Back to Journals » OncoTargets and Therapy » Volume 17
Clinical Response of Advanced Lung Adenocarcinoma with Class III BRAF G466V Missense Mutation to Dabrafenib and Trametinib: A Case Report
Authors Fang R , Xu S, Gong J, Liao Z
Received 16 November 2023
Accepted for publication 11 January 2024
Published 23 January 2024 Volume 2024:17 Pages 27—31
DOI https://doi.org/10.2147/OTT.S448132
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Nagashree Seetharamu
Ruoxin Fang,* Sha Xu,* Jun Gong, Zhengkai Liao
Department of Radiation and Medical Oncology, Zhongnan Hospital of Wuhan University, Hubei Key Laboratory of Tumor Biological Behaviors, Hubei Cancer Clinical Study Center, Wuhan, Hubei, 430071, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhengkai Liao, Department of Radiation and Medical Oncology, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, 430071, People’s Republic of China, Email [email protected]
Aim: BRAF is a pivotal driver gene in cancer development. Based on this, the combination of dabrafenib and trametinib was approved for treating NSCLC patients with BRAFV600E mutations. However, the majority of BRAF mutations in lung cancer are non-V600E variants, particularly class III mutants, which currently lack targeted therapeutic options and result in unfavorable clinical outcomes.
Case Presentation: We present a case of advanced lung adenocarcinoma with a class III BRAFG466V mutation. The patient experienced significant pleural and pericardial effusion, leading to chest tightness and an inability to lie flat. Severe pain and limited mobility from lumbar destruction seriously affected the patient’s quality of life. Due to the patient’s intolerance to chemotherapy, dabrafenib and trametinib combination therapy was chosen. After three months of targeted therapy, the patient’s overall condition significantly improved, enabling self-care, and achieving partial response (PR) as an indicator of treatment efficacy.
Conclusion: The combination therapy of dabrafenib and trametinib demonstrates remarkable clinical benefits for lung adenocarcinoma patients with the BRAFG466V mutation. Targeted therapy should be considered for patients with BRAF class III mutations, especially those in poor general condition and may not tolerate chemotherapy.
Keywords: case report, NSCLC, BRAF G466V, targeted therapy, dabrafenib, trametinib
Introduction
The emergence of targeted tyrosine kinase inhibitor (TKI) therapy has significantly improved survival outcomes and quality of life in advanced lung cancer patients, particularly those with lung adenocarcinoma.1 Common driver genes in lung cancer include EGFR, ALK, ROS1, etc. The BRAF gene mutation is relatively rare, accounting for approximately 4.5% of all lung cancer cases, with over 50%, classified as non-V600E mutations.2
The BRAF’s basal kinase activity surpasses that of other RAF family members.3 RAF kinases transmit signals from Ras to downstream dual-specificity protein kinases MEK1 and MEK2, which then phosphorylate and activate serine/threonine-specific protein kinases MAPK1/ERK1 and MAPK2/ERK2.4 This pathway is closely related to cellular proliferation, differentiation, survival, cytoskeleton and other functions.5 As a result, BRAF has become a crucial focus for oncology therapy. The BRAFV600E mutation is the most prevalent alteration in the BRAF gene. Currently, dabrafenib and trametinib are approved for treating non-small cell lung cancer (NSCLC) patients with BRAFV600E mutation.6 However, effective targeted therapy for non-V600E mutations of BRAF is still under development. Studies in advanced melanoma with rare BRAF mutations have shown that BRAF inhibitors combined with MEK inhibitors (BRAF/MEK inhibitors) resulted in a median overall survival (OS) of 11.3 months,7 suggesting potential efficiency for non-V600 mutations, but evidence in NSCLC is limited.
The BRAF mutations can be categorized into three distinct groups: class I comprises autonomously activating BRAFV600 mutations in the RAS pathway. In contrast, class II and III include BRAFnon-V600 mutations. Class III mutations hinder kinase activity, relying on RAS activation, and wild-type CRAF functions as an enhancer of the RAS signaling pathway.8 Recent findings indicate that NSCLC patients with BRAF class III mutations have a poorer response to platinum-containing chemotherapies compared to those with BRAFV600E mutations.9 The BRAFG466V mutation, is a class III alteration, potentially confers increased tumor aggressiveness10 and lacks targeted therapeutic options.
Here, we describe a novel case of advanced lung adenocarcinoma harboring the rare BRAFG466V mutation. This case exhibited a substantial positive reaction to initial targeted treatment with dabrafenib and trametinib. Our report adheres to the CARE checklist.11
Case Presentation
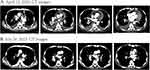
On April 11, 2023, a 68-year-old man with a history of heavy smoking and hypertension, visited Zhongnan Hospital of Wuhan University. He had worsening lower back pain for the past 2 months, reaching a severity where he experienced intense back pain, chest constriction, and respiratory distress. Consequently, he was unable to live independently, spending over half of his day confined to bed. His Eastern Cooperative Oncology Group (ECOG) score had reached 3, significantly impacting daily functioning. Imaging tests revealed a solid mass in the lower lobe of his right lung, multiple lymph node metastases in the mediastinum, significant pericardial and pleural effusion, and metastases to the left adrenal gland and multiple bones (Figures 1A and S1).
Due to the risk of cardiac tamponade, we immediately performed a pericardial puncture and a pathological examination of the bloody pericardial effusion. Immunohistochemical results were as follows: MOC31 (+), CK7 (+), P40 (-), NapsinA (+), TTF-1 (+), Muc4 (+), PD-L1 tumor proportion score (TPS)=0 (Figure 2). The patient’s pathological diagnosis was metastatic lung adenocarcinoma. Combined with the above examinations, the patient’s final diagnosis was lung adenocarcinoma (cT2N3M1c, stage IVB, TNM AJCC/UICC 8th edition).12 Due to unbearable low back pain, we performed vertebral radiotherapy for him (Figure S2), and symptoms improved after radiotherapy. Next-generation sequencing (NGS) analysis of pericardial effusion samples revealed BRAF mutation in exon 11, c.1397G>T, p.G466V, with variant allele frequency (VAF) of 62.85% (Figure S3 and Table S1). No other genetic mutations were found. Considering the patient’s compromised health and potential chemotherapy intolerance, we initiated first-line targeted therapy with oral dabrafenib and trametinib on April 29, 2023, following appropriate informed consent. It is noteworthy that dual BRAF/MEK inhibitors are currently approved exclusively for NSCLC cases harboring the BRAFV600E mutation.
 |
Figure 2 Histopathologic examination of a paraffin-embedded tissue section from pericardial effusion. (A) Hematoxylin-eosin (HE) staining. Scale bars: 50μm. Black arrow: cancer cells; Green arrow: immune cells. (B) TTF-1 (+). (C) P40 (-). (D) NapsinA (+). (E) Muc4 (+). (F) MOC31 (+). (G) CK7 (+). (H) PD-L1 tumor proportion score (TPS)=0. Technical details are provided in Table S2. |
The CT assessment conducted after three months of treatment showed complete resolution of pericardial and pleural effusions, a reduction in the size of the right lung mass and adrenal metastasis, as well as improved bone integrity compared to baseline. Tumor response evaluation demonstrated a partial response (PR)13(Figures 1B and S1). Additionally, the patient’s overall condition significantly improved, with complete alleviation of chest tightness and dyspnea. After strenuous activity, the patient only experienced mild lumbago, and his ECOG score reached 0–1. Currently, the patient has not exhibited any common adverse effects associated with the combination therapy of dabrafenib and trametinib, while long-term efficacy is still under surveillance.
Discussion
With the increasing importance of targeted therapy in cancer treatment, NGS technology is garnering greater attention. Nowadays, routine NGS detection encompasses an expanding repertoire of genes, including EGFR, ALK, KRAS, ROS1, RET, MET, BRAF and ERBB2.1 BRAF belongs to the RAF family of serine/threonine kinases, which also includes ARAF and CRAF.3 The RAF family operates downstream of KRAS and directly phosphorylates MEK, which, in turn, phosphorylates ERK.14 BRAF, with its high kinase activity, is a crucial target for tumor therapy.3
BRAF mutations can be categorized into three types: Class I mutations, known as BRAFV600 mutations, act independently of RAS signaling and function as monomers. Class II mutations, known as BRAFnonV600 mutations, also function independently of RAS but transmit signals as constitutive dimers. BRAF class III mutations exhibit low or no kinase activity and rely on RAS activation to amplify the RAS signaling pathway.10 The most common BRAF mutation is BRAFV600E, but in NSCLC, almost half of the mutations are BRAFnonV600E mutations.2 Dabrafenib and trametinib inhibit BRAF and MEK, and in their Phase II trial, they demonstrated a 64% response rate and a median response duration of 10.4 months in previously untreated NSCLC patients with BRAFV600E mutations.15 Moreover, a recently published prospective study has indicated that NSCLC with BRAFnonV600E mutations, especially class III, exhibits a diminished therapeutic response compared to V600E after platinum-containing chemotherapies.9 Therefore, there is still an unmet need for targeted therapy in NSCLC patients with BRAFnonV600E mutations, and the efficacy of dabrafenib in combination with trametinib for these mutations is yet to be determined.
The BRAFG466V mutation is categorized as a class III mutation, and several studies have investigated the efficacy of BRAF/MEK inhibitors against this mutation. In a phase II study, three NSCLC patients with the BRAFG466V mutation did not respond to vemurafenib.16 Another phase II study included one patient with BRAFG466V mutation in NSCLC who experienced disease progression after trametinib treatment.17 Additionally, Noeparast et al reported a case whose gemcitabine combined with cisplatin chemotherapy resulted in only 7 months of OS. This study also suggested that dabrafenib combined with trametinib effectively inhibited ERK activity in cytological experiments under the BRAFG466V mutation.18 Although preclinical studies suggested that wide-type RAS could inhibit the efficacy of BRAF inhibitors,8 a recent meta-analysis showed that the combination of BRAF and MEK inhibitors shows higher clinical activity than BRAF or EGFR inhibitors alone for BRAF class II and III mutations.19 Therefore, it is highly likely that BRAF/MEK inhibitors would be effective in the treatment of NSCLC with BRAFG466V mutation. Considering the unsatisfactory outcomes of platinum-containing immuno-chemotherapy or single-drug trametinib for BRAFG466V, and taking into account the patient’s poor general condition, which may hinder their ability to tolerate chemotherapy, the family declined this treatment option. As a result, we explored an alternative approach using dabrafenib in combination with trametinib for the patient’s treatment.
We present, for the first time, the efficacy of this combination in a patient with lung adenocarcinoma carrying BRAFG466V mutation. This confirms its efficacy and suggests that BRAFG466V may also be a driver gene for lung cancer. In addition to immuno-chemotherapy, targeted therapy should also be a treatment option for this mutation, particularly in patients with PD-L1 negativity and compromised general condition who may have limited tolerance towards chemotherapy. The reasons for the treatment’s effectiveness are multifaceted. Although the BRAFG466V mutation itself impairs kinase activity, it can activate MEK by forming a dimer with CRAF, leading to ERK activation.8 Trametinib appears to be more effective than other MEK inhibitors in cells with KRAS mutations and CRAF-mediated ERK activation which could give trametinib an advantage in treating the BRAFG466V mutation.20 Additionally, dabrafenib has some inhibitory effect on CRAF,21 which to some extent explains why dabrafenib combined with trametinib may be effective for BRAFG466V mutation and other class III mutations.
However, Rustgi et al’s results suggested that BRAF/MEK inhibitors could not completely block the ERK pathway activated in patients with BRAFnonV600 mutations,22 suggesting that the efficacy of BRAF/MEK inhibitors for BRAFnonV600 mutations may still be limited. At present, for NSCLC patients with BRAFnonV600 mutations, immuno-chemotherapy is still the first choice. Targeted therapy, such as BRAF/MEK inhibitors or MEK inhibitors combined with anti-EGFR monoclonal antibodies may be a potential treatment option. Meanwhile, the efficacy of targeted therapy combined with immunotherapy is also worth exploring. Although the treatment strategy of dabrafenib combined with trametinib was effective for BRAFG466V mutation in our report, the long-term efficacy of this patient needs further observation. For BRAFnonV600 mutations, we still need more targeted treatment methods to improve the prognosis of this part of patients.
Conclusion
In patients with advanced lung adenocarcinoma with the BRAFG466V mutation, dabrafenib in combination with trametinib resulted in partial remission. Targeted therapy should be considered for patients with BRAF class III mutations, especially for patients with PD-L1 negativity or a poor general condition who are unable to tolerate chemotherapy.
Ethics Statement
Approval was obtained from the clinical ethics committee of Zhongnan Hospital of Wuhan University (approval number 2023162K). The patient provided informed consent, agreeing to the publication of this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the college of American pathologists, the international association for the study of lung cancer, and the association for molecular pathology. J Thorac Oncol. 2018;13(3):323–358. doi:10.1016/j.jtho.2017.12.001
2. Sheikine Y, Pavlick D, Klempner SJ, et al. BRAF in lung cancers: analysis of patient cases reveals recurrent BRAF Mutations, fusions, kinase duplications, and concurrent alterations. JCO Precis Oncol. 2018;2:
3. Fiskus W, Mitsiades N. B-raf inhibition in the clinic: present and future. Annu Rev Med. 2016;67(1):29–43. doi:10.1146/annurev-med-090514-030732
4. Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol. 2011;82(3):201–209. doi:10.1016/j.bcp.2011.05.015
5. Nguyen-Ngoc T, Bouchaab H, Adjei AA, Peters S. BRAF alterations as therapeutic targets in non-small-cell lung cancer. J Thorac Oncol. 2015;10(10):1396–1403. doi:10.1097/jto.0000000000000644
6. Planchard D, Kim TM, Mazieres J, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, Phase 2 trial. Lancet Oncol. 2016;17(5):642–650. doi:10.1016/s1470-2045(16)00077-2
7. Menzer C, Menzies AM, Carlino MS, et al. Targeted therapy in advanced melanoma with rare BRAF mutations. J Clin Oncol. 2019;37(33):3142–3151. doi:10.1200/jco.19.00489
8. Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature. 2017;548(7666):234–238. doi:10.1038/nature23291
9. Sakai T, Matsumoto S, Ueda Y, et al. Clinico-genomic features and targetable mutations in non-small cell lung cancers harboring BRAF non-V600E mutations: a multi-institutional genomic screening study (LC-SCRUM-Asia). J Thorac Oncol. 2023;18(11):1538–1549. doi:10.1016/j.jtho.2023.07.024
10. Fontana E, Valeri N. Class(y) dissection of BRAF heterogeneity: beyond non-V600. Clin Cancer Res. 2019;25(23):6896–6898. doi:10.1158/1078-0432.Ccr-19-2732
11. Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218–235. doi:10.1016/j.jclinepi.2017.04.026
12. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The Eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203. doi:10.1016/j.chest.2016.10.010
13. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
14. Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29(15):2046–2051. doi:10.1200/jco.2010.33.1280
15. Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18(10):1307–1316. doi:10.1016/s1470-2045(17)30679-4
16. Mazieres J, Cropet C, Montané L, et al. Vemurafenib in non-small-cell lung cancer patients with BRAF(V600) and BRAF(nonV600) mutations. Ann Oncol. 2020;31(2):289–294. doi:10.1016/j.annonc.2019.10.022
17. Johnson DB, Zhao F, Noel M, et al. Trametinib activity in patients with solid tumors and lymphomas harboring BRAF non-V600 mutations or fusions: results from NCI-MATCH (EAY131). Clin Cancer Res. 2020;26(8):1812–1819. doi:10.1158/1078-0432.Ccr-19-3443
18. Noeparast A, Teugels E, Giron P, et al. Non-V600 BRAF mutations recurrently found in lung cancer predict sensitivity to the combination of Trametinib and Dabrafenib. Oncotarget. 2017;8(36):60094–60108. doi:10.18632/oncotarget.11635
19. Dankner M, Wang Y, Fazelzad R, et al. Clinical activity of mitogen-activated protein kinase-targeted therapies in patients with non-V600 BRAF-mutant tumors. JCO Precis Oncol. 2022;6(6):e2200107. doi:10.1200/po.22.00107
20. Lito P, Saborowski A, Yue J, et al. Disruption of CRAF-mediated MEK activation is required for effective MEK inhibition in KRAS mutant tumors. Cancer Cell. 2014;25(5):697–710. doi:10.1016/j.ccr.2014.03.011
21. King AJ, Arnone MR, Bleam MR, et al. Dabrafenib; preclinical characterization, increased efficacy when combined with trametinib, while BRAF/MEK tool combination reduced skin lesions. PLoS One. 2013;8(7):e67583. doi:10.1371/journal.pone.0067583
22. Rustgi N, Maria A, Toumbacaris N, et al. Combined RAF and MEK inhibition to treat activated non-V600 BRAF-altered advanced cancers. Oncologist. 2023;24:oyad247. doi:10.1093/oncolo/oyad247
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.