate CSF inflammatory changes in all types of AE. Recent research revealed that IL-10, IL-1β, and MCP1 were the molecules that distinguished NMDAR-E, LGI1-E, and GABABR-E most. IL-10, an anti-inflammatory cytokine predominantly produced by monocytes, inhibits the production of pro-inflammatory cytokines, including GM-CSF, IL-6, TNF, etc.26 The presence of elevated IL-10 expression in NMDAR-E correlates favorably with disease severity.27 This trend was not observed between LGI1-E and the control group of healthy individuals.28 In contrast, the expression of IL-10 was substantially higher in LGI1-E than in NMDAR-E and GABABR-E, and in all AE subtypes, IL-10 expression correlated insignificantly with the severity of CSF inflammatory parameters, such as a higher CSF lymphocyte count and QAlb. This might suggest that LGI1-E with mild inflammatory responses, and NMDAR-E and GABABR-E with severe inflammatory responses due to IL-10 anti-inflammatory potential. IL-1β is involved in numerous biological processes, such as cell division and differentiation.29 IL-1β differentially induced perturbations in blood-brain barrier (BBB) permeability by inducing modifications in junctional complex organization and transcellular trafficking.30 Accordingly, in our cohort, GABABR-E patients with elevated serum IL-1β were more likely to develop vast blood-CSF barrier dysfunction and a higher CSF lymphocyte count. Chemokine MCP1 levels are elevated in the CNS and CSF of patients with neuroinflammatory disorders characterized by breakdown of the BBB and leukocyte infiltration of the CNS.30 It aids host defense by attracting monocytes and macrophages to the site of inflammation.31 MCP1 is required for anti-NMDAR IgG migration to the brain in AE,32 and MCP1 mRNA expression is elevated in the hippocampus of mice with anti-NMDAR antibody-induced recurrent seizures.33 Plasma levels of MCP1 were elevated in LGI1-E patients, but not in NMDAR-E or GABABR-E patients11,34 MCP1 expression was significantly higher in NMDAR-E than in LGI1-E and GABABR-E, but there was no correlation with elevated CSF lymphocyte count and blood-CSF barrier dysfunction. This may be due to the small sample size of NMDAR-E in our cohort for measuring serum MCP1 expression.
Due to the nature of the study design, the retrospective analyses of data collected during routine clinical practice generate a variety of possible biases. A significant limitation of this study is the small sample size within subgroups due to the low prevalence of AE, especially for the cytokine test, which may have constrained our conclusions and contributed to the exploratory nature of this study.
Conclusion
In conclusion, we demonstrate that NMDAR-E, LGI1-E, and GABABR-E CSF patterns differ significantly, indicating divergent immunopathogeneses. We provide evidence of a more severe inflammatory response in NMDAR-E and GABABR-E with frequent polyspecific immune activation than in LGI1-E. Concerning diagnostic considerations, we demonstrate that the presence of elevated CSF leukocytes, age-adjusted QAlb, and severe function impairment (mRS≥3) at disease onset renders the diagnosis of LGI1-E extremely implausible. Finally, we demonstrated the association between several novel cytokines distinct from NMDAR-E, LGI1-E, and GABABR-E and CSF inflammatory responses. Prospective research on treatment responses and long-term outcomes, as well as analysis of cytokines and relapse samples, could further validate our results.
Abbreviations
AE, autoimmune encephalitis; NMDAR-E, anti-N-methyl-D-aspartate receptor encephalitis; LGI1-E, anti-leucine-rich glioma-inactivated 1 encephalitis; GABABR-E, anti-gamma aminobutyric acid-B receptor encephalitis; OCB, oligoclonal bands; QAlb, CSF/serum albumin ratios; CSF, cerebrospinal fluid; CASE, the Clinical Assessment Scale in Autoimmune Encephalitis; mRS, The modified Rankin scale; MOG, myelin oligodendrocyte glycoprotein; AQP4, aquaporin 4; PCA, principal component analysis; OPLS-DA, orthogonal partial least squares discriminant analysis; VIP, variable importance in projection; IQR, interquartile range; AUCs, the area under the receiver operating characteristic curves; OR, odds ratios; CIs, confidence intervals; BBB, the blood-brain barrier.
Ethics Approval and Consent to Participate
Our study complied with the Declaration of Helsinki and Basel Declaration, and was approved by Institutional Review Board of The First Affiliated Hospital of Shandong First Medical University and Qilu Hospital of Shandong University, and patient consent was acquired prior to the initiation of experiment.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81601020), the Natural Science Foundation of Shandong Province, China (No. ZR2016HP04, No.ZR2019MH062, No.ZR2022QH045), and China Postdoctoral Science Foundation (2021M691227).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shan W, Yang H, Wang Q. Neuronal Surface Antibody-Medicated Autoimmune Encephalitis (Limbic Encephalitis) in China: a Multiple-Center, Retrospective Study. Front Immunol. 2021;12:621599. doi:10.3389/fimmu.2021.621599
2. Gu Y, Zhong M, He L, et al. Epidemiology of Antibody-Positive Autoimmune Encephalitis in Southwest China: a Multicenter Study. Front Immunol. 2019;10:2611. doi:10.3389/fimmu.2019.02611
3. Xu X, Lu Q, Huang Y, et al. Anti-NMDAR encephalitis: a single-center, longitudinal study in China. Neurol Neuroimmunol Neuroinflamm. 2020;7(1). doi:10.1212/NXI.0000000000000633.
4. Ghimire P, Khanal UP, Gajurel BP, et al. Anti-LGI1, anti-GABABR, and Anti-CASPR2 encephalitides in Asia: a systematic review. Brain Behav. 2020;10(10):e1793. doi:10.1002/brb3.1793
5. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74. doi:10.1016/S1474-4422(10)70253-2
6. Blinder T, Lewerenz J. Cerebrospinal Fluid Findings in Patients With Autoimmune Encephalitis-A Systematic Analysis. Front Neurol. 2019;10:804. doi:10.3389/fneur.2019.00804
7. Hoftberger R, Titulaer MJ, Sabater L, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. 2013;81(17):1500–1506. doi:10.1212/WNL.0b013e3182a9585f
8. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. doi:10.1016/S1474-4422(15)00401-9
9. Lim JA, Lee ST, Moon J, et al. Development of the clinical assessment scale in autoimmune encephalitis. Ann Neurol. 2019;85(3):352–358. doi:10.1002/ana.25421
10. Qiao S, Xie Y, Li H, et al. Cytokines/chemokines and soluble immune checkpoint molecules in anti-GABA(B) receptor encephalitis. Mult Scler Relat Disord. 2022;68:104234.
11. Qiao S, Zhang SC, Li HY, et al. Cytokines/chemokines and immune checkpoint molecules in anti-leucine-rich glioma-inactivated 1 encephalitis. Neurol Sci. 2023;44(3):1017–1029. doi:10.1007/s10072-022-06526-6
12. Reiber H. Flow rate of cerebrospinal fluid (CSF)--a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci. 1994;122(2):189–203. doi:10.1016/0022-510X(94)90298-4
13. Worley B, Powers R. PCA as a practical indicator of OPLS-DA model reliability. Curr Metabolomics. 2016;4(2):97–103. doi:10.2174/2213235X04666160613122429
14. Waterman CL, Currie RA, Cottrell LA, et al. An integrated functional genomic study of acute phenobarbital exposure in the rat. BMC Genomics. 2010;11(1):9. doi:10.1186/1471-2164-11-9
15. Chen B, Qin C, Ji S, Tian D, Zhang M, Bu B. Modified models to distinguish central nervous system demyelinating diseases with brain lesions. Mult Scler Relat Disord. 2021;52:102965. doi:10.1016/j.msard.2021.102965
16. Durr M, Nissen G, Suhs KW, et al. CSF Findings in Acute NMDAR and LGI1 Antibody-Associated Autoimmune Encephalitis. Neurol Neuroimmunol Neuroinflamm. 2021;8(6). doi:10.1212/NXI.0000000000001086.
17. Nissen MS, Ryding M, Nilsson AC, et al. CSF-Neurofilament Light Chain Levels in NMDAR and LGI1 Encephalitis: a National Cohort Study. Front Immunol. 2021;12:719432. doi:10.3389/fimmu.2021.719432
18. Zrzavy T, Hoftberger R, Wimmer I, Berger T, Rommer P, Macher S. Longitudinal CSF Findings in Autoimmune Encephalitis-A Monocentric Cohort Study. Front Immunol. 2021;12:646940. doi:10.3389/fimmu.2021.646940
19. Troscher AR, Klang A, French M, et al. Selective Limbic Blood-Brain Barrier Breakdown in a Feline Model of Limbic Encephalitis with LGI1 Antibodies. Front Immunol. 2017;8:1364. doi:10.3389/fimmu.2017.01364
20. Klang A, Schmidt P, Kneissl S, et al. IgG and complement deposition and neuronal loss in cats and humans with epilepsy and voltage-gated potassium channel complex antibodies. J Neuropathol Exp Neurol. 2014;73(5):403–413. doi:10.1097/NEN.0000000000000063
21. Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133(Pt 6):1655–1667. doi:10.1093/brain/awq113
22. Panda PK, Sharawat IK, Ramachandran A, et al. Validity and prognostic utility of clinical assessment scale for autoimmune encephalitis (CASE) score in children with autoimmune encephalitis. Brain Dev. 2023;45(1):8–15. doi:10.1016/j.braindev.2022.09.009
23. Macher S, Bsteh G, Hoftberger R, Berger T, Rommer P, Zrzavy T. Clinical scales in autoimmune encephalitis-A retrospective monocentric cohort study. Ann Clin Transl Neurol. 2023;10(10):1768–1775. doi:10.1002/acn3.51865
24. Zhang Y, Tu E, Yao C, Liu J, Lei Q, Lu W. Validation of the Clinical Assessment Scale in Autoimmune Encephalitis in Chinese Patients. Front Immunol. 2021;12:796965. doi:10.3389/fimmu.2021.796965
25. Denkinger MD, Leins H, Schirmbeck R, Florian MC, Geiger H. HSC Aging and Senescent Immune Remodeling. Trends Immunol. 2015;36(12):815–824. doi:10.1016/j.it.2015.10.008
26. Ouyang W, O’Garra A. IL-10 Family Cytokines IL-10 and IL-22: from Basic Science to Clinical Translation. Immunity. 2019;50(4):871–891. doi:10.1016/j.immuni.2019.03.020
27. Liu J, Liu L, Kang W, et al. Cytokines/Chemokines: potential Biomarkers for Non-paraneoplastic Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Front Neurol. 2020;11:582296. doi:10.3389/fneur.2020.582296
28. Lin YT, Yang X, Lv JW, Liu XW, Wang SJ. CXCL13 Is A Biomarker Of Anti-Leucine-Rich Glioma-Inactivated Protein 1 Encephalitis Patients. Neuropsychiatr Dis Treat. 2019;15:2909–2915. doi:10.2147/NDT.S222258
29. Weber A, Wasiliew P, Kracht M. Interleukin-1beta (IL-1beta) processing pathway. Sci Signal. 2010;3(105):56.
30. Versele R, Sevin E, Gosselet F, Fenart L, Candela P. TNF-alpha and IL-1beta Modulate Blood-Brain Barrier Permeability and Decrease Amyloid-beta Peptide Efflux in a Human Blood-Brain Barrier Model. Int J Mol Sci. 2022;23(18). doi:10.3390/ijms231810235
31. Higgins KR, Kovacevic W, Stokes L. Nucleotides regulate secretion of the inflammatory chemokine CCL2 from human macrophages and monocytes. Mediators Inflamm. 2014;2014:293925. doi:10.1155/2014/293925
32. Wesselingh R, Butzkueven H, Buzzard K, Tarlinton D, O’Brien TJ, Monif M. Innate Immunity in the Central Nervous System: a Missing Piece of the Autoimmune Encephalitis Puzzle? Front Immunol. 2019;10:2066. doi:10.3389/fimmu.2019.02066
33. Taraschenko O, Fox HS, Eldridge E, et al. Monoclonal Antibodies From Anti-NMDA Receptor Encephalitis Patient as a Tool to Study Autoimmune Seizures. Front Neurosci. 2021;15:710650. doi:10.3389/fnins.2021.710650
34. Liba Z, Kayserova J, Elisak M, et al. Anti-N-methyl-D-aspartate receptor encephalitis: the clinical course in light of the chemokine and cytokine levels in cerebrospinal fluid. J Neuroinflammation. 2016;13(1):55. doi:10.1186/s12974-016-0507-9
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.