Back to Journals » International Journal of Nanomedicine » Volume 15
Biodegradable Polymers for Gene-Delivery Applications
Authors Chen CK, Huang PK, Law WC , Chu CH, Chen NT , Lo LW
Received 8 July 2019
Accepted for publication 4 February 2020
Published 30 March 2020 Volume 2020:15 Pages 2131—2150
DOI https://doi.org/10.2147/IJN.S222419
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Chih-Kuang Chen,1 Ping-Kuan Huang,2 Wing-Cheung Law,3 Chia-Hui Chu,4 Nai-Tzu Chen,5 Leu-Wei Lo4
1Department of Materials and Optoelectronic Science, National Sun Yat-Sen University, Kaohsiung 80424, Taiwan; 2Department of Fiber and Composite Materials, Feng Chia University, Taichung 40724, Taiwan; 3Department of Industrial and Systems Engineering, The Hong Kong Polytechnic University, Hong Kong SAR, People’s Republic of China; 4Institute of Biomedical Engineering and Nanomedicine, National Health Research Institutes, Zhunan 35053, Taiwan; 5Institute of New Drug Development, China Medical University, Taichung 40402, Taiwan
Correspondence: Chih-Kuang Chen
Department of Materials and Optoelectronic Science, National Sun Yat-Sen University, Kaohsiung 80424, Taiwan
Tel +886-7-5252000 ext. 4060
Fax +886-7-5254099
Email [email protected]
Leu-Wei Lo
Institute of Biomedical Engineering and Nanomedicine, National Health Research Institutes, Zhunan 35053, Taiwan
Tel +886-37-246166 ext. 37115
Email [email protected]
Abstract: Gene-based therapies have emerged as a new modality for combating a myriad of currently incurable diseases. However, the fragile nature of gene therapeutics has significantly hampered their biomedical applications. Correspondingly, the development of gene-delivery vectors is of critical importance for gene-based therapies. To date, a variety of gene-delivery vectors have been created and utilized for gene delivery. In general, they can be categorized into viral- and non-viral vectors. Due to safety issues associated with viral vectors, non-viral vectors have recently attracted much more research focus. Of these non-viral vectors, polymeric vectors, which have been preferred due to their low immunogenicity, ease of production, controlled chemical composition and high chemical versatility, have constituted an ideal alternative to viral vectors. In particular, biodegradable polymers, which possess advantageous biocompatibility and biosafety, have been considered to have great potential in clinical applications. In this context, the aim of this review is to introduce the recent development and progress of biodegradable polymers for gene delivery applications, especially for their chemical structure design, gene delivery capacity and additional biological functions. Accordingly, we first define and categorize biodegradable polymers, followed by describing their corresponding degradation mechanisms. Various types of biodegradable polymers resulting from natural and synthetic polymers will be introduced and their applications in gene delivery will be examined. Finally, a future perspective regarding the development of biodegradable polymer vectors will be given.
Keywords: gene therapy, gene delivery, non-viral vectors, polymeric vectors, biodegradable polymers
Introduction
Gene therapy has emerged as a new and efficient modality to combat inherited and acquired diseases, such as genetic disorders, cancer, cardiovascular diseases, neurological disorders, and diabetes mellitus.1–6 In gene therapy, therapeutic deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) gene molecules are capable of altering defective genes, modifying missing genes, and silencing mutated genes.7–9 With an increasing understanding of illness-causing genes, gene therapy has become a new hope for treating various currently incurable diseases.10 However, the fragile nature of therapeutic genes has significantly hampered the applicability of gene therapy.2,7 For example, naked therapeutic genes readily lose their original bioactivity via serum nuclease attack.11 Additionally, with a negatively-charged property, hydrophilic feature and high molecular weight, therapeutic genes exhibit very poor cellular membrane permeability, low cell-uptake, as well as limited stability during blood circulation.12 Beyond the mentioned obstacles, a number of extracellular or intracellular biological barriers associated with gene therapy still need to be overcome. These barriers include fast clearance, immune response, lack of specificity to targeted tissues and cells, an endosomal escape issue, and an unpacking problem.10 In this context, gene-delivery vectors in gene therapy play an essential role because they are capable of delivering therapeutic genes into targeted cells, unpacking genes intracellularly, and ensuring that the genes conduct eventual transfection processes.13,14 Correspondingly, the use of proper gene-delivery vectors enables therapeutic genes to exhibit their maximal transfection efficiency, but minimal adverse effects to patients.15
To date, gene-delivery vectors can be approximately categorized into two major types, including viral and non-viral vectors.7 Despite their high gene-delivery efficiency, the development of viral vectors is significantly impeded. This is because the use of viral vectors has raised considerable safety concerns, such as immune responses, inflammatory responses, toxicity problems, and insertional mutagenesis.16,17 In contrast, non-viral vectors, which offer non-immunogenicity, low cost, improved loading capacity and high chemical versatility, have garnered significant attention for gene delivery in recent years.18,19 Various types of non-viral vectors have been employed for gene-delivery applications, including cationic polymers (CPs), lipids, and inorganic nanoparticles.20,21 Of these non-viral vectors, CPs, which are distinguished from other vectors by their high chemical versatility, have gained the greatest interest for use as an alternative to viral vectors.22–24 In general, CPs are capable of forming poplyplexs with negatively charged genes such as siRNA and plasmid DNA (pDNA) via electrostatic interaction (Figure 1).25 These polyplexes with a size around a few hundred nanometers are further taken up by cells through endocytosis mechanisms, for example, phagocytosis, clathrin-mediated endocytosis and micropinocytosis or a fusion mechanism.26 Following the cell uptake, polyplexes have to escape from acidic intracellular compartments such as lysosome and endosome via the proton-sponge mechanism or the swelling and local mechanical disruption mechanism.27,28 Sequentially, polyplexes need to unpack their gene payloads such as siRNA and pDNA via the degradation of CPs.29 The unpacked siRNA is then degrading sequence-specific messenger RNA (mRNA) through the formation of RNA-induced silencing complex (RISC) in cytosol, followed by inhibiting the activity of pathogen proteins.30 The unpacked pDNA, however, has to translocate to the cell nucleus for the transcription mechanism and subsequent protein expression.26
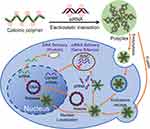 |
Figure 1 Gene delivery mechanism of polyplexes. |
On the basis of the mentioned delivery mechanism, various polymers have been utilized to create CPs. For example, conventional polymeric vectors, such as polyethylenimines (PEI), poly(2-N-(dimethylaminoethyl) methacrylate) (PDMAEMA), and poly(
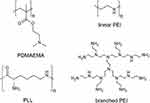 |
Figure 2 Chemical structures of conventional CPs for gene delivery. |
Although a myriad of CP-based vectors have been developed, the key factor affecting them for subsequent clinical applications is cytotoxicity.2,33 Previous reports have demonstrated that the size and positive charges of CPs are two major factors causing cytotoxicity.33,34 CPs exhibiting a high molecular weight and non-degradable bonds may accumulate in normal cells, and such accumulation may adversely affect the transportation and metabolic activities of normal cells, eventually resulting in high cytotoxicity.35,36 Moreover, most of the commonly-used CP-based vectors, such as PEI, PDMAEMA and PLL, consist of non-degradable vinyl (-C-C-) and amide bonds, which are incapable of being split into low molecular weight residues in a physiological environment for further body clearance (Figure 2).9 The non-degradable feature of such kind of CPs thus causes significant accumulation in the human body, especially after repeated administration.37 Moreover, unpacking of the loaded genes of CPs inside of targeted cells is also substantially affected by their degradability.38,39 Correspondingly, it is difficult for non-degradable CPs, which generally display high toxicity, to exhibit their maximal gene-delivery efficiency, further limiting their ultimate clinical uses. An alternative approach is to use their low molecular counterparts. Having a low molecular weight, such kind of CPs is able to be eliminated from the human body.40 However, a decrease in molecular weight of CPs would reduce their complexation ability to therapeutic genes, thereby affecting the transfection efficiency to targeted cells.37 Additionally, the colloidal stability of CPs in a serum-containing environment is also reduced by decreasing the molecular weight.40 Such a compromise, however, is undesirable when designing and exploiting CP-based vectors for gene delivery.
To overcome the aforementioned challenges, biodegradable polymers have been employed as constituent materials for creating CPs for gene-delivery applications.41 With a biodegradability feature, biodegradable CPs can therefore benefit gene-delivery processes by lowering their accumulation amount in treated cells and reducing their toxicity to targeted cells and tissues.1,37,42 Furthermore, their biodegradability can also be utilized as an effective tool to unpack and release the loaded therapeutic genes in the cytosol of treated cells.43,44 Consequently, the development of biodegradable CPs is of great importance for gene-delivery applications.
In this review, a brief introduction to biodegradable polymers will be given firstly. In general, biodegradable polymers can be categorized into three groups, including natural polymers, synthetic polymers, and bioreducible polymers. Their degradation in physiological environments depends highly on hydrolysis of the polymer backbone or breaking down of the incorporated labile linkages. The degradation of polymer structures, in turn, enables biodegradable CPs to be eliminated from the body via an excretion pathway, and thus guarantees bio-safety of biodegradable CPs. Accordingly, the progress and development of the three mentioned types of biodegradable CPs in gene-delivery applications will be reviewed. Future perspective regarding the development and design of next-generation biodegradable CP-based gene-delivery vectors will also be presented.
Biodegradable Polymers
Over the past two decades, biodegradable polymers have been extensively utilized for a broad array of biomedical applications, such as gene therapy, drug delivery, tissue engineering, and bionanotechnology.45–47 Given that repeated administration is often requisite in gene therapy, the use of biodegradable polymeric vectors in gene therapy is relatively more important than that in other applications.37 According to resources, biodegradable polymers can generally be divided into major groups, including natural and synthetic polymers.48,49 Both groups possess their own advantages and disadvantages. For example, natural polymers are highlighted for their great biocompatibility, bioactivity, and cell-activated proteolytic degradation.46,49 However, it is difficult to precisely purify, characterize, and identify the chemical structure and composition of natural polymers that are acquired from natural resources, thus resulting in a batch-to-batch variation problem.45,48 Such variation problem further increases the difficulty of developing new gene-delivery formulations due to the biomedical influence caused by the difficulty of clearly confirming the material composition. Additionally, their high bioactivity also results in a strong immunogenic response.46 On the other hand, synthetic polymers are characterized by their biological inert property and precisely-controlled chemical structure. As a result, they are capable of exhibiting high batch-to-batch uniformity and great chemical versatility.50 Nonetheless, due to the biological inert feature, the lack of biological cues constitutes a problem that limits the progress and development of synthetic polymers.51 Correspondingly, the incorporation of functionalities into the polymeric structure of synthetic polymers for enhancing their interactions with targeted cells and tissues has become an important topic in the polymer science field.52 Moreover, as compared with natural polymers, synthetic polymers can be subjected to hydrolysis without requiring the presence of enzymes.46
Regarding biodegradable natural polymers, they generally can be acquired from two major natural sources, including proteins and polysaccharides.45 Proteins that consist of polypeptides generally exhibit a three-dimensional folded structure and are a major component of many natural tissues, including skin, musculoskeletal, vascular, and lung tissues of bovine, porcine, equine, and human.46,53 In addition, they can also be obtained from blood plasma, as well as microbial fermentation.45 Due to their great biocompatible and biodegradable properties, protein-based vectors, such as gelatin and albumin, have been widely employed as vectors for gene-delivery applications. Their chemical structures are shown in Figure 3. Another important class of biodegradable natural polymers is polysaccharides. Polysaccharides are composed of glucose units joined together through glycosidic linkages, and are gaining significant attention from researchers due to their particular biological functions, such as cell signaling and immune recognition.46 Additionally, with reactive groups on the glucose units, polysaccharides can be converted to a variety of polysaccharide-based derivatives through elegant chemical modification techniques.54 The resulting derivatives can thus be conferred with desired biomedical functions. Typical examples of biodegradable polysaccharides for gene-delivery applications include chitosan (CS), hyaluronic acid (HA), dextran, and β-cyclodextrin (β-CD). Their chemical structures are shown in Figure 3.
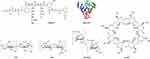 |
Figure 3 Chemical structures of natural biodegradable polymers for gene delivery. |
Concerning biodegradable synthetic polymers, they generally possess hydrolytically-labile chemical bonds in their polymer backbone. Such labile chemical bonds, such as esters, anhydrides, carbonates, amides and urethanes, lead to the nomenclature of the corresponding biodegradable polymers, for example, polyesters, polyanhydrides, polycarbonates, polyamides, and polyurethanes.55 Through hydrolysis, biodegradable polymers can also be degraded into a metabolite, which can be further excreted from the body.56 Taking polylactide (PLA), a kind of polyester, as an example, its major degradation product via hydrolysis is lactic acid, which can be rapidly eliminated from body and has no significant adverse effects on the body.57 Accordingly, PLA has been extensively used as various types of biomaterials for a wide range of biomedical uses.58 In general, two major polymerization methods exist for creating biodegradable polymers, including step polymerization and ring-opening polymerization (ROP).46,57,59 In step polymerization, monomers with two reactive functional groups are required in advance. In addition, the removal of accompanying side products, such as water, is generally essential to the polymerization.59 Step polymerization is extensively utilized to synthesize a myriad of biodegradable synthetic polymers for gene-delivery applications, such as poly(4-hydroxy-L-proline ester) (PHP), poly[α-(4-aminobutyl)-L-glycolic acid] (PAGA), poly(β-amino esters) (PBAE), and polyurethane (PU) (Figure 4). ROP, which is known for its high monomer conversion and living characterization, has also been widely used for synthesizing biodegradable gene-delivery polymers,59–61 such as poly(δ-valerolactone) (PVL), aminated poly(α-hydroxy acids) (PAHA), polyphosphoester (PPE), PLA, and polycarbonate (PC) (Figure 4). Nonetheless, the necessity of preparing ring-like monomers for ROP constitutes a critical issue affecting the success of ROP because the synthetic procedure of such monomers is generally quite complex.61 Thus, the optimization of ROP procedures and monomer synthesis remains a challenge.
 |
Figure 4 Chemical structures of synthetic biodegradable polymers for gene delivery. |
Another type of biodegradable polymers for gene delivery is termed bioreducible polymers.9 Such kind of polymers degrade themselves into small molecular-weight fragments via bonding cleavages instead of hydrolysis. Correspondingly, degradable linkages, such as ester, disulfide, imine, carbamate, amide, and ketal must be introduced into cationic polymer skeletons.1 Such degradable bonds can break down their bonding via chemical signals from targeted cells and tissues.62 Of these chemical signals, redox, which can trigger the cleavage of disulfide bonds, is the most frequently used chemical signal for designing bioreducible polymers.33,63 Representative bioreducible polymers with an incorporation of disulfide bonds include degradable PEI derivatives, poly(amido ethylenimine) (PAA), and poly(cystaminebisacrylamide-diaminohexane) (poly(CBA-DAH)).
Biodegradable Natural Polymers for Gene Delivery
Protein-Based Vectors
In protein-based gene-delivery vectors, gelatin and albumin are the two most commonly-used proteins. Characterized by their low antigenicity and high biocompatibility, gelatin polymers have been widely used to prepare gene-delivery vectors.64 For instance, Kim and coworkers prepared polymerized siRNA (poly-siRNA) through self-polymerization of thiol groups at the 5′-end of sense and anti-sense strands of siRNA (Figure 5).65 The resulting polymerized siRNA was further encapsulated by thiolated gelatin (tGel) nanoparticles with a self-crosslinking structure, leading to the formation of poly-siRNA/tGel nanoparticles (psi-tGel NPs). Such NPs can protect siRNA molecules from enzymatic degradation and down-regulate target gene-expression in melanoma cells. Moreover, they also showed enhanced tumor accumulation as compared with naked siRNA in a tumor-bearing mice model. In particular, they possess very low in vivo cytotoxicity.
 |
Figure 5 Synthetic strategy of poly-siRNA. |
Chougule and coworkers used gelatin nanocarriers (GNCs) to load STAT6 siRNA (S6S) to obtain a nanoformulation for inhibiting a cell proliferation-related gene STAT6 in A549 cancer cells.66 The characteristics of such nanoformulation, including size, charge, loading efficiency, stability, cytotoxicity, and gene-silencing ability, have been fully analyzed. They also demonstrated that such nanoformulation can effectively silence STAT6 gene-expression, subsequently resulting in considerable destruction of A549 cancer cells. Moreover, Amiji’s research group attempted to conduct chemical modification to enhance the gene-delivery efficiency of gelatin-based carriers.67 They first created poly(ethylene glycol)-modified (PEGylated) gelatin NPs, followed by using such NPs to deliver pDNA encoding green fluorescent protein in NIH-3T3 cells. Due to the delivery, strong green fluorescent protein expression was observed from the treated cells. Moreover, Amiji and coworkers utilized gelatin-based NPs to deliver therapeutic genes through an oral administration route.68 They first loaded TNF-α specific siRNA into gelatin NPs, followed by entrapping these NPs into poly(ε-caprolactone) (PCL) microspheres to form a gelatin-based oral system. Such microspheres were further introduced into the body of mice by oral administration. In vivo results showed that such system successfully silenced TNF-α gene-expression, thus leading to a decreased colonic level of TNF-α. Tabata and coworkers employed a coacelvation method to prepare siRNA-loaded collagen NPs.69 Specifically, such NPs were subjected to cross-linked treatment via the use of glutaraldehyde (GA). By increasing the concentration of the used GA, the cross-linking extent was increased, effectively slowing down the release rate of the loaded siRNA. The sustained siRNA release from the NPs can thus further lead to a prolonged time period of gene suppression.
Due to the lack of positively-charged motifs,70 cationic polymers are generally required for preparing albumin-based gene-delivery vectors. For example, Shastri and coworkers first prepared a polyplex composed of pDNA and PEI, followed by incorporating the polyplex with albumin.71 The transfection efficiency of the resulting ternary polyplexes was then studied in HeLa cells. Results demonstrated that the introduction of albumin played a significant role in polyplex dissociation and transfection rate. Stenzel used an elegant chemical approach to create bovine serum albumin (BSA)-PDMAEMA bioconjugates.72 The resulting bioconjugates demonstrated their ability to efficiently condense anticancer oligonucleotide, resulting in a new ternary gene-delivery formulation. Due to the albumin corona, such formulation can effectively protect the cationic polymer and genetic drug, thus giving rise to reduced toxicity of the cationic polymer and enhanced gene-transfection efficiency. Without the use of cationic polymers, Gorjup prepared pDNA-loaded human serum albumin (HAS) NPs via a desolvation technique.73 In particular, such NPs were further subjected to cross-linked treatment via GA, as well as surface ligand modification. Results showed that both the crosslinking process and used ligand profoundly affected the transfection efficiency of the NPs. This is because such crosslinking was capable of improving the pDNA loading capacity and stability of the NPs. Moreover, the surface modification via Tat enables the NPs with an enhanced binding ability and an increased uptake level to the targeted cells. Using a chemical modification technique, Sun and coworkers successfully synthesized cationic bovine serum albumin (CBSA) and utilized such CBSA as gene-delivery vectors without the need to use other cationic polymers.74 CBSA has demonstrated great protection ability to Bcl2-specific siRNA. Due to its great encapsulation efficiency, the resulting CBSA/siRNA NPs exhibited an efficient gene-silencing effect that induces notable cancer cell apoptosis, and subsequently inhibits tumor growth in a B16 lung metastasis model.
Polysaccharide-Based Vectors
For polysaccharide-based gene-delivery vectors, CS, consisting of randomly distributed β-(1-4)-linked D-glucosamine and N-acetyl-D-glucosamine, has been used more extensively than other kinds of polysaccharides due to its positively-charged nature. However, due to the low pKa values (approximately 6.3–6.4), CS possesses very limited solubility in a physiological condition, as well as low complexation ability to therapeutic genes.75,76 To overcome this inherent challenge, chemical modifications of pristine CS are generally necessary to enhance its gene-delivery efficiency. As a result, various CS derivatives have been developed for gene delivery. For example, trimethyl chitosan (TCS) was synthesized via quaternization of the CS backbone by Thanou and coworkers.77 The resulting TCS exhibited great solubility over a wide pH range and conferred a cationic characteristic to the polymer. Through the endowed cationic feature, TCS has successfully delivered pDNA into various cancer cells and demonstrated promising transfection results. They also observed that, with an increase of quanterization degree, both transfection efficiency and cytotoxicity of TCS were increased. To reduce cytotoxicity via quanterization, various hydrophilic polymers poly(N-isopropylacrylamide)78 and polyethylene glycol (PEG)79 have been grafted to the backbone of TCS. In addition, such grafting can also improve the stability of the resulting gene/TCS nanoobjects.
In addition to CS derivatives, CS-based graft polymers have also been used for gene-delivery applications. For example, Hu grafted PEI on the backbone of CS via an amide linker.80 The resulting chitosan-linked-PEI (CP) effectively bound pDNA, leading to the formation of DNA/CP complexes. Such DNA/CP complexes have demonstrated efficient transfection efficiency to various cancer cells and reduced cytotoxicity. Specifically, a reduced tumor growth rate was observed when DNA/CP complex-treated cancer cells were inoculated in mice. Tabata and coworkers have prepared CS/PEI NPs via emulsifier-free emulsion polymerization.81 pDNA was successfully loaded into such NPs and delivered to mesenchymal stem cells (MSCs). The NPs demonstrated promising transfection efficiency, as well as low cytotoxicity. Moreover, living polymerization techniques, such as atom transfer radical polymerization (ATRP) and reversible addition-fragmentation chain transfer polymerization (RAFT), have been employed to create cationic polymer-grafted CS via a “grating from” strategy. For example, Xu and coworkers used a comb-shaped polymer composed of CS as the backbone and PDMAEMA as the side chain via ATRP.75 The resulting comb-shaped polymer showed great pDNA delivery efficiency, as well as reduced cytotoxicity in various cells. Chen and coworkers first synthesized p(PEGMA-co-DMAEMA) via RAFT, followed by using a “graft onto” strategy to graft the polymer, as well as folic acid (a targeted moiety) on the backbone of TCS.82 As shown in Figure 6, the resulting TCS-g-p(PEGMA-co-DMAEMA)-FA exhibited improved water solubility and great pDNA packaging ability, leading to an enhanced level of gene-transfection efficiency as compared with the controls in 293T cells.
 |
Figure 6 Preparation of TCS-g-poly(PEGMA-co-DMAEMA)-FA/pDNA polyplexes. |
Other polysaccharide-based polymers, such as HA, β-CD and dextran, were reported to be used as gene-delivery vectors. Due to the lack of cationic resources, these mentioned polymers have to be modified with other cationic polymers for gene delivery or to be utilized as a part of gene carrier systems. Due to the specificity to the CD44 protein, HA not only can be utilized as a therapeutic agent carrier, but also an active targeting ligand to various cells. Shieh and coworkers conjugated poly(
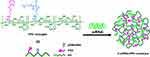 |
Figure 7 Preparation of C-siRNA-HPD complexes. |
In addition to HA, β-CD polymers, that are composed of seven D(+)-glucose units linked by α-1,4-linkages, have been widely used as non-viral vectors for gene delivery. This is because they are noted for their distinctive material features. First, with an inner cavity, β-CD polymers are capable of loading hydrophobic drugs and hydrophilic genes simultaneously. Additionally, they can serve as a core for constructing unique polymeric architectures, such as star-shaped polymers. Moreover, they also possess high availability for supramolecular chemistry applications. For example, Ma and coworkers synthesized a new cyclodextrin derivative (CD-PLLD) consisting of a β-CD core and poly(
Biodegradable Synthetic Polymers for Gene Delivery
Polyesters
Aliphatic polyesters, which are recognized for their biodegradability, have been widely employed in various biomedical products, such as sutures, tissue-engineering scaffolds, and drug-delivery carriers.46 In recent years, different types of polyesters varying according to their monomer unit have been used ubiquitously for gene-delivery applications. Commonly-used polyester-based gene-delivery polymers include PHP, PAGA, PBAE, PVL, aminated PAHA, PPE, and PLA. For example, as the first biodegradable polymer to be used with gene-delivery vectors, PHP was synthesized by Langer91 and Park92 and their coworkers. Degradation results showed that PHP can be degraded into a degradation product with half of its original molecular weight in less than 2 h in a physiological environment. Moreover, complete degradation of PHP into its corresponding monomers was reported to take three months. PHP showed an ability to effectively bind DNA and to possess comparable transfection efficiency to a conventional transfection agent PLL. More importantly, the transfection efficiency of PHP-based polyplexes was not significantly affected by serum proteins. Additionally, Lim and coworkers synthesized PAGA via step polymerization, followed by utilizing the new polymer to form polyplexes with DNA.93,94 Such polyplexes exhibited three-fold higher transfection efficiency than the commonly-used transfection agent PLL. Compared with PLL, PAGA had significantly reduced cytotoxicity.
PBAE polymers that are synthesized via Michael addition of amines and acrylate esters were first created and utilized as gene-delivery vectors by Langer and coworkers.95 Relevant reports showed that PBAE exhibited comparable transfection efficiency with commonly-used transfection agent PEI, but very low cytotoxicity. To optimize the chemical structure of PBAE in terms of transfection efficiency, Langer and coworkers further designed a myriad of Michael addition combinations based on various primary amines or secondary amines to an array of bisacrylates.96,97 Through the combinations, a library of PBAE-based derivatives has been successfully created and evaluated for transfection efficiency for systematically screening out better PBAE derivatives. Their findings regarding chemical-structure influence further elicited the development of new types of polymeric gene-delivery systems, such as lipidoids, which are an oligocationic polymer with hydrophobic modification.98,99 The concept of hydrophobic modification was further applied to other cationic polymers to enhance their transfection efficiency, reduce their cytotoxicity, and improve their colloidal stability.11 PVL-based polymers have also recently been exploited as gene-delivery vectors. For instance, He and coworkers conducted a ring-opening polymerization of tertiary amine-bearing valerolactone and alkylated valerolactone monomers, resulting in the creation of new functional PVL copolymers.100 They demonstrated that functional PVL copolymers can effectively complex with pDNA to form pDNA/PVL polyplexes. In addition, the monomer arrangement and aliphatic side chain length of the functional PVL copolymers had a significant influence on their transfection efficiency. The best functional PVL copolymer with an optimal chemical structure can have 2.2-fold higher transfection efficiency than PEI. For the preparation of aminated PAHA, Cheng and coworkers first carried out a ring-opening polymerization of 5-(4-(prop-2-yn-1-yloxy)benzyl)-1,3-dioxolane-2,4-dione (1), an O-carboxyanhydride derived from tyrosine, followed by functionalizing the alkyne functionality of 1 with 2-aminoethanethiol hydrochloride (AET) through thiol-yne “click” photochemistry.101 The resulting poly(1)-g-AET2 (PAET), a newly developed aminated PAHA polymer, displayed excellent cell penetration and gene-delivery properties along with low toxicity.
With the backbone recognizable to enzymes and analogous to natural nucleic acids, PPE polymers have been selected as candidate vectors for gene delivery.102 For example, Wang and coworkers synthesized poly(2-aminoethyl ethylene phosphate) (PPEEA), a type of PPE, to build up a multilayer coating with DNA.103 Due to the slow degradation of phosphoester bonds, such multilayer can sustainably release its loaded DNA for up to months. With a PPE composition and gene-loading capacity, the multilayer is capable of facilitating mouse osteoblast cell adhesion on its surface and prolonging gene expression in the attached osteoblast cells. Such material features can therefore benefit further biomedical applications, such as implanted materials or scaffolds. Wang and coworkers further synthesized a PCL-b-PPEEA copolymer, followed by converting it and PCL-PEG to form a new PPE-based micelle system.104–106 Such micellar system was capable of binding siRNA and treating hypoxic tumors and lung cancer via a RNA interference (RNAi) mechanism. Furthermore, Wang and coworkers used a ring-opening polymerization method to synthesize a new triblock copolymer PEG-b-PCL-b-PPEEA, followed by converting the polymer into a micelle.107 In addition to loading siRNA, the micelle was able to encapsulate a hydrophobic anticancer drug paclitaxel at the same time. As a result, PEG-b-PCL-b-PPEEA micelle systems are able to be utilized as a carrier for drug/gene combinational therapies for combating cancer-relating challenges and issues.
Having a long history of safety in humans and versatile biomedical applications, PLA has been broadly used as a constituent material for an array of biomedical products, including sutures, scaffolds, surgical tools, drug-delivery carriers, etc.58 However, PLA has rarely been used as a gene-delivery vector, due to the lack of cationic resources on its backbone. To address this issue, Cheng and coworkers first synthesized allyl functional polylactide via a ring-opening polymerization of allyl functional lactide monomers, followed by partially or fully converting ally functionalities into tertiary amines, resulting in the creation of cationic polylactides (CPLAs).108,109 CPLAs have been successfully utilized as gene-delivery vectors for siRNA and pDNA in cancer cells and various tissue cells. Moreover, through a proper particulate process and chemical structure design, CPLA can be further transferred into nanocapsules110 for drug/gene co-delivery (Figure 8) and nanoparticles111 with enhanced in vivo stability. Chen and coworkers further prepared a biodegradable cationic polyester vector (BCPV) formulation based on CPLA polymers for siRNA delivery in cancer cells.112–114 The targeted mutated genes they are concerned with in various cancer cells comprise K-ras, BCR–ABL and Notch1. Results showed that BCPV-mediated siRNA delivery and therapy effectively inhibited cell proliferation, migration and invasion of different cancer cells, including pancreatic and leukemic cancer cells. Recently, BCPV-mediated siRNA therapy has further demonstrated great efficiency in suppressing the tumor growth of pancreatic cancer in a xenograft mice model.115
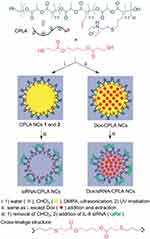 |
Figure 8 Synthesis of CPLA nanocapsules, and the co-loading of hydrophilic siRNA and hydrophobic anticancer drugs. Chen C-K, Law WC, Aalinkeel R, et al. Biodegradable cationic polymeric nanocapsules for overcoming multidrug resistance and enabling drug-gene co-delivery to cancer cells. Nanoscale. 2014;6 (3):1567–1572. Reproduced by permission of The Royal Society of Chemistry.110 |
Polycarbonates
Having high biocompatibility, low inherent cytotoxicity and tunable mechanical properties, polycarbonates (PCs) have emerged as an attractive choice for gene-delivery applications.116 For example, He and coworkers first synthesized poly(5-methyl-5-allyloxycarbonyl-trimethylene carbonate) (PMAC) via the catalysis of immobilized porcine pancreas lipase (IPPL).117 Sequentially, the allyl functionality of PMAC was converted to epoxide groups, resulting in the preparation of PMAC-O, which was further modified by PEI to create PMAC-g-PEI copolymers. As compared with PEI, PMAC-g-PEI showed enhanced transfection efficiency and much lower cytotoxicity in 293T cells. Moreover, they introduced 5.5-dimethyltrimethylene carbonate (DTC) into the backbone of PMAC-g-PEI, resulting in the preparation of P(MAC-co-DTC)-g-PEI.118 The synthetic route of P(MAC-co-DTC)-g-PEI is shown in Figure 9. Results revealed that the introduction of DTC monomer units enabled P(MAC-co-DTC)-g-PEI to control its charge density and hydrophobicity, which further affected the unpacking and endosomal escape of the loaded DNA. As a result, the most promising P(MAC-co-DTC)-g-PEI polymer for in vivo gene delivery has been successfully determined based on a corresponding biological evaluation.
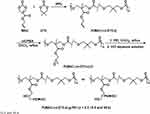 |
Figure 9 Synthetic route of P(MAC-co-DTC)-g-PEI copolymers. Reprinted with permission from He F, Wang CF, Jiang T, Han B, Zhuo RX. Poly[(5-methyl-5-allylox-ycarbonyl-trimethylene carbonate)-co-(5,5-dimethyl-trimethylene car-bonate)] with grafted polyethylenimine as biodegradable polycations for efficient gene delivery. Biomacromolecules. 2010;11(11): 3028–3035. Copyright (2010) American Chemical Society.118 |
Hedrick and coworkers synthesized new cationic PCs via an organocatalytic ring-opening polymerization of functional cyclic carbonates containing alkyl halide side chains, followed by a subsequent functionalization step with bis-tertiary amines.119 With a successful loading of DNA, such new PCs displayed high gene-transfection efficiency, even in the presence of serum, as well as minimal cytotoxicity in the various tested cells. Moreover, the new cationic PC was incorporated with PEG segments to create a new cationic PC-b-PEG-b-cationic PC triblock copolymer.120 The new triblock copolymer exhibited several favorable physicochemical and biological properties, such as enhanced in vitro stability and improved gene-expression efficiency. More importantly, such triblock copolymer is highly biocompatible, thus inducing minimal cytotoxicity and hemolysis. Utilizing a similar synthesis method, Yang and coworkers designed and synthesized galactose- and glucose-functionalized cationic polycarbonate diblock copolymers (Gal-APC and Glu-APC).121 Gal has been reported to have high affinity to asialoglycoprotein receptors (ASGP-Rs), which are overexpressed on the hepatocyte cell surface. As compared with the control Glu-APC/DNA complexes, transfection efficiency was higher in ASGP-R-positive hepatocyte cells due to the targeting ability resulting from the functionalized Gal.
Piel and coworkers designed and synthesized a series of new guanidinium- and morpholino-functionalized biocompatible and biodegradable PC vectors for siRNA delivery in HeLa cells.122 The influence of the modified functionality and the chemical structure of the PC vectors on the capacity to form polyplexes with siRNA and to decrease the expression of two specified genes expression has been systematically investigated. Moreover, such new PC vectors were modified with PEG chains on their polymeric structure, followed by complexing with siRNA to form polyplexes.123 Such modification successfully overcame the extracellular barriers, thus resulting in effective in vivo results after an intravenous injection. Results showed that the polyplexes exhibited no significant influence on hemolysis and coagulation. In vivo biodistribution results revealed enhanced siRNA accumulation at the tumor site in mice by using the polyplexes as the siRNA delivery vector.
Polyurethanes
Having elasticity, flexibility and biodegradability, PUs constitute a very attractive material for tissue-engineering applications.124 PUs have also been reported to have high potential for use as gene-delivery carriers. For example, Shau and coworkers synthesized 2-diethylaminoethylaminepolyurethane (LGEA-PU), consisting of PEG segments and tertiary amines, via step polymerization and post-chemical modification.125 LGEA-PUs were capable of complexing DNA to form LGEA-PU59/DNA complexes that efficiently transfected COS-7 cells. It was also found that the molecular weight of LGEA-PUs had a significant effect on transfection ability. Their results displayed that LGEA-PU59 with a moderate molecular weight was capable of forming LGEA-PU59/DNA complexes with a particle size of 110–120 nm. When the molecular weight is lower than LGEA-PU59, such LGEA-PUs are hard to form complexes with DNA due to insufficient electrostatic interaction. In contrast, as the molecular weight is higher than LGEA-PU59, those LGEA-PUs are difficult in releasing the complexed DNA, then causing a limited transfection efficiency. Using a similar synthesis strategy, Cherng and coworkers created a variety of cationic PUs containing tertiary amines in the backbone and primary, secondary, or tertiary amines on the side chain.126 These cation PUs that exhibited comparable transfection efficiency with commonly-used cationic polymers, such as PEI or PDMAEMA, are noted for their non-cytotoxicity and biodegradability. Such cationic PUs thus possess high applicability for in vivo gene-delivery. Sequentially, Shau and coworkers used an elegant chemical approach to synthesize poly(ester-co-urethane) with a PEI800 polymer on the side chain (PEU-g-PEI800).127 In addition to its great biodegradability, PEU-g-PEI800 exhibited higher transfection efficiency as compared with PEI when delivering DNA in COS-7 cells. Furthermore, Chiou and coworkers employed PU-PEI to deliver miRNA (miR145) in brain tumors.128 Such PU-PEI-mediated miR145 delivery successfully inhibited the tumorigenesis of glioblastomas, one of the most common primary brain tumors. In addition to brain tumors, they also utilized the PU-PEI-mediated miR145 therapy to treat lung cancer.129 In vivo results showed that the PU-PEI-mediated miR145 delivery to xenograft tumors effectively reduced tumor growth and metastasis, sensitized tumors to chemoradiotherapies, and prolonged the survival time of tumor-bearing mice. Additionally, PU-PEI has also been used for siRNA delivery for treating metastasis of head and neck squamous cell carcinoma.130 Targeting the two upregulated levels of proteins, EZH2 and Oct4, in cancer stem cells, PU-PEI-mediated gene therapy with siEZH2 and siOct4 as the treat agents was capable of reducing tumor growth and metastasis in vivo.
Bioreducible Polymers for Gene Delivery
With the development of polymer chemistry, a myriad of cationic polymers have been exploited as gene-delivery vectors. Relevant transfection results indicated that cationic polymers with a high molecular weight show better gene complexing ability, cell uptake, and transfection efficiency. However, polymers possessing low molecular weight exhibited weaker complexing ability, but reduced toxicity. To overcome this challenge, bioreducible polymers have been created as a new type of non-viral gene-delivery systems. When designing a bioreducible polymer, degradable bonds, such as disulfide, imine, carbamate, amide, and ketal are incorporated into the backbone or the side chain of cationic polymers, which are noted for their transfection efficiency.1 With the incorporation of degradable bonds, it is not necessary for the constituent polymers for cationic polymers exhibiting high transfection efficiency to have high molecular weights. The resulting cationic polymers can thus effectively ameliorate the cytotoxicity problem. Among these degradable bonds, disulfide bonds are the most frequently used linkages to be incorporated into the polymeric structure of cationic polymers. This is because cationic polymers with disulfide linkages are capable of being stable in an extracellular oxidizing environment, but rapidly break down their polymer chains or particulate structures in the reducing environment of the cytoplasm, thus effectively unpacking their loaded gene therapeutics and completing the desired function mechanism of the loaded genes.9 Due to their noted transfection efficiency, cationic polymers, such as PEI, PAA, and poly(CBA-DAH), have been extensively used for designing and creating bioreducible polymers for gene delivery.
For the preparation of PEI-based bioreducible polymers, Lee and coworkers utilized small molecular weight PEI as the precursor, followed by cross-linking it using two disulfide-containing cross-linkers, including dithiobis(succinimidylpropionate) (DSP) and dimethyl·3,3′-dithiobispropionimidate·2HCl (DTBP).131 The resulting cross-linked product showed improved transfection results in CHO cells due to the triggered release of the loaded DNA via the reductive cleavage of the disulfide bonds. Zhong and coworkers produced thiolated PEI (PEI-SH) from 800 Da PEI, followed by converting it into disulfide cross-linked PEI (PEI-SS) via oxidation.132 PEI-SS was capable of effectively binding and condensing pDNA to form nano-sized polyplexes. Results demonstrated that PEI-SS polyplexes possessed higher gene-transfection efficiency and lower cytotoxicity than their non-degradable counterparts. Jiang and coworkers synthesized disulfide-containing cross-linked polyethylenimines (PEI-SS-CLs) via click chemistry as gene-delivery vectors.133 An azide-terminated PEI was first synthesized from a low-molecular-weight PEI (1.8 kDa), followed by converting it into a high-molecular weight disulfide-containing cross-linked PEI through the introduction of a dialkyne cross-linker and the conducting of a click reaction. Results revealed that the cross-linked PEI possessed both higher gene-transfection efficiency and lower cytotoxicity than PEI (25 kDa). Consequently, this new PEI bioreducible polymer can constitute an attractive candidate for further in vivo evaluations. Jiang and coworkers further prepared polyaspartamide-based disulfide-containing brushed polyethylenimine derivatives P(Asp-Az)x-SS-PEIs.134 Azide-functional poly(aspartic acid) derivatives and monoalkyneterminated PEI with disulfide linkages were first synthesized, followed by synthesizing P(Asp-Az)x-SS-PEIs consisting of poly(aspartic acid) main chain and disulfide-connected PEI pendants via click chemistry. In vitro results showed that the reducible P(Asp-Az)x-SS-PEIs not only exhibited much lower cytotoxicity, but also possessed higher transfection activity as compared with the control of non-degradable 25 kDa PEI. Moreover, degradable bonds can also be utilized as a linker between stealthy coating layers, such as PEG and cationic polymers. For instance, Bauhuber and coworkers used an elegant chemistry approach to synthesize a library of PEG-SS-PEI diblock copolymers, in which the PEG block linked the PEI block via disulfide bonds.135 They reported that the non-degradable PEG-S-PEI (with the link of sulfide between the PEG and PEI blocks) copolymers with a PEG content of higher than 50% lost their transfection ability. Meanwhile, at the same PEG contents, PEG-SS-PEI diblock copolymers can still restore their transfection ability, indicating that the intracellular cleavage of the PEG domain is crucial for the transfection process. Liu and coworkers synthesized a novel reducible targeted gene vector c(RGDyK)-poly(ethyleneglycol)-SS-polyethylenimine (RGD-PEG-SS-PEI), which has a disulfide bond as the linkage for the PEG and PEI blocks.136 The synthetic route of RGD-PEG-SS-PEI is shown in Figure 10. Sequentially, RGD-PEG-SS-PEI successfully bound pDNA to form RGD-PEG-SS-PEI/pDNA complexes. As compared with mPEG-PEI (without disulfide linkages)/pDNA complexes, RGD-PEG-SS-PEI/pDNA complexes displayed improved levels of transfection efficiency and reduced cytotoxicity in U87 cells in vitro, and enhanced levels of gene expression in the brain of intracranial U87 glioblastoma-bearing mice, as well.
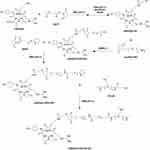 |
Figure 10 Synthesis of RGD-PEG-SS-PEI. Reprinted with permission from Lei Y, Wang J, Xie C, et al. Glutathione-sensitive RGD-poly(ethy-lene glycol)-SS-polyethylenimine for intracranial glioblastoma tar- geted gene delivery. J Gene Med. 2013;15(8–9):291–305. Copyright © 2013 John Wiley and Sons.136 |
Relative to PEIs, bioreducible PAAs are known for their great biocompatibility and enhanced gene-transfection efficiency due to their reducible disulfide-containing backbone. In general, PAAs are synthesized by step polymerization (Michael addition) of cystaminebisacrylamide (CBA) and different aliphatic amines. For example, Kim and coworkers synthesized three types of disulfide-containing PAA from step polymerization of CBA and three different amine monomers, including ethylenediamine (EDA), diethylenetriamine (DETA), and triethylenetetramine (TETA).137 These PAAs were capable of complexing with DNA to form well-defined polyplexes with a positively-charged surface and exhibiting higher transfection efficiency than the positive control. In particular, one of the PAAs resulting from CBA and TETA exhibited even higher transfection efficiency than 25 kDa branched PEI in the presence of serum. Due to the importance of the incorporated disulfide bond of PAAs, the influence of the disulfide linkage density of PAAs on gene delivery has thus garnered significant interest from researchers. For example, Engbersen and coworkers created a series of PAAs resulting from the step polymerization of CBA and 1-(2-aminoethyl) piperazine (AEP) or N,N’-hexamethylenebisacrylamide (HMBA)/CBA and AEP.138 With such monomer combination, the disulfide linkage content in the backbone of the resulting PAAs can thus be precisely tuned from 0 mol% to 63 mol%. Results revealed that poly(HMBA/CBA-AEP) copolymers had higher transfection efficiency and lower cytotoxicity in COS-7 cells as compared to poly(HMBA-AEP) homopolymers, which do not have disulfide linkages incorporated in the main chain. Below the disulfide content of 20 mol%, the transfection efficiency and cell viability of poly(HMBA/CBA-AEP) were increased by increasing the disulfide content, indicating the importance of disulfide linkages in the gene-delivery performance of bioreducible PAAs. Oupický and coworkers synthesized reducible hyperbranched poly(amido amine)s (RHBs) by Michael addition of N,N-dimethylaminodipropylenetriamine (DMDPTA) and two bisacrylamide monomers CBA and HMBA.139 By controlling the molar feeding ratio of CBA and HBMA, the density of disulfide linkages of the resulting RHB can be well controlled. Results showed that the disulfide content in RHB significantly affected the molecular weight of the degradation products, ease of the polyplex disassembly, cytotoxicity, and transfection activity. Based on the found correlations, optimized formulations of RHB-based polyplexes have been verified. Moreover, Oupický and coworkers synthesized PAAs with a controlled disulfide linkage content, followed by studying the effect of the disulfide linkage content on PAA cytotoxicity.140 Results indicated that the toxicity of bioreducible PAAs was decreased in a linear fashion by increasing the disulfide content. Evaluating in two cell lines, lower toxicity of bioreducible PAAs was observed in cells containing higher glutathione. By using a thiol blocker DTNB, the amount of exofacial plasma membrane thiols thus was controlled. In contrast, it was found that the presence of exofacial plasma membrane thiols increased toxicity of bioreducible PAAs. This is most likely due to the enhanced levels of cell membrane disrupting resulting from the increased covalently bonded attachment of bioreducible PAAs via the thiol-disulfide reaction with the membrane thiols. The decoration of stealthy coatings, such as PEG, has also been performed on bioreducible PAAs. For example, Kim and coworkers prepared p(TETA/CBA) and p(TETA/CBA)-g-PEG2k, followed by mixing them into a formulation for polyplex formation.141 It was found that an increase of PEG amount in the formulation adversely affects polyplex formation. However, optimal formulations can be confirmed via the proposed facile approach when simultaneously considering the stability and bioactivity of the resulting polyplexes.
poly(CBA-DAHs), another type of bioreducible polymer, consists of CBA and diamines. Kim and coworkers are the first to prepare poly(CBA-DAHs) for gene-delivery applications.142 Through the selection of diamines, the length of oligoethylene spacer -(CH2)- was allowed to be varied in the main chain of the resulting poly(CBA-DAHs). Results showed that the poly(CBA-DAHs) with an oligoethylene spacer of -(CH2)3- had higher transfection efficiency than those with an oligoethylene spacer of -(CH2)2-, indicating the importance of polymer architectures of poly(CBA-DAHs) in gene delivery. Poly(CBA-DAHs) can also be synthesized by Michael addition of CBA and N-boc protected diamines with various aliphatic chain lengths.143 Following polymerization and deprotection, poly(CBA-DAHs) with disulfide bonds and tertiary amine groups in the backbone, and pendant primary amine groups on the side chains have been successfully prepared. Results revealed that poly(CBA-DAHs) with a hexaethylene spacer exhibited the highest transfection efficiency as compared to poly(CBA-DAHs) with different spacer lengths.
Conclusions and Perspectives
In this review, we first presented a definition and categorization of biodegradable polymers. Relevant degradation mechanisms of biodegradable polymers have also been described. Various biodegradable polymers categorized by natural polymers and synthetic polymers have been highlighted for their chemical structure and gene-delivery applications. Structure-property relationships of these biodegradable polymers have also been organized and analyzed. Currently, biodegradability constitutes the most critical material feature affecting the clinical applicability of final biomedical products because it substantially influences their biocompatibility and biosafety. Considering the necessity of repeated administration in gene-based therapies, biodegradability is particularly critical for gene-delivery vectors. Accordingly, the use of biodegradable polymers for gene delivery and therapies has attracted great research attention. Despite possessing favorable biodegradability, biocompatibility and non-toxicity, the development of biodegradable polymer vectors from in vitro to in vivo and eventual clinical trials still faces an immense challenge. This is because such medical translation needs to overcome different types of biological barriers. To overcome these barriers, it is suggested that future investigations of biodegradable polymer vectors focus on the following five main directions: (1) a clear structure-function relationship of developing biodegradable polymer vectors should be clearly elucidated. Based on this understanding, a chemical structural design for creating new vectors can be much more rational and effective; (2) the resulting polyplexes have to possess enhanced in vivo stability to avoid rapid clearance during blood circulation; (3) targeting functions and imaging ability need to be incorporated into developing biodegradable polymer vectors; (4) more elaborated in vivo assessments should be performed for developing biodegradable polymer vectors for reducing the currently large gap between in vitro results and in vivo performance; and (5) additional investigations into the control of release profiles of the loaded genes of biodegradable polymer vectors are requisite. Due to the importance of gene unpacking in the entire transfection process, it is necessary to comprehensively study precisely how loaded genes are released from vectors. Moreover, the design of smart vectors capable of releasing their loaded gene payloads in a timely and spatially-controlled manner is crucial for the development of next-generation gene-delivery vectors.
Acknowledgments
The authors are thankful for the financial support from The Mister of Science and Technology, Taiwan (MOST 106-2221-E-224-058; MOST 107-2221-E-110-079-MY2; MOST 108-2632-E-035-001) and the Hong Kong Polytechnic University (G-YBMY).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Islam MA, Park TE, Singh B, et al. Major degradable polycations as carriers for DNA and siRNA. J Controlled Release. 2014;193:74–89. doi:10.1016/j.jconrel.2014.05.055
2. Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109(2):259–302. doi:10.1021/cr800409e
3. Yin H, Song CQ, Dorkin JR, et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotechnol. 2016;34(3):328–333. doi:10.1038/nbt.3471
4. Hoban MD, Orkin SH, Bauer DE. Genetic treatment of a molecular disorder: gene therapy approaches to sickle cell disease. Blood. 2016;127(7):839–848. doi:10.1182/blood-2015-09-618587
5. Nakamura T, Yamada Y, Sato Y, Khalil IA, Harashima H. Innovative nanotechnologies for enhancing nucleic acids/gene therapy: controlling intracellular trafficking to targeted biodistribution. Biomaterials. 2019;218:119329. doi:10.1016/j.biomaterials.2019.119329
6. Revia RA, Stephen ZR, Zhang MQ. Theranostic nanoparticles for RNA-based cancer treatment. Acc Chem Res. 2019;52(6):1496–1506. doi:10.1021/acs.accounts.9b00101
7. Keles E, Song Y, Du D, Dong WJ, Lin YH. Recent progress in nanomaterials for gene delivery applications. Biomater Sci. 2016;4(9):1291–1309. doi:10.1039/C6BM00441E
8. Donkuru M, Badea I, Wettig S, Verrall R, Elsabahy M, Foldvari M. Advancing nonviral gene delivery: lipid- and surfactant-based nanoparticle design strategies. Nanomedicine. 2010;5(7):1103–1127. doi:10.2217/nnm.10.80
9. Ullah I, Muhammad K, Akpanyung M, et al. Bioreducible, hydrolytically degradable and targeting polymers for gene delivery. J Mater Chem B. 2017;5(18):3253–3276. doi:10.1039/C7TB00275K
10. Lee SJ, Kim MJ, Kwon IC, Roberts TM. Delivery strategies and potential targets for siRNA in major cancer types. Adv Drug Delivery Rev. 2016;104:2–15. doi:10.1016/j.addr.2016.05.010
11. Liu ZH, Zhang ZY, Zhou CR, Jiao YP. Hydrophobic modifications of cationic polymers for gene delivery. Prog Polym Sci. 2010;35(9):1144–1162. doi:10.1016/j.progpolymsci.2010.04.007
12. Iyer AK, Duan ZF, Amiji MM. Nanodelivery systems for nucleic acid therapeutics in drug resistant tumors. Mol Pharmaceutics. 2014;11(8):2511–2526. doi:10.1021/mp500024p
13. Aied A, Greiser U, Pandit A, Wang WX. Polymer gene delivery: overcoming the obstacles. Drug Discovery Today. 2013;18(21–22):1090–1098. doi:10.1016/j.drudis.2013.06.014
14. Kim B, Park J-H, Sailor MJ. Rekindling RNAi therapy: materials design requirements for in vivo siRNA delivery. Adv Mater. 2019;31:1903637. doi:10.1002/adma.201903637
15. O’Rorke S, Keeney M, Pandit A. Non-viral polyplexes: scaffold mediated delivery for gene therapy. Prog Polym Sci. 2010;35(4):441–458. doi:10.1016/j.progpolymsci.2010.01.005
16. Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–358. doi:10.1038/nrg1066
17. Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–555. doi:10.1038/nrg3763
18. Pandey AP, Sawant KK. Polyethylenimine: A versatile, multifunctional non-viral vector for nucleic acid delivery. Mat Sci Eng C. 2016;68:904–918. doi:10.1016/j.msec.2016.07.066
19. Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18(1):33–37. doi:10.1038/71889
20. Kim HJ, Kim A, Miyata K, Kataoka K. Recent progress in development of siRNA delivery vehicles for cancer therapy. Adv Drug Delivery Rev. 2016;104:61–77. doi:10.1016/j.addr.2016.06.011
21. Moss KH, Popova P, Hadrup SR, Astakhova K, Taskova M. Lipid nanoparticles for delivery of therapeutic RNA oligonucleotides. Mol Pharmaceutics. 2019;16(6):2265–2277. doi:10.1021/acs.molpharmaceut.8b01290
22. Yue YN, Wu C. Progress and perspectives in developing polymeric vectors for in vitro gene delivery. Biomater Sci. 2013;1(2):152–170. doi:10.1039/C2BM00030J
23. Wang YY, Ye MZ, Xie RS, Gong SQ. Enhancing the in vitro and in vivo stabilities of polymeric nucleic acid delivery nanosystems. Bioconjugate Chem. 2019;30(2):325–337. doi:10.1021/acs.bioconjchem.8b00749
24. Shi BY, Zheng M, Tao W, et al. Challenges in DNA delivery and recent advances in multifunctional polymeric DNA delivery systems. Biomacromolecules. 2017;18(8):2231–2246. doi:10.1021/acs.biomac.7b00803
25. Rose VL, Mastrotto F, Mantovani G. Phosphonium polymers for gene delivery. Polym Chem. 2017;8(2):353–360. doi:10.1039/C6PY01855F
26. Rinkenauer AC, Schubert S, Traeger A, Schubert US. The influence of polymer architecture on in vitro pDNA transfection. J Mater Chem B. 2015;3(38):7477–7493. doi:10.1039/C5TB00782H
27. Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Controlled Release. 2011;151(3):220–228. doi:10.1016/j.jconrel.2010.11.004
28. Rehman ZU, Hoekstra D, Zuhorn IS. Mechanism of polyplex- and lipoplex-mediated delivery of nucleic acids: real-time visualization of transient membrane destabilization without endosomal lysis. ACS Nano. 2013;7(5):3767–3777. doi:10.1021/nn3049494
29. Zhou ZX, Liu XR, Zhu DC, et al. Nonviral cancer gene therapy: delivery cascade and vector nanoproperty integration. Adv Drug Delivery Rev. 2017;115:115–154. doi:10.1016/j.addr.2017.07.021
30. Williford JM, Wu J, Ren Y, Archang MM, Leong KW, Mao HQ. Recent advances in nanoparticle-mediated siRNA delivery. Annu Rev Biomed Eng. 2014;16(1):347–370. doi:10.1146/annurev-bioeng-071813-105119
31. Jager M, Schubert S, Ochrimenko S, Fischer D, Schubert US. Branched and linear poly(ethylene imine)-based conjugates: synthetic modification, characterization, and application. Chem Soc Rev. 2012;41(13):4755–4767. doi:10.1039/c2cs35146c
32. Dzmitruk V, Apartsin E, Ihnatsyeu-Kachan A, Abashkin V, Shcharbin D, Bryszewska M. Dendrimers show promise for siRNA and microRNA therapeutics. Pharmaceutics. 2018;10(3):126. doi:10.3390/pharmaceutics10030126
33. Lungwitz U, Breunig M, Blunk T, Gopferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60(2):247–266. doi:10.1016/j.ejpb.2004.11.011
34. Cai JG, Yue YA, Rui D, Zhang YF, Liu SY, Wu C. Effect of chain length on cytotoxicity and endocytosis of cationic polymers. Macromolecules. 2011;44(7):2050–2057. doi:10.1021/ma102498g
35. Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Delivery Rev. 2006;58(4):467–486. doi:10.1016/j.addr.2006.03.007
36. Fischer D, Li YX, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24(7):1121–1131. doi:10.1016/S0142-9612(02)00445-3
37. Luten J, van Nostruin CF, De Smedt SC, Hennink WE. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J Controlled Release. 2008;126(2):97–110. doi:10.1016/j.jconrel.2007.10.028
38. Schaffer DV, Fidelman NA, Dan N, Lauffenburger DA. Vector unpacking as a potential barrier for receptor-mediated polyplex gene delivery. Biotechnol Bioeng. 2000;67(5):598–606. doi:10.1002/(ISSN)1097-0290
39. Zhang YX, Zhou J, Ma SN, He YY, Yang J, Gu ZW. Reactive oxygen species (ROS)-degradable polymeric nanoplatform for hypoxia-targeted gene delivery: unpacking DNA and reducing toxicity. Biomacromolecules. 2019;20(5):1899–1913. doi:10.1021/acs.biomac.9b00054
40. Kunath K, von Harpe A, Fischer D, et al. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J Controlled Release. 2003;89(1):113–125. doi:10.1016/S0168-3659(03)00076-2
41. Kim K, Chen WCW, Heo Y, Wang YD. Polycations and their biomedical applications. Prog Polym Sci. 2016;60:18–50. doi:10.1016/j.progpolymsci.2016.05.004
42. Xiang YG, Oo NNL, Lee JP, Li ZB, Loh XJ. Recent development of synthetic nonviral systems for sustained gene delivery. Drug Discov Today. 2017;22(9):1318–1335. doi:10.1016/j.drudis.2017.04.001
43. Arote RB, Jiang HL, Kim YK, Cho MH, Choi YJ, Cho CS. Degradable poly(amido amine)s as gene delivery carriers. Expert Opin Drug Delivery. 2011;8(9):1237–1246. doi:10.1517/17425247.2011.586333
44. Wang Y, Zhang S, Benoit DSW. Degradable poly(ethylene glycol) (PEG)-based hydrogels for spatiotemporal control of siRNA/nanoparticle delivery. J Controlled Release. 2018;287:58–66. doi:10.1016/j.jconrel.2018.08.002
45. Doppalapudi S, Jain A, Khan W, Domb AJ. Biodegradable polymers-an overview. Polym Adv Technol. 2014;25(5):427–435. doi:10.1002/pat.v25.5
46. Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32(8–9):762–798. doi:10.1016/j.progpolymsci.2007.05.017
47. Chen C-K, Lin W-J, Hsia Y, Lo L-W. Synthesis of polylactide-based core–shell interface cross-linked micelles for anticancer drug delivery. Macromol Biosci. 2017;17(3):1600191. doi:10.1002/mabi.v17.3
48. Vert M. Degradable polymers in medicine: updating strategies and terminology. Int J Artif Organs. 2011;34(2):76–83. doi:10.5301/IJAO.2011.6400
49. Pashuck ET, Stevens MM. Designing regenerative biomaterial therapies for the clinic. Sci Transl Med. 2012;4(160):160sr4. doi:10.1126/scitranslmed.3002717
50. Sabir MI, Xu XX, Li L. A review on biodegradable polymeric materials for bone tissue engineering applications. J Mater Sci. 2009;44(21):5713–5724. doi:10.1007/s10853-009-3770-7
51. Liu XH, Holzwarth JM, Ma PX. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol Biosci. 2012;12(7):911–919. doi:10.1002/mabi.201100466
52. Tian HY, Tang ZH, Zhuang XL, Chen XS, Jing XB. Biodegradable synthetic polymers: preparation, functionalization and biomedical application. Prog Polym Sci. 2012;37(2):237–280. doi:10.1016/j.progpolymsci.2011.06.004
53. Olsen D, Yang CL, Bodo M, et al. Recombinant collagen and gelatin for drug delivery. Adv Drug Delivery Rev. 2003;55(12):1547–1567. doi:10.1016/j.addr.2003.08.008
54. Hu Y, Li Y, Xu FJ. Versatile functionalization of polysaccharides via polymer grafts: from design to biomedical applications. Acc Chem Res. 2017;50(2):281–292. doi:10.1021/acs.accounts.6b00477
55. Liu QY, Jiang L, Shi R, Zhang LQ. Synthesis, preparation, in vitro degradation, and application of novel degradable bioelastomers-A review. Prog Polym Sci. 2012;37(5):715–765. doi:10.1016/j.progpolymsci.2011.11.001
56. Li YL, Maciel D, Rodrigues J, Shi XY, Tomas H. Biodegradable polymer nanogels for drug/nucleic acid delivery. Chem Rev. 2015;115(16):8564–8608. doi:10.1021/cr500131f
57. Williams CK. Synthesis of functionalized biodegradable polyesters. Chem Soc Rev. 2007;36(10):1573–1580. doi:10.1039/b614342n
58. Tyler B, Gullotti D, Mangraviti A, Utsuki T, Brem H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv Drug Delivery Rev. 2016;107:163–175. doi:10.1016/j.addr.2016.06.018
59. Pretula J, Slomkowski S, Penczek S. Polylactides-methods of synthesis and characterization. Adv Drug Delivery Rev. 2016;107:3–16. doi:10.1016/j.addr.2016.05.002
60. Wang YC, Yuan YY, Du JZ, Yang XZ, Wang J. Recent progress in polyphosphoesters: from controlled synthesis to biomedical applications. Macromol Biosci. 2009;9(12):1154–1164. doi:10.1002/mabi.200900253
61. Brannigan RP, Dove AP. Synthesis, properties and biomedical applications of hydrolytically degradable materials based on aliphatic polyesters and polycarbonates. Biomater Sci. 2017;5(1):9–21. doi:10.1039/C6BM00584E
62. Bulmus V, Woodward M, Lin L, Murthy N, Stayton P, Hoffman A. A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. J Controlled Release. 2003;93(2):105–120. doi:10.1016/j.jconrel.2003.06.001
63. Matsumoto S, Christie RJ, Nishiyama N, et al. Environment-responsive block copolymer micelles with a disulfide cross-linked core for enhanced siRNA delivery. Biomacromolecules. 2009;10(1):119–127. doi:10.1021/bm800985e
64. Zhao H, Lin ZY, Yildirimer L, Dhinakar A, Zhao X, Wu J. Polymer-based nanoparticles for protein delivery: design, strategies and applications. J Mater Chem B. 2016;4(23):4060–4071. doi:10.1039/C6TB00308G
65. Lee SJ, Yhee JY, Kim SH, Kwon IC, Kim K. Biocompatible gelatin nanoparticles for tumor-targeted delivery of polymerized siRNA in tumor-bearing mice. J Controlled Release. 2013;172(1):358–366. doi:10.1016/j.jconrel.2013.09.002
66. Youngren SR, Tekade RK, Gustilo B, Hoffmann PR, Chougule MB. STAT6 siRNA matrix-loaded gelatin nanocarriers: formulation, characterization, and ex vivo proof of concept using adenocarcinoma cells. Biomed Res Int. 2013;2013:858946. doi:10.1155/2013/858946
67. Kaul G, Amiji M. Cellular interactions and in vitro DNA transfection studies with poly(ethylene glycol)-modified gelatin nanoparticles. J Pharm Sci. 2005;94(1):184–198. doi:10.1002/jps.20216
68. Kriegel C, Amiji M. Oral TNF-alpha gene silencing using a polymeric microsphere-based delivery system for the treatment of inflammatory bowel disease. J Controlled Release. 2011;150(1):77–86. doi:10.1016/j.jconrel.2010.10.002
69. Ishikawa H, Nakamura Y, Jo J, Tabata Y. Gelatin nanospheres incorporating siRNA for controlled intracellular release. Biomaterials. 2012;33(35):9097–9104. doi:10.1016/j.biomaterials.2012.08.032
70. Torrano AA, Pereira AS, Oliveira ON, Barros-Timmons A. Probing the interaction of oppositely charged gold nanoparticles with DPPG and DPPC langmuir monolayers as cell membrane models. Colloid Surface B. 2013;108:120–126. doi:10.1016/j.colsurfb.2013.02.014
71. Syga MI, Nicoli E, Kohler E, Shastri VP. Albumin incorporation in polyethylenimine-DNA polyplexes influences transfection efficiency. Biomacromolecules. 2016;17(1):200–207. doi:10.1021/acs.biomac.5b01308
72. Jiang YY, Lu HX, Khine YY, Dag A, Stenzel MH. Polyion complex micelle based on albumin-polymer conjugates: multifunctional oligonucleotide transfection vectors for anticancer chemotherapeutics. Biomacromolecules. 2014;15(11):4195–4205. doi:10.1021/bm501205x
73. Look J, Wilhelm N, von Briesen H, et al. Ligand-modified human serum albumin nanoparticles for enhanced gene delivery. Mol Pharmaceutics. 2015;12(9):3202–3213. doi:10.1021/acs.molpharmaceut.5b00153
74. Han JF, Wang Q, Zhang ZR, Gong T, Sun X. Cationic bovine serum albumin based self-assembled nanoparticles as siRNA delivery vector for treating lung metastatic cancer. Small. 2014;10(3):524–535. doi:10.1002/smll.v10.3
75. Ping YA, Liu CD, Tang GP, et al. Functionalization of chitosan via atom transfer radical polymerization for gene delivery. Adv Funct Mater. 2010;20(18):3106–3116. doi:10.1002/adfm.201000177
76. Chen C-K, Huang S-C. Preparation of reductant–responsive N-maleoyl-functional chitosan/poly(vinyl alcohol) nanofibers for drug delivery. Mol Pharmaceutics. 2016;13(12):4152–4167. doi:10.1021/acs.molpharmaceut.6b00758
77. Kean T, Roth S, Thanou M. Trimethylated chitosans as non-viral gene delivery vectors: cytotoxicity and transfection efficiency. J Controlled Release. 2005;103(3):643–653. doi:10.1016/j.jconrel.2005.01.001
78. Mao ZW, Ma L, Yan J, Yan M, Gao CY, Shen JC. The gene transfection efficiency of thermoresponsive N,N,N-trimethyl chitosan chloride-g-poly(N-isopropylacrylamide) copolymer. Biomaterials. 2007;28(30):4488–4500. doi:10.1016/j.biomaterials.2007.06.033
79. Germershaus O, Mao SR, Sitterberg J, Bakowsky U, Kissel T. Gene delivery using chitosan, trimethyl chitosan or polyethylenglycol-graft-trimethyl chitosan block copolymers: establishment of structure-activity relationships in vitro. J Controlled Release. 2008;125(2):145–154. doi:10.1016/j.jconrel.2007.10.013
80. Gao JQ, Zhao QQ, Lv TF, et al. Gene-carried chitosan-linked-PEI induced high gene transfection efficiency with low toxicity and significant tumor-suppressive activity. Int J Pharm. 2010;387(1–2):286–294. doi:10.1016/j.ijpharm.2009.12.033
81. Pimpha N, Sunintaboon P, Inphonlek S, Tabata Y. Gene delivery efficacy of polyethyleneimine-introduced chitosan shell/poly(methyl methacrylate) core nanoparticles for rat mesenchymal stem cells. J Biomater Sci Polym Ed. 2010;21(2):205–223. doi:10.1163/156856209X415503
82. Ren HQ, Liu S, Yang JX, et al. N,N,N-trimethylchitosan modified with well defined multifunctional polymer modules used as pDNA delivery vector. Carbohydr Polym. 2016;137:222–230. doi:10.1016/j.carbpol.2015.10.085
83. Lin WJ, Lee WC, Shieh MJ. Hyaluronic acid conjugated micelles possessing CD44 targeting potential for gene delivery. Carbohydr Polym. 2017;155:101–108. doi:10.1016/j.carbpol.2016.08.021
84. Yoon HY, Kim HR, Saravanakumar G, et al. Bioreducible hyaluronic acid conjugates as siRNA carrier for tumor targeting. J Controlled Release. 2013;172(3):653–661. doi:10.1016/j.jconrel.2013.09.008
85. Liu G, Choi KY, Bhirde A, et al. Sticky nanoparticles: a platform for siRNA delivery by a bis(zinc(II) dipicolylamine)-functionalized, self-assembled nanoconjugate. Angew Chem Int Ed. 2012;51(2):445–449. doi:10.1002/anie.201105565
86. Liu T, Xue W, Ke B, Xie MQ, Ma D. Star-shaped cyclodextrin-poly(L-lysine) derivative co-delivering docetaxel and MMP-9 siRNA plasmid in cancer therapy. Biomaterials. 2014;35(12):3865–3872. doi:10.1016/j.biomaterials.2014.01.040
87. Xiu KM, Yang JJ, Zhao NN, Li JS, Xu FJ. Multiarm cationic star polymers by atom transfer radical polymerization from beta-cyclodextrin cores: influence of arm number and length on gene delivery. Acta Biomater. 2013;9(1):4726–4733. doi:10.1016/j.actbio.2012.08.020
88. Wen YT, Zhang ZX, Li J. Highly efficient multifunctional supramolecular gene carrier system self-assembled from redox-sensitive and zwitterionic polymer blocks. Adv Funct Mater. 2014;24(25):3874–3884. doi:10.1002/adfm.v24.25
89. Wang ZH, Li WB, Ma J, Tang GP, Yang WT, Xu FJ. Functionalized nonionic dextran backbones by atom transfer radical polymerization for efficient gene delivery. Macromolecules. 2011;44(2):230–239. doi:10.1021/ma102419e
90. Wang ZH, Zhu Y, Chai MY, Yang WT, Xu FJ. Biocleavable comb-shaped gene carriers from dextran backbones with bioreducible ATRP initiation sites. Biomaterials. 2012;33(6):1873–1883. doi:10.1016/j.biomaterials.2011.11.027
91. Putnam D, Langer R. Poly(4-hydroxy-L-proline ester): low-temperature polycondensation and plasmid DNA complexation. Macromolecules. 1999;32(11):3658–3662. doi:10.1021/ma982004i
92. Lim YB, Choi YH, Park JS. A self-destroying polycationic polymer: biodegradable poly(4-hydroxy-L-proline ester). J Am Chem Soc. 1999;121(24):5633–5639. doi:10.1021/ja984012k
93. Lim YB, Kim CH, Kim K, Kim SW, Park JS. Development of a safe gene delivery system using biodegradable polymer, poly[alpha-(4-aminobutyl)-l-glycolic acid]. J Am Chem Soc. 2000;122(27):6524–6525. doi:10.1021/ja001033h
94. Lim YB, Han SO, Kong HU, et al. Biodegradable polyester, poly[alpha-(4 aminobutyl)-l-glycolic acid], as a non-toxic gene carrier. Pharm Res. 2000;17(7):811–816. doi:10.1023/A:1007552007765
95. Akinc A, Anderson DG, Lynn DM, Langer R. Synthesis of poly(beta-amino ester)s optimized for highly effective gene delivery. Bioconjugate Chem. 2003;14(5):979–988. doi:10.1021/bc034067y
96. Akinc A, Lynn DM, Anderson DG, Langer R. Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. J Am Chem Soc. 2003;125(18):5316–5323. doi:10.1021/ja034429c
97. Anderson DG, Lynn DM, Langer R. Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angew Chem Int Ed. 2003;42(27):3153–3158. doi:10.1002/anie.200351244
98. Akinc A, Zumbuehl A, Goldberg M, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26(5):561–569. doi:10.1038/nbt1402
99. Love KT, Mahon KP, Levins CG, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA. 2010;107(21):1864–1869. doi:10.1073/pnas.0910603106
100. Song L, Ding AX, Zhang KX, Gong B, Lu ZL, He L. Degradable polyesters via ring-opening polymerization of functional valerolactones for efficient gene delivery. Org Biomol Chem. 2017;15(31):6567–6574. doi:10.1039/C7OB00822H
101. Zhang ZH, Yin LC, Xu YX, et al. Facile functionalization of polyesters through thiol-yne chemistry for the design of degradable, cell-penetrating and gene delivery dual-functional agents. Biomacromolecules. 2012;13(11):3456–3462. doi:10.1021/bm301333w
102. Yilmaz ZE, Jerome C. Polyphosphoesters: new trends in synthesis and drug delivery applications. Macromol Biosci. 2016;16(12):1745–1761. doi:10.1002/mabi.v16.12
103. Lu ZZ, Wu J, Sun TM, Ji J, Yan LF, Wang J. Biodegradable polycation and plasmid DNA multilayer film for prolonged gene delivery to mouse osteoblasts. Biomaterials. 2008;29(6):733–741. doi:10.1016/j.biomaterials.2007.10.033
104. Liang S, Yang XZ, Du XJ, et al. Optimizing the size of micellar nanoparticles for efficient siRNA delivery. Adv Funct Mater. 2015;25(30):4778–4787. doi:10.1002/adfm.201501548
105. Mao CQ, Xiong MH, Liu Y, et al. Synthetic lethal therapy for KRAS mutant non-small-cell lung carcinoma with nanoparticle-mediated CDK4 siRNA delivery. Mol Ther. 2014;22(5):964–973. doi:10.1038/mt.2014.18
106. Liu XQ, Xiong MH, Shu XT, Tang RZ, Wang J. Therapeutic delivery of siRNA silencing HIF-1 alpha with micellar nanoparticles inhibits hypoxic tumor growth. Mol Pharmaceutics. 2012;9(10):2863–2874. doi:10.1021/mp300193f
107. Sun TM, Du JZ, Yao YD, et al. Simultaneous delivery of siRNA and paclitaxel via a “two-in-one” micelleplex promotes synergistic tumor suppression. ACS Nano. 2011;5(2):1483–1494. doi:10.1021/nn103349h
108. Chen C-K, Law WC, Aalinkeel R, et al. Well-defined degradable cationic polylactide as nanocarrier for the delivery of siRNA to silence angiogenesis in prostate cancer. Adv Healthcare Mater. 2012;1(6):751–761. doi:10.1002/adhm.201200094
109. Jones CH, Chen C-K, Jiang M, Fang L, Cheng C, Pfeifer BA. Synthesis of cationic polylactides with tunable charge densities as nanocarriers for effective gene delivery. Mol Pharmaceutics. 2013;10(3):1138–1145. doi:10.1021/mp300666s
110. Chen C-K, Law WC, Aalinkeel R, et al. Biodegradable cationic polymeric nanocapsules for overcoming multidrug resistance and enabling drug-gene co-delivery to cancer cells. Nanoscale. 2014;6(3):1567–1572. doi:10.1039/C3NR04804G
111. Chen C-K, Jones CH, Mistriotis P, et al. Poly(ethylene glycol)-block-cationic polylactide nanocomplexes of differing charge density for gene delivery. Biomaterials. 2013;34(37):9688–9699. doi:10.1016/j.biomaterials.2013.08.063
112. Yang CB, Panwar N, Wang YC, et al. Biodegradable charged polyester-based vectors (BCPVs) as an efficient non-viral transfection nanoagent for gene knockdown of the BCR-ABL hybrid oncogene in a human chronic myeloid leukemia cell line. Nanoscale. 2016;8(17):9405–9416. doi:10.1039/C6NR00996D
113. Yang CB, Hu R, Anderson T, et al. Biodegradable nanoparticle-mediated K-ras down regulation for pancreatic cancer gene therapy. J Mater Chem B. 2015;3(10):2163–2172. doi:10.1039/C4TB01623H
114. Yang CB, Chan KK, Lin WJ, et al. Biodegradable nanocarriers for small interfering ribonucleic acid (siRNA) co-delivery strategy increase the chemosensitivity of pancreatic cancer cells to gemcitabine. Nano Res. 2017;10(9):3049–3067. doi:10.1007/s12274-017-1521-7
115. Lin GM, Chen CK, Yin F, et al. Biodegradable nanoparticles as siRNA carriers for in vivo gene silencing and pancreatic cancer therapy. J Mater Chem B. 2017;5(18):3327–3337. doi:10.1039/C6TB03116A
116. Chen W, Meng FH, Cheng R, Deng C, Feijen J, Zhong ZY. Advanced drug and gene delivery systems based on functional biodegradable polycarbonates and copolymers. J Controlled Release. 2014;190:398–414. doi:10.1016/j.jconrel.2014.05.023
117. Wang CF, Lin YX, Jiang T, He F, Zhuo RX. Polyethylenimine-grafted polycarbonates as biodegradable polycations for gene delivery. Biomaterials. 2009;30(27):4824–4832. doi:10.1016/j.biomaterials.2009.05.053
118. He F, Wang CF, Jiang T, Han B, Zhuo RX. Poly[(5-methyl-5-allyloxycarbonyl-trimethylene carbonate)-co-(5,5-dimethyl-trimethylene carbonate)] with grafted polyethylenimine as biodegradable polycations for efficient gene delivery. Biomacromolecules. 2010;11(11):3028–3035. doi:10.1021/bm1008525
119. Ong ZY, Fukushima K, Coady DJ, Yang YY, Ee PLR, Hedrick JL. Rational design of biodegradable cationic polycarbonates for gene delivery. J Controlled Release. 2011;152(1):120–126. doi:10.1016/j.jconrel.2011.01.020
120. Yang C, Ong ZY, Yang YY, Ee PLR, Hedrick JL. Novel biodegradable block copolymers of poly(ethylene glycol) (PEG) and cationic polycarbonate: effects of PEG configuration on gene delivery. Macromol Rapid Commum. 2011;32(22):1826–1833. doi:10.1002/marc.201100350
121. Ong ZY, Yang C, Gao SJ, Ke XY, Hedrick JL, Yang YY. Galactose-functionalized cationic polycarbonate diblock copolymer for targeted gene delivery to hepatocytes. Macromol Rapid Commum. 2013;34(21):1714–1720. doi:10.1002/marc.201300538
122. Frere A, Kawalec M, Tempelaar S, et al. Impact of the structure of biocompatible aliphatic polycarbonates on siRNA transfection ability. Biomacromolecules. 2015;16(3):769–779. doi:10.1021/bm501676p
123. Frere A, Baroni A, Hendrick E, et al. PEGylated and functionalized aliphatic polycarbonate polyplex nanoparticles for intravenous administration of HDAC5 siRNA in cancer therapy. ACS Appl Mater Interfaces. 2017;9(3):2181–2195. doi:10.1021/acsami.6b15064
124. Guan JJ, Fujimoto KL, Sacks MS, Wagner WR. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. 2005;26(18):3961–3971. doi:10.1016/j.biomaterials.2004.10.018
125. Shau MD, Tseng SJ, Yang TF, Cherng JY, Chin WK. Effect of molecular weight on the transfection efficiency of novel polyurethane as a biodegradable gene vector. J Biomed Mater Res Part A. 2006;77A(4):736–746. doi:10.1002/jbm.a.30605
126. Hung WC, Da Shau M, Kao HC, Shih MF, Cherng JY. The synthesis of cationic polyurethanes to study the effect of amines and structures on their DNA transfection potential. J Controlled Release. 2009;133(1):68–76. doi:10.1016/j.jconrel.2008.09.082
127. Liu XY, Ho WY, Hung WJ, Shau MD. The characteristics and transfection efficiency of cationic poly (ester-co-urethane) - short chain PEI conjugates self-assembled with DNA. Biomaterials. 2009;30(34):6665–6673. doi:10.1016/j.biomaterials.2009.08.052
128. Yang YP, Chien Y, Chiou GY, et al. Inhibition of cancer stem cell-like properties and reduced chemoradioresistance of glioblastoma using microRNA145 with cationic polyurethane-short branch PEI. Biomaterials. 2012;33(5):1462–1476. doi:10.1016/j.biomaterials.2011.10.071
129. Chiou GY, Cherng JY, Hsu HS, et al. Cationic polyurethanes-short branch PEI-mediated delivery of Mir145 inhibited epithelial-mesenchymal transdifferentiation and cancer stem-like properties and in lung adenocarcinoma. J Controlled Release. 2012;159(2):240–250. doi:10.1016/j.jconrel.2012.01.014
130. Lo WL, Chien Y, Chiou GY, et al. Nuclear localization signal-enhanced RNA interference of EZH2 and Oct4 in the eradication of head and neck squamous cell carcinoma-derived cancer stem cells. Biomaterials. 2012;33(14):3693–3709. doi:10.1016/j.biomaterials.2012.01.016
131. Gosselin MA, Guo WJ, Lee RJ. Efficient gene transfer using reversibly cross-linked low molecular weight polyethylenimine. Bioconjugate Chem. 2001;12(6):989–994. doi:10.1021/bc0100455
132. Peng Q, Zhong ZL, Zhuo RX. Disulfide cross-linked polyethylenimines (PEI) prepared via thiolation of low molecular weight PEI as highly efficient gene vectors. Bioconjugate Chem. 2008;19(2):499–506. doi:10.1021/bc7003236
133. Liu J, Jiang XL, Xu L, Wang XM, Hennink WE, Zhuo RX. Novel reduction-responsive cross-linked polyethylenimine derivatives by click chemistry for nonviral gene delivery. Bioconjugate Chem. 2010;21(10):1827–1835. doi:10.1021/bc100191r
134. Zhang GY, Liu J, Yang QZ, Zhuo RX, Jiang XL. Disulfide-containing brushed polyethylenimine derivative synthesized by click chemistry for nonviral gene delivery. Bioconjugate Chem. 2012;23(6):1290–1299. doi:10.1021/bc300133r
135. Bauhuber S, Liebl R, Tomasetti L, Rachel R, Goepferich A, Breunig M. A library of strictly linear poly(ethylene glycol)-poly(ethylene imine) diblock copolymers to perform structure-function relationship of non-viral gene carriers. J Controlled Release. 2012;162(2):446–455. doi:10.1016/j.jconrel.2012.07.017
136. Lei Y, Wang J, Xie C, et al. Glutathione-sensitive RGD-poly(ethylene glycol)-SS-polyethylenimine for intracranial glioblastoma targeted gene delivery. J Gene Med. 2013;15(8–9):291–305. doi:10.1002/jgm.2726
137. Christensen LV, Chang CW, Kim WJ, et al. Reducible poly(amido ethylenimine)s designed for triggered intracellular gene delivery. Bioconjugate Chem. 2006;17(5):1233–1240. doi:10.1021/bc0602026
138. Lin C, Zhong ZY, Lok MC, et al. Linear poly(amido amine)s with secondary and tertiary amino groups and variable amounts of disulfide linkages: synthesis and in vitro gene transfer properties. J Controlled Release. 2006;116(2):130–137. doi:10.1016/j.jconrel.2006.09.009
139. Chen J, Wu C, Oupicky D. Bioreducible hyperbranched poly(amido amine)s for gene delivery. Biomacromolecules. 2009;10(10):2921–2927. doi:10.1021/bm900724c
140. Wu C, Li J, Zhu Y, Chen J, Oupicky D. Opposing influence of intracellular and membrane thiols on the toxicity of reducible polycations. Biomaterials. 2013;34(34):8843–8850. doi:10.1016/j.biomaterials.2013.07.095
141. Brumbach JH, Lin C, Yockman J, et al. Mixtures of poly(triethylenetetramine/cystamine bisacrylamide) and poly(triethylenetetramine/cystamine bisacrylamide)-g-poly(ethylene glycol) for improved gene delivery. Bioconjugate Chem. 2010;21(10):1753–1761. doi:10.1021/bc900522x
142. Ou M, Xu RZ, Kim SH, Bull DA, Kim SW. A family of bioreducible poly(disulfide amine)s for gene delivery. Biomaterials. 2009;30(29):5804–5814. doi:10.1016/j.biomaterials.2009.06.050
143. Ou M, Wang XL, Xu RZ, Chang CW, Bull DA, Kim SW. Novel biodegradable poly(disulfide amine)s for gene delivery with high efficiency and low cytotoxicity. Bioconjugate Chem. 2008;19(3):626–633. doi:10.1021/bc700397x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
