Back to Journals » Journal of Inflammation Research » Volume 17
Coactosin-Like Protein 1 (COTL1) Could Be an Immunological and Prognostic Biomarker: From Pan-Cancer Analysis to Low-Grade Glioma Validation
Received 19 December 2023
Accepted for publication 14 March 2024
Published 19 March 2024 Volume 2024:17 Pages 1805—1820
DOI https://doi.org/10.2147/JIR.S453509
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xiaoyun Wang,1,2 Yuwei Bai,1,2 Bei Wang1,2
1Institute of Integration of Traditional Chinese and Western Medicine, The Hospital Affiliated to Jiangnan University, Wuxi, People’s Republic of China; 2Wuxi School of Medicine, Jiangnan University, Wuxi, People’s Republic of China
Correspondence: Bei Wang, Email [email protected]
Background: Cancer represents a widespread global health challenge impacting millions of individuals worldwide. Identifying new targets for cancer treatment is a crucial step in developing more effective therapies. Among these potential targets, Coactosin-like protein 1 (COTL1), a cytoskeleton-associated protein with critical roles in cell migration, adhesion, and signaling, has shown involvement in tumor progression.
Methods: GSCA, TIMER, SangerBox database were used to explore the COTL1 expression across different tumor types. We employed the TCGA Pan-Atlas Cancer Genomics Dataset, which is available through the cBioportal platform, to explore genetic alterations in COTL1. We conduct a comprehensive analysis of COTL1, encompassing gene expression, clinical prognosis, RNA modification, immunotherapy, and cancer stemness through SangerBox database. Clinical samples were validated using immunohistochemistry.
Results: Our analysis revealed that COTL1 is highly expressed in most cancers and correlates with decreased survival in Glioma, Glioblastoma multiforme, and pan-kidney cohorts. Furthermore, COTL1 was found to be associated with DNA and RNA stemness in 20 and 22 different tumor types, respectively. Additionally, COTL1 showed positive correlations with immunological checkpoints and immune infiltration cells. It was also linked to tumor mutation burden (TMB), microsatellite instability (MSI), neoantigen (NEO), and programmed death ligand 1 (PD-L1), all of which are potential targets for immunotherapies. Moreover, a favorable relationship was demonstrated between genomic-instability markers such as heterozygosity (LOH), homologous recombination deficiency (HRD), and mutant allele tumor heterogeneity (MATH) with COTL1. Furthermore, our findings confirmed a positive correlation between COTL1 expression, CD8, and PD-L1 in LGG, as well as an association of high COTL1 expression with decreased patient survival in LGG.
Conclusion: Based on these compelling findings, COTL1 may hold significant clinical implications for the development of novel cancer therapies and serve as a potential target for tumors associated with immunotherapy in the future.
Keywords: COTL1, pan-cancer analysis, cancer therapy, prognosis biomarker, immune cell infiltration
Introduction
Cancer poses a formidable challenge to global health, causing substantial morbidity and mortality and placing a heavy burden on society.1 Current cancer treatment approaches encompass a wide range, from surgery, chemotherapy, and radiotherapy to targeted therapy and immunotherapy. Despite some successes, patient prognosis remains suboptimal, with drug resistance, adverse effects, and other complications contributing to poor outcomes.2 To address these challenges, there is an urgent need to identify new therapeutic targets and sensitive tumor biomarkers for improved cancer diagnosis and management. Such efforts are crucial for advancing our understanding of this complex disease and enhancing patient outcomes.
COTL1 is a small cytoplasmic actin-binding protein3–5 belonging to the ADF/cofilin family, critical for regulating actin dynamics and cytoskeleton organization.6–8 COTL1 is implicated in various cellular processes like cell migration, adhesion, and signaling, and recent studies suggest its involvement in cancer progression. Upregulation of COTL1 has been observed in several cancer types, including breast, lung, and pancreatic cancer, and has been associated with poor prognosis and increased metastasis in some cases.9–13 However, the precise mechanisms underlying COTL1’s contribution to cancer progression are not yet fully understood.
This study aims to investigate the potential association of COTL1 with cancer progression and development through pan-cancer analysis. The study also explores COTL1’s role in immunotherapy and its involvement in cancer epigenetics and stemness. The findings of this study may offer valuable insights into potential therapeutic targets for cancer treatment, contributing to the existing knowledge base and underscoring the importance of COTL1 in cancer biology. These findings may have significant clinical implications for the development of novel cancer therapies and provide important information for further exploration of COTL1’s role in cancer progression and development.
Materials and Methods
COTL1 Expression Analysis
To evaluate the expression of COTL1 in various cancers, SangerBox14 and TIMER15 databases were utilized. Additionally, the GSCA16 online database was instrumental in examining the expression of COTL1 across different pathological stages.
Survival Analysis
The SangerBox database, a comprehensive and freely accessible tool for analyzing data from The Cancer Genome Atlas (TCGA), was employed to explore the correlation between COTL1 expression and survival analysis across various cancers. This encompassed overall survival (OS), disease-specific survival (DSS), disease-free interval (DFI), and progression-free interval (PFI) analyses, providing valuable insights into the potential clinical implications of COTL1 expression.
Genetic Alteration
In our analysis, we employed the TCGA Pan-Atlas Cancer Genomics Dataset, which is available through the cBioportal platform, to explore genetic alterations in COTL1.17 This dataset offers a comprehensive and integrated resource for analyzing genomic data across a wide range of cancer types, providing a valuable tool for studying the molecular mechanisms underlying various cancers.
RNA Modification
The SangerBox database was utilized to perform an in-depth analysis of RNA methylation modifications, with a specific focus on N1-methyladenosine (m1A), 5-methylcytidine (m5C), and N6-methyladenosine (m6A). These modifications are associated with three distinct types of genes: writers, readers, and erasers. Additionally, we examined the expression of COTL1 in connection with genes involved in RNA methylation alterations across various types of tumors, providing crucial insights into the potential role of COTL1 in RNA regulatory processes within the context of cancer.
Genomic Instability
The current study utilized the online data analysis tool SangerBox to explore the potential association between COTL1 expression and genomic instability across various cancers. We investigated the relationships between COTL1 gene expression and several genomic instability parameters, including Homologous Recombination Deficiency (HRD), Loss of Heterozygosity (LOH), neoantigen load (NEO), Mutant-Allele Tumor Heterogeneity (MATH), tumor purity, ploidy, DNA stemness score (DNAss), and RNA stemness score (RNAss) in the context of multiple cancer types. This comprehensive analysis aimed to shed light on the potential impact of COTL1 expression on genomic instability in diverse cancer settings.
Immune Analysis
To further our understanding of the tumor microenvironment and its relationship with COTL1 expression, we utilized the SangerBox online database. Specifically, we explored the correlation between seven types of immune cells, five categories of immune regulatory factors, 47 immune checkpoint genes, and COTL1 expression. These investigations provided critical insights into the potential link between COTL1 expression and various immune factors, further elucidating the clinical implications of COTL1 as a therapeutic target in the context of cancer immunotherapy.
Patients and Tissue Specimens
The clinical samples utilized in this study were acquired from the affiliated hospital of Jiangnan University in China. These formalin-fixed, paraffin-embedded samples were obtained from 52 patients between 2012 and 2023. Importantly, all sample collection procedures were performed with explicit patient consent, and the study was conducted in compliance with the approval of the ethics committee of the affiliated hospital of Jiangnan University.
Immunohistochemistry Analysis
In this study, the immunohistochemistry assay was performed with minor modifications to a previously described protocol.13 Primary antibodies against COTL1 (1:400, Proteintech, #17119-1-AP), CD8 (1:500, Proteintech, #66868-1-AP), and PD-1 (1:500, Proteintech, #28076-1-AP) were used in the assay.
Statistical Analysis
The Wilcoxon test was employed to investigate the differential expression analyses of COTL1 between tumors and normal tissues. To compare survival curves, Log rank tests were conducted to calculate hazard ratio (HR) and log-rank p-value within the Kaplan-Meier plotter. Spearman correlation was utilized to examine the association between gene expression levels.
Results
COTL1 expression in human cancers
In this study, we utilized the TIMER 2 and SangerBox databases to investigate COTL1 expression across 37 different types of cancers. Our analysis demonstrated significant upregulation of COTL1 expression in 21 cancer types, including Glioblastoma multiforme (GBM, p=7.0e-84), Glioma (GBMLGG, P= 5.9e-218), Brain Lower Grade Glioma (LGG, p=4.7e-174), Uterine Corpus Endometrial Carcinoma (UCEC, p=0.02), Breast invasive carcinoma (BRCA, p=1.0e-23), Cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC, p=1.7e-4), Esophageal carcinoma (ESCA, p=1.0e-63), Stomach and Esophageal carcinoma (STES, p=3.0e-143), Colon adenocarcinoma (COAD, p=1.0e-25), Colon adenocarcinoma/Rectum adenocarcinoma Esophageal carcinoma (COADREAD, p=3.8e-24), Prostate adenocarcinoma (PRAD, p=7.8e-8), Stomach adenocarcinoma (STAD, p=2.3e-29), Head and Neck squamous cell carcinoma (HNSC, p=2.6e-16), Liver hepatocellular carcinoma (LIHC, p=1.9e-5), Skin Cutaneous Melanoma (SKCM, p=2.9e-56), Bladder Urothelial Carcinoma (BLCA, p=0.02), Ovarian serous cystadenocarcinoma (OV, p=5.1e-21), Pancreatic adenocarcinoma (PAAD, p=1.7e-52), Testicular Germ Cell Tumors (TGCT, p=6.7e-46), Uterine Carcinosarcoma (UCS, p=3.1e-9), Adrenocortical carcinoma (ACC, p=0.03), and Cholangiocarcinoma (CHOL, p=2.8e-5). Conversely, COTL1 was found to be down-regulated in 5 types of cancers, namely Lung adenocarcinoma (LUAD, p=3.0e-9), Lung squamous cell carcinoma (LUSC, p=4.1e-36), Acute Lymphoblastic Leukemia (ALL, p=2.0e-56), Acute Myeloid Leukemia (LAML, p=1.3e-30), and Kidney Chromophobe (KICH, p=5.8e-24) (Figure 1A and B).
To validate the COTL1 expression in Thyroid carcinoma (THCA) and Kidney renal papillary cell carcinoma (KIRP), which showed inconsistent results in TIMER and SangerBox, we cross-validated using UALCAN databases. The results confirmed a significant down-regulation of COTL1 expression in both THCA and KIRP, emphasizing the importance of utilizing multiple databases to ensure data accuracy and the need for further investigation into the potential role of COTL1 in these tumors (Supplementary Figure 1).
Furthermore, we performed an analysis using the GSCA database to explore the association between COTL1 mRNA expression and pathological stages in KIRC and SKCM. The findings revealed a significant correlation between COTL1 expression and pathological stages in both cancer types (Figure 1C and D), providing additional evidence supporting the potential role of COTL1 in cancer progression. Utilizing such databases enables the comprehensive exploration of large-scale genomic data, aiding in the identification of potential biomarkers and therapeutic targets for tumors.
Survival analysis
In our comprehensive pan-cancer analysis, we sought to investigate the potential prognostic value of COTL1 expression across different cancer types. Our analysis of overall survival (OS) revealed a significant correlation between higher COTL1 expression and poor prognosis in nine types of tumors, including GBMLGG (p=1.4e-24, HR=2.24 (1.92,2.61)), LGG (p=2.7e-10, HR=2.08 (1.66,2.61)), KIRP (p=0.04, HR=1.56 (1.02,2.37)), KIPAN (p=1.5e-3, HR=1.22 (1.08,1.39)), MESO (p=0.01, HR=1.49 (1.10,2.01)), UVM (p=4.0e-5, HR=2.63 (1.60,4.33)), LAML (p=8.7e-3, HR=1.18 (1.04,1.34)), ACC (p=3.4e-4, HR=1.91 (1.34,2.73)), and ALL-R (p=0.01, HR=1.19 (1.04,1.36)) (Figure 2A). Additionally, disease-specific survival (DSS) analysis indicated that elevated COTL1 expression was associated with poor prognosis in seven types of tumors, such as GBMLGG (p=5.0e-24, HR=2.31 (1.97,2.71)), LGG (p=4.2e-10, HR=2.13 (1.69,2.69)), KIRP (p=0.03, HR=1.85 (1.08,3.16)), KIPAN (p=0.02, HR=1.21 (1.03,1.42)), GBM (p=0.03, HR=1.44 (1.03,2.02)), UVM (p=5.5e-5, HR=2.79 (1.63,4.78)), and ACC (p=8.2e-4, HR=1.86 (1.29,2.68)) (Figure 2B). Furthermore, the analysis of disease-free interval (DFI) and progression-free interval (PFI) identified COTL1 as a high-risk factor in several cancers, such as PAAD, HNSC, LGG, GBMLGG, and ACC, while it was noted as a low-risk factor in LIHC (Figure 2C and D). These compelling findings strongly suggest that COTL1 expression may serve as a potential prognostic biomarker across a range of tumor types.
 |
Figure 2 Survival level analysis of COTL1 in different tumor tissues. (A) Overall survival map. (B) Disease-specific survival. (C) Disease-free interval. (D) Progression-free interval. |
Genetic alteration
Upon investigation using the cBioPortal database, we found that COTL1 mutations were present in 1.4% of 26 types of cancers, with deep deletion being the most common type of mutation and embryonal carcinoma showing the highest alteration frequency (Figure 3A). The analysis of mutation sites revealed an overall mutation frequency of 0.2, with the T96M amino acid sequence being the most frequently altered (Figure 3B and C). Additionally, the impact of COTL1 mutations on cancer prognosis was evaluated, and the results indicated that there was no significant association between COTL1 mutation and survival (Supplementary Figure 2).
COTL1 and RNA methylation modification markers
The aim of this study was to investigate the potential association between COTL1 and RNA methylation alterations. RNA methylation is a common post-transcriptional modification critical in various biological processes, including gene expression regulation, RNA stability, and protein translation. Specifically, this study focused on three types of RNA methylation modifications, namely m1A, m5C, and m6A, and their association with COTL1. The findings of this research showed that there is a strong positive correlation between COTL1 and most of the genes involved in modifying RNA methylation (Figure 4). This result suggests that COTL1 may play a crucial role in regulating RNA methylation in cancer tissues. However, a few genes, including SKCM, CHOL, UCS, and THYM, did not show significant correlation with cancer tissues. Overall, these valuable insights into the potential role of COTL1 in RNA methylation alteration, and its implications in cancer development and progression, suggest the need for more research to elucidate the molecular mechanisms underlying this association. Additionally, exploring the therapeutic implications of targeting COTL1 in cancer treatment may provide a promising avenue for future breakthroughs in cancer therapy.
 |
Figure 4 Correlation of RNA modifications (m1A, m5C, and m6A) and COTL1 expression in different types of cancer (*p<0.05). |
Genomic-instability parameters
The current study aims to delve deeper into the potential relationship between COTL1 gene expression and genomic heterogeneity, encompassing tumor mutational burden (TMB), mutational and copy number alteration burden (MATH), microsatellite instability (MSI), tumor purity, ploidy, homologous recombination deficiency (HRD), and loss of heterozygosity (LOH). Regarding tumor mutational burden (TMB), a notable significant association was identified in ten types of tumors, with seven showing a positive correlation including COAD, COADREAD, BRCA, STES, KIPAN, OV, and ACC, while LIHC, SKCM, and CHOL exhibited a significant negative correlation (Figure 5A). Concerning microsatellite instability (MSI), a significant association was observed in seven tumors, with two showing a positive correlation (STES and STAD) and five demonstrating a negative correlation (GBMLGG, KIPAN, LUAD, OV, and TGCT) (Figure 5B). Additionally, a significant negative association in neoantigen load (NEO) was noted in one tumor, CHOL (Figure 5C).
 |
Figure 5 Pan-cancer analysis of COTL1 in correlation with tumor heterogenicity markers. (A) TMB. (B) MSI. (C) NEO. (D) Purity. |
Tumor tissues comprise diverse cell types, influencing tumor initiation and progression. Tumor purity, representing the proportion of tumor cells in a sample, correlates with clinical features and genomic expression. There was a significant positive correlation in 34 tumors and a negative correlation in 34 tumors, including GBM, GBMLGG, LGG, CESC, and others (Figure 5D).
Homologous recombination deficiency (HRD) has a profound impact on treatment options and prognosis for various tumors. The correlation between COTL1 expression and HRD status was significant in ten tumors, with a positive correlation in three tumors (BRCA, SARC, and PAAD) and a negative correlation in seven tumors (GBM, GBMLGG, STES, and others) (Figure 6A). Polyploidy, a cancer hallmark, was significantly correlated with COTL1 expression in seven tumors, with two showing a positive correlation (SARC and KIPAN) and five displaying a negative correlation (LUAD, LUSC, and others) (Figure 6B).
 |
Figure 6 Pan-cancer analysis of COTL1 in correlation with genomic-instability markers. (A) HRD. (B) Ploidy. (C) LOH. (D) MATH. |
Loss of heterozygosity (LOH) is a chromosomal event that can result in the loss of an entire gene and its adjacent chromosomal region. In this study, we investigated the relationship between COTL1 expression and LOH. Our findings revealed a significant correlation between these two factors in seven tumors. Notably, we observed a positive correlation in two tumors (SARC and KIPAN) and a negative correlation in five tumors (LUAD, LUSC, MESO, OV, and BLCA) (Figure 6C). Moreover, we examined the association between COTL1 expression and the mutant-allele tumor heterogeneity (MATH) score, which effectively represents the deviation of the distribution of minor allele frequency (MAF) values of tumor-specific variant sites. Our results demonstrated a significant positive correlation between COTL1 expression and MATH in one tumor (LAML) and a significant negative correlation in nine tumors (GBMLGG, LGG, LUAD, STES, KIPAN, STAD, KIRC, PAAD, and TGCT) (Figure 6D). These findings provide important insights into the molecular mechanisms underlying tumor heterogeneity and suggest that COTL1 may play a critical role in regulating the progression of various cancers. By analyzing these genomic features, we aim to gain a better understanding of the underlying mechanisms that may contribute to the observed association between COTL1 expression and cancer progression.
COTL1 showed a positive association with tumor stemness
Tumor stemness encompasses the self-renewal and differentiation abilities of specific cells within a tumor, resembling normal stem cells. These cells are implicated in driving tumor growth, metastasis, and resistance to conventional cancer treatments. Tumor stemness involves intricate genetic and epigenetic changes within the tumor microenvironment, making it crucial to comprehend its mechanisms for advancing cancer treatments. In this study, we examined the relationship between COTL1 expression and tumor stemness, focusing on DNAss and RNAss. Our findings uncovered a significant correlation between COTL1 expression and DNAss in 20 tumors, with four tumors displaying a positive correlation (GBMLGG, LGG, BRCA, and UVM) and 16 tumors showing a negative correlation (CESC, LUAD, COAD, and others) (Figure 7A). Moreover, a significant negative association between COTL1 expression and RNAss was observed in 22 tumors such as GBMLGG, LGG, CESC, and others (Figure 7B). These insights shed light on the potential role of COTL1 in tumor stemness and may hold implications for the development of innovative cancer treatment strategies.
COTL1 positively correlated with immune infiltration cells
In this study, our primary objective was to investigate the relationship between COTL1 expression and tumor immune cell infiltration by leveraging two publicly available databases, namely TIMER and EPIC. Our analysis based on TIMER data unveiled a noteworthy correlation between COTL1 expression and immune cell infiltration across all 38 tumors. Notably, the majority of these correlations were positive, implying that heightened COTL1 expression was linked to increased immune cell infiltration (Figure 8A). Likewise, our analysis utilizing EPIC data demonstrated a significant association between COTL1 expression and immune cell infiltration in 43 out of 44 tumors, with the exception of SKCM (Figure 8B). These compelling findings suggest that COTL1 may indeed exert influence over the regulation of immune cell infiltration in a diverse array of tumors. Further investigations are warranted to unravel the underlying mechanisms and clinical implications of these observations.
 |
Figure 8 Correlation of COTL1 expression with tumor immune cell infiltration. (A) Based on TIMER data. (B) Based on EPIC data. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. |
COTL1 associated with immunoregulatory genes and immune checkpoint genes
Immunoregulatory genes are responsible for regulating the immune system, maintaining the delicate balance between immune activation and suppression, and preventing the immune system from mounting attacks on the body’s own tissues, thus averting autoimmunity. These genes encompass cytokines, chemokines, and immune cell receptors, crucial for coordinating immune responses and governing inflammation. Mutations or dysregulation of these genes can instigate immune disorders, including allergies, autoimmune diseases, or immunodeficiencies.18 This paper focuses on the association between COTL1 expression and immunoregulatory genes, which encompass chemokines, receptors, MHCS, immunosuppression, and immune activation. Immunoregulatory genes are vital for maintaining immune balance and preventing autoimmune reactions. The study’s analysis uncovered a significant positive correlation between COTL1 expression and immunomodulatory genes across various tumors (Figure 9). Additionally, a notable positive association between COTL1 expression and immune checkpoint genes was observed in nearly all tumors (Figure 10). These findings shed light on the potential role of COTL1 in regulating immune responses within tumors and highlight its potential as a therapeutic target for cancer treatment. Such insights could pave the way for more precise and efficacious immunotherapeutic interventions.
 |
Figure 9 Correlation of COTL1 expression and immunoregulatory genes in pan-cancer analysis (*p<0.05). |
 |
Figure 10 Correlation between COTL1 expression with immunological checkpoints gene (*p<0.05). |
COTL1 is associated with poor prognosis, immune infiltration in LGG
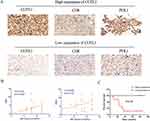
In a study aiming to gain further insight into the expression of COTL1 in low-grade glioma (LGG), 52 randomly selected tumor tissues from LGG patients underwent IHC analysis. The analysis revealed that 63% of the tumor tissues exhibited high expression of COTL1, while 39% showed low expression based on the IHC scoring criteria. Furthermore, the study explored the correlation between COTL1 expression, immune infiltration, and immune checkpoint genes. In specimens from the same patient, the study evaluated immune infiltration and PD-L1 expression in serial sections. The findings indicated a significantly higher number of CD8+ lymphocytes infiltrating the tissues of patients with high COTL1 expression compared to those with low COTL1 expression. Additionally, a positive correlation between PD-L1 protein expression levels and COTL1 expression levels was observed (Figure 11A and B). To assess the impact of COTL1 on patient prognosis in LGG, the tumor tissues were categorized into high and low COTL1 expression groups, and the clinical follow-up data of the patients were analyzed using Kaplan-Meier survival analysis and the Log rank test. The results demonstrated that patients with high COTL1 expression were associated with a worse prognosis compared to those with low COTL1 expression (Figure 11C).
Discussion
COTL1 has a wide range of protein interactions, including actin, profilin, and LIMK1, and it has been demonstrated to regulate actin filament turnover by binding to actin filaments and promoting depolymerization.3,5 Additionally, COTL1 plays a vital role in coordinating the formation and stability of focal adhesions, which are crucial for cell adhesion and migration.19,20 Initially identified as highly expressed in pancreatic cancer tissues, COTL1 has since been implicated in various cancer types, with confirmed up-regulation in glioblastoma tissues and PAAD.7,12 These consistent findings underscore the potential significance of COTL1 in the development and progression of multiple cancer types. Furthermore, while Jeong et al reported a significant correlation between high COTL1 expression and low metastatic potential in non-small cell lung cancer, our study revealed down-regulated COTL1 expression in both LUAD and LUSC, suggesting a potential tumor-suppressive role of COTL1 in lung tumors.21 However, the role of COTL1 in breast cancer appears to be more complex, with inconsistent findings indicating both inhibitory effects on breast cancer cell growth and high expression in breast cancer.11,13 These conflicting results suggest a potential dual role for COTL1, akin to TGFβ, underscoring the complexity of its involvement in tumor biology. Consequently, further research is imperative to confirm the role of COTL1 in breast cancer. Overall, these insights highlight the potential of COTL1 as a valuable biomarker for cancer diagnosis.
Our study uncovers a substantial correlation between COTL1 expression and the survival of cancer patients across various tumor types. Specifically, heightened COTL1 expression was identified as a significant high-risk factor in GBMLGG, LGG, and KIPAN, manifesting as poor outcomes in OS, DSS, DFI, and PFI. Moreover, our analysis highlights the unfavorable prognosis associated with elevated COTL1 expression in UVM and KIPAN, indicated by diminished OS, DSS, and PFI. Additionally, we established COTL1 as a potential prognostic biomarker for LGG. Collectively, these findings indicate COTL1’s potential as a promising prognostic marker for multiple tumor types. Tumor stemness, pivotal for tumor cell growth, demonstrates a positive association with COTL1 expression, leading to heightened resistance to chemoradiotherapy and ultimately contributing to poorer patient prognosis. Insight into the relationship between COTL1 and tumor stemness presents an opportunity to develop more effective therapeutic strategies for cancer treatment.
Tumor immune cell infiltration, encompassing the influx of immune cells like T cells, B cells, natural killer cells, macrophages, and dendritic cells into the tumor microenvironment, holds critical importance in the immune response to cancer, as it can facilitate the recognition and elimination of cancer cells.22 However, the impact of tumor immune cell infiltration on tumor progression and immunosuppression varies based on the type and function of the infiltrating immune cells. Therefore, comprehending the mechanisms governing tumor immune cell infiltration is pivotal for the development of effective cancer immunotherapies. A previous study has reported a significant positive correlation between ALDH2 expression and the activities of immunological B cells and CD4+ T cells in the context of lung cancer.23 Recent studies have suggested the potential involvement of COTL1 in modulating the immune response to tumors. Notably, one study identified a significant increase in COTL1 expression in tumor-associated macrophages (TAMs) in breast cancer patients.13 TAMs are integral in regulating the immune response to tumors, and their presence is often linked to poor prognosis. The study revealed that heightened COTL1 expression in TAMs was associated with a more immunosuppressive phenotype, indicating COTL1’s potential contribution to the immunosuppressive tumor microenvironment. Additionally, the study indicated that COTL1 expression was upregulated in response to treatment with immune checkpoint inhibitors (ICIs) in breast cancer patients. ICIs function by blocking specific receptors on immune cells, enhancing their ability to recognize and combat cancer cells. Immune checkpoint inhibition, particularly the targeted modulation of the PD-1/PD-L1 and CTLA-4 inhibitory pathways, has emerged as a central focus in the investigation of GBM-induced immunosuppression.24 The positive association between COTL1 expression and a favorable response to ICIs suggests that COTL1 may play a role in bolstering the immune response against tumors. Our investigation has unveiled a notable correlation between COTL1 and various immune parameters, including immune cell infiltration, immunoregulatory genes, and immune checkpoint genes. These findings underscore the potential of COTL1 as a promising target for the development of novel cancer immunotherapies. However, further research is imperative to elucidate the precise mechanisms underlying COTL1’s involvement in the immune response to tumors and its viability as a therapeutic target.
In this groundbreaking study, we have conducted the first comprehensive analysis of COTL1 gene expression, clinical prognosis, RNA modification, immunotherapy, and cancer stemness. Our primary objective was to unravel the intricate role of COTL1 in tumorigenesis and to offer valuable insights for the future of individualized therapy. Through meticulous examination of various facets of COTL1, encompassing its expression levels, clinical significance, RNA modifications, immunotherapeutic potential, and association with cancer stemness, our study has advanced the understanding of the molecular mechanisms underpinning its involvement in cancer development and progression. These significant findings carry profound implications for the advancement of targeted therapies and the formulation of personalized treatment strategies for cancer patients.
Ethical Approval and Consent to Participate
This work was approved by the affiliated hospital of Jiangnan University. Our study complies with the Declaration of Helsinki.
Funding
This work was supported by the National Natural Science Foundation of China (No.31801171, 82273434), and Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (No. HB2020039, BJ2023058).
Disclosure
The authors declare no competing interests.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 2020;21(3):e168–e178. doi:10.1016/S1470-2045(19)30823-X
3. Provost P, Samuelsson B, Radmark O. Interaction of 5-lipoxygenase with cellular proteins. Proc Natl Acad Sci U S A. 1999;96(5):1881–1885. doi:10.1073/pnas.96.5.1881
4. Provost P, Doucet J, Hammarberg T, Gerisch G, Samuelsson B, Radmark O. 5-Lipoxygenase interacts with coactosin-like protein. J Biol Chem. 2001;276(19):16520–16527. doi:10.1074/jbc.M011205200
5. Provost P, Doucet J, Stock A, Gerisch G, Samuelsson B, Radmark O. Coactosin-like protein, a human F-actin-binding protein: critical role of lysine-75. Biochem J. 2001;359(Pt 2):255–263. doi:10.1042/bj3590255
6. Doucet J, Provost P, Samuelsson B, Radmark O. Molecular cloning and functional characterization of mouse coactosin-like protein. Biochem Biophys Res Commun. 2002;290(2):783–789. doi:10.1006/bbrc.2001.6236
7. Nakatsura T, Senju S, Ito M, Nishimura Y, Itoh K. Cellular and humoral immune responses to a human pancreatic cancer antigen, coactosin-like protein, originally defined by the SEREX method. Eur J Immunol. 2002;32(3):826–836. doi:10.1002/1521-4141(200203)32:3<826::AID-IMMU826>3.0.CO;2-Y
8. Rakonjac M, Fischer L, Provost P, et al. Coactosin-like protein supports 5-lipoxygenase enzyme activity and up-regulates leukotriene A4 production. Proc Natl Acad Sci U S A. 2006;103(35):13150–13155. doi:10.1073/pnas.0605150103
9. Antoniou E, Margonis GA, Angelou A, Zografos GC, Pikoulis E. Cytokine networks in animal models of colitis-associated cancer. Anticancer Res. 2015;35(1):19–24.
10. Guo S, Yang P, Jiang X, et al. Genetic and epigenetic silencing of mircoRNA-506-3p enhances COTL1 oncogene expression to foster non-small lung cancer progression. Oncotarget. 2017;8(1):644–657. doi:10.18632/oncotarget.13501
11. Xia L, Xiao X, Liu WL, et al. Coactosin-like protein CLP/Cotl1 suppresses breast cancer growth through activation of IL-24/PERP and inhibition of non-canonical TGFbeta signaling. Oncogene. 2018;37(3):323–331. doi:10.1038/onc.2017.342
12. Shao S, Fan Y, Zhong C, Zhu X, Zhu J. Coactosin-Like Protein (COTL1) Promotes Glioblastoma (GBM) Growth in vitro and in vivo. Cancer Manag Res. 2020;12:10909–10917. doi:10.2147/CMAR.S246030
13. Wang B, Zhao L, Chen D. Coactosin-like protein in breast carcinoma: friend or foe? J Inflamm Res. 2022;15:4013–4025. doi:10.2147/JIR.S362606
14. Shen W, Song Z, Zhong X, et al. Sangerbox: a comprehensive, interaction-friendly clinical bioinformatics analysis platform. iMeta. 2022;1(3):e36. doi:10.1002/imt2.36
15. Li T, Fan J, Wang B, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–e110. doi:10.1158/0008-5472.CAN-17-0307
16. Liu CJ, Hu FF, Xia MX, Han L, Zhang Q, Guo AY. GSCALite: a web server for gene set cancer analysis. Bioinformatics. 2018;34(21):3771–3772. doi:10.1093/bioinformatics/bty411
17. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):l1. doi:10.1126/scisignal.2004088
18. Dixit A, Balakrishnan B, Karande AA. Immunomodulatory activity of glycodelin: implications in allograft rejection. Clin Exp Immunol. 2018;192(2):213–223. doi:10.1111/cei.13096
19. Liu M, Li G, Wang M, et al. Further studies about Coactosin-like protein-1 affecting the migration of mouse neocortical neurons. J Mole Histol. 2018;49(5):519–530. doi:10.1007/s10735-018-9790-3
20. Li G, Yin Y, Chen J, et al. Coactosin-like protein 1 inhibits neuronal migration during mouse corticogenesis. J Veter Sci. 2018;19(1):21–26. doi:10.4142/jvs.2018.19.1.21
21. Jeong HC, Kim GI, Cho SH, et al. Proteomic analysis of human small cell lung cancer tissues: up-regulation of coactosin-like protein-1. J Prot Res. 2011;10(1):269–276. doi:10.1021/pr100714b
22. Thorsson V, Gibbs DL, Brown SD, et al. The Immune Landscape of Cancer. Immunity. 2018;48(4):812–830 e814. doi:10.1016/j.immuni.2018.03.023
23. Tran TO, Vo TH, Lam LHT, Le NQK. ALDH2 as a potential stem cell-related biomarker in lung adenocarcinoma: comprehensive multi-omics analysis. Comput. Struct. Biotechnol. J. 2023;21:1921–1929. doi:10.1016/j.csbj.2023.02.045
24. Bianconi A, Palmieri G, Aruta G, et al. Updates in glioblastoma immunotherapy: an overview of the current clinical and translational scenario. Biomedicines. 2023;11(6):1520. doi:10.3390/biomedicines11061520
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.