Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
A Grading System of Microvascular Invasion for Patients with Hepatocellular Carcinoma Undergoing Liver Resection with Curative Intent: A Multicenter Study
Authors Wang H , Chen JJ, Yin SY, Sheng X, Wang HX, Lau WY, Dong H, Cong WM
Received 1 November 2023
Accepted for publication 15 January 2024
Published 24 January 2024 Volume 2024:11 Pages 191—206
DOI https://doi.org/10.2147/JHC.S447731
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mohamed Shaker
Han Wang,1,* Jun-Jie Chen,2,* Shu-Yi Yin,3,* Xia Sheng,4 Hong-Xia Wang,5 Wan Yee Lau,6 Hui Dong,1 Wen-Ming Cong1
1Department of Pathology, Shanghai Eastern Hepatobiliary Surgery Hospital, Naval Medical University, Shanghai, People’s Republic of China; 2Department of Radiology, Shanghai Eastern Hepatobiliary Surgery Hospital, Naval Medical University, Shanghai, People’s Republic of China; 3Department of Pathology, Shanghai Changhai Hospital, Naval Medical University, Shanghai, People’s Republic of China; 4Department of Pathology, Minhang Hospital, Fudan University, Shanghai, People’s Republic of China; 5Department of Pathology, Jiading District Central Hospital, Shanghai University of Medicine & Health Sciences, Shanghai, People’s Republic of China; 6Faculty of Medicine, Chinese University of Hong Kong, Hong Kong, China
*These authors contributed equally to this work
Correspondence: Hui Dong; Wen-Ming Cong, Department of Pathology, Shanghai Eastern Hepatobiliary Surgery Hospital, Naval Medical University, 225 Changhai Road, Shanghai, 200438, People’s Republic of China, Tel +86-021-81875191 ; +86-021-81875192, Email [email protected]; [email protected]
Background: Microvascular invasion (MVI) is closely correlated with poor clinical outcomes in patients with hepatocellular carcinoma (HCC). A grading system of MVI is needed to assist in the management of HCC patient.
Methods: Multicenter data of HCC patients who underwent liver resection with curative intent was analyzed. This grading system was established by detected number and distance from tumor boundary of MVI. Survival outcomes were compared among patients in each group. This system was verified by time-receiver operating characteristic curve, time-area under the curve, calibration curve, and decision curve analyses. Cox regression analysis was performed to study the associated factors of prognosis. Logistic analysis was used to study the predictive factors of MVI.
Results: All patients were classified into 4 groups: M0: no MVI; M1: 1~5 proximal MVIs (≤ 1 cm from tumor boundary); M2a: > 5 proximal MVIs (≤ 1 cm from tumor boundary); M2b: ≥ 1 distal MVIs (> 1 cm from tumor boundary). The recurrence-free survival (RFS), overall survival (OS), and early RFS rates among all the individual groups were significantly different. Based on the number of proximal MVI (0~5 vs > 5), patients in the M2b group were further divided into two subgroups which also showed different prognosis. Multiple methods showed this grading system to be significantly better than the MVI two-tiered system in prognostic evaluation. Four multivariate models for RFS, OS, early RFS, late RFS, and a predictive model of MVI were then established and were shown to satisfactorily evaluate prognosis and have a great discriminatory power, respectively.
Conclusion: This MVI grading system could precisely evaluate prognosis of HCC patients after liver resection with curative intent and it could be employed in routine pathological reports. The severity of MVI from both adjacent and distant from tumor boundary should be stated. A hypothesis about two occurrence modes of distal MVI was proposed.
Keywords: hepatocellular carcinoma, microvascular invasion, hepatectomy, hepatic resection, pathology
Graphical Abstract:
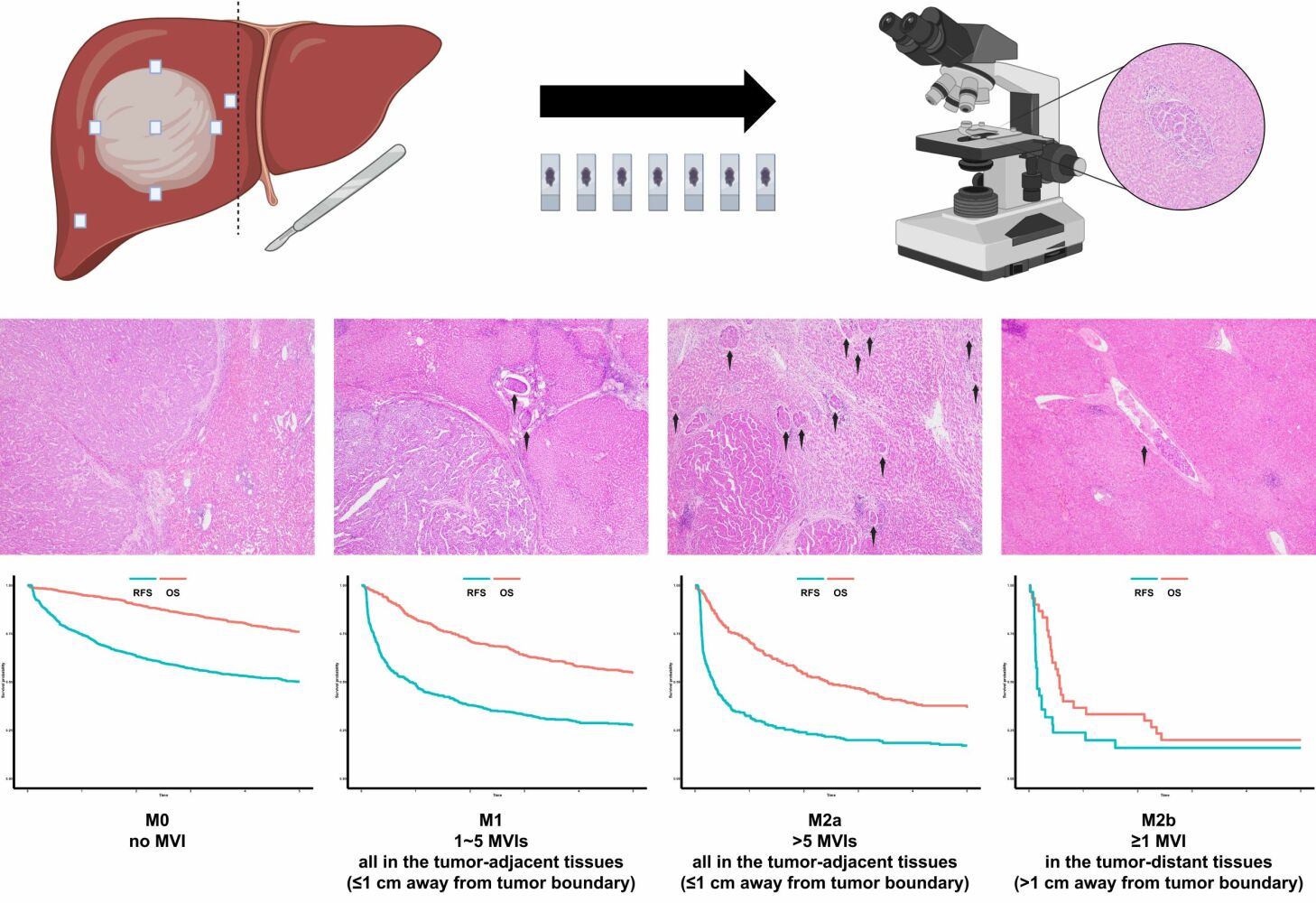
Introduction
Liver cancer is constantly one of the leading causes of cancer-related morbidity and mortality worldwide. Hepatocellular carcinoma (HCC) represents the most common histological subtype of liver cancer.1 In 2019, there were approximately 747,000 cases of HCC worldwide and 480,000 deaths were attributable to HCC.2 Although tremendous advances have been achieved in early detection of HCC by screening, as well as targeted therapy and immunotherapy, the overall survival (OS) of all patients with HCC was still poor with a median of 6~10 months.3 Even for those patients with early stages of HCC who can undergo curative liver resection, postoperative recurrence is one of the most common reasons leading to death. Tumor relapse occurs in at least 70% of HCC patients after hepatic resection and caused 82.5% of death.4 As a consequence, pragmatic clinicopathological parameters relating to tumor biological behavior should be studied to come up with a more personalized intervention approach and surveillance for different populations of HCC patients.
Microvascular invasion (MVI) is widely accepted as an essential pathological feature that predict prognosis of each stage of HCC5–7 as it stands for more extensive dissemination beyond the macroscopic range of tumor and a lower probability of R0 resection.8 There have been many attempts to improve prognosis of HCC patients with MVI, including postoperative adjuvant transcatheter arterial chemoembolization (TACE),9 hepatic arterial infusion chemotherapy (HAIC),10 stereotactic body radiotherapy,11 and immunotherapy.12 However, curative liver resection could be sufficient to achieve good survival outcomes for some patients with MVIs, and these patients may receive unnecessary adjuvant therapy. On the other hand, adjuvant therapies have been shown to improve the prognosis of HCC patients with MVI.13 Therefore, a pragmatic MVI grading system to stratify clinical assessment is indispensable.
The incidences of MVI in resected HCC specimens were significantly different in various studies.14 Even excluding the influence of tumor staging, the reported MVI detection rate for a solitary small HCC varied greatly from 12.4 to 37.3%,15 which illustrated that there were some biases in the previous assessments of MVI. For a MVI grading which can become widely accepted, a standardized gross specimen sampling method is the foremost prerequisite to achieve a stable MVI detection rate. In 2015, our group first put forward the seven-point baseline sampling protocol for HCC16 and this has been routinely used in the pathology departments of the vast majority of Chinese medical centers.17
This study aimed to study a cohort of 1546 HCC patients who underwent liver resection with curative intent coming from multiple centers to establish an implementable MVI grading system closely related to recurrence risks and survival benefits which could be used as a supplement to the “Practice guidelines for the pathological diagnosis of primary liver cancer”.16
Materials and Methods
Patients
The demographic, clinical, ultrasonic and pathologic data of consecutive patients with histologically confirmed primary HCC who underwent liver resection with curative intent in four medical centers from July 2015 to January 2016 were retrospectively reviewed (Shanghai Eastern Hepatobiliary Surgery Hospital, Shanghai Changhai Hospital, Minhang Hospital, Jiading District Central Hospital). HCC patients with portal invasion or extrahepatic metastasis underwent liver resection were also included in this study when the surgeons evaluated that all the tumor lesions could be completely removed (eg HCC patients with Cheng’s classification type I and II portal vein tumor thrombus and resectable liver tumors; HCC patients with resectable metastatic sites of abdominal lymph node, adrenal gland, or peritoneum).18,19 Patients with recurrent HCC or preoperative anticancer treatment were excluded in this study. For multinodular tumors, parameters were recorded based on the tumor with the largest dimension. The ultrasonic assessment criteria of tumor echo, tumor boundary and intratumoral echo referred to the previous study.20 Tumor differentiation was classified by the Edmondson-Steiner grading.21 The definition of non-anatomical/anatomical resection was based on the Brisbane 2000 Nomenclature of Liver Anatomy and Resections and the 2020 update.22,23 The Barcelona Clinic Liver Cancer (BCLC) staging and Tumor-Node-Metastasis (TNM) classification were evaluated by the latest version.24,25
Follow-Up
Patients were followed up once every 3 months for the first 2 years after hospital discharge and once every 4 to 6 months thereafter. At each follow-up visit, serum levels of alpha-fetoprotein (AFP) and hepatitis B virus deoxyribonucleic acid (HBV DNA), liver function, and radiological examination of liver were conducted. Tumor recurrence was clinically suspected with a progressive elevation of serum AFP levels and/or ultrasonographic detection of a new tumor lesion. The diagnosis of recurrence was made with a dynamic computerized tomography scan or magnetic resonance imaging, which demonstrated contrast enhancement in the arterial phase and wash-out in the venous phase, or with hepatic angiography, which disclosed high tumor vascularity. For patients who were diagnosed to have recurrence or progressive diseases, suitable treatments were decided by clinicians in consultation with the patients according to the liver function, location and number of recrudescent tumors. The study endpoints were recurrence-free survival (RFS) and OS. RFS was defined by the date of surgery to the date when recurrence was confirmed. Early RFS and late RFS were divided by the date at 2 years after surgery.26 OS was defined as the duration between surgery and the last follow-up visit or death. Follow-up was terminated 5 years after surgery.
MVI Grading System
The seven-point baseline sampling protocol and diagnostic criteria of MVI were performed according to the “Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update”.16 Four experienced pathologists assessed the pathologic sections independently. When the assessment results were controversial, immunohistochemical markers of Hep Par-1, Arginase-1, Glypican-3 would be conducted to confirm the tumor foci of HCC and immunohistochemical marker of CD34 would be used to determine whether the tumor foci were in vessel. This MVI grading system was classified based on the number and distribution of MVI. The specifics were as follows: M0: no MVI; M1: 1~5 MVIs, all in the tumor-adjacent tissues (proximal MVI, ≤1 cm away from tumor boundary); M2a: >5 MVIs, all in the tumor-adjacent tissues (proximal MVI, ≤1 cm away from tumor boundary); M2b: ≥1 MVI, in the tumor-distant tissues (distal MVI, >1 cm away from tumor boundary) (Figure 1). Intratumoral MVI were not counted because it has been shown to have no prognostic value.27
 |
Figure 1 Diagnostic criteria of the MVI grading system. (A) M0; (B). M1; (C) M2a; (D). M2b; (E) Hep Par-1; (F). Arginase-1; (G) Glypican-3; (H) CD34; Arrow: MVI; Magnification of all images: 40×. |
Statistical Analysis
Continuous variables (presented as median [interquartile range] or mean ± standard deviation) and categorical variables (presented as number of cases and percentage) were compared by the Mann–Whitney test or Student’s t-test, and the Chi-squared test or Fisher’s exact test, respectively. Kolmogorov–Smirnov tests and Levene’s tests were used to judge the normal distribution and homogeneity of variance of the data, respectively. The Kaplan–Meier curve, Log rank tests were used to analyze recurrence and survival of different groups of patients. Time-receiver operating characteristic (time-ROC) curve, time-area under the curve (time-AUC), calibration curve, and decision curve were performed to exhibit the power of this MVI grading system and compared with the MVI two-tiered system (presence/absence of MVI). ROC curve analyses were conducted to determine the AUC. Comparisons between their AUCs were performed using the DeLong test. Hosmer–Lemeshow test was conducted to evaluate the calibration of models. Univariate and multivariate prognostic analyses were conducted by the Cox proportional hazards regression, and presented with forest plots. Multivariate models were further demonstrated by the time-AUC and decision curve analysis. Univariate and multivariate binary logistic regression analyses were executed to screen for MVI-related predictive factors and the power of multivariate model was displayed by ROC curve. Factors with P values of less than 0.157 in the univariate analysis were included in the multivariate analysis for further screening.28 A P value less than 0.05 was considered as statistically significant. All statistical analyses were employed using the SPSS software (IBM SPSS Statistics, USA, version 27.0) and the R-project (R Foundation for Statistical Computing, Vienna, Austria, version 3.4.4).
Results
Baseline Characteristics
For the crude cohort of 1546 patients, there were 1320 (85.4%) men and 226 (14.6%) women with a median age of 53.0 years. The median [interquartile range] of the tumor diameter was 4.6 [3.0; 8.1] cm, and HCC patients with single nodular and multiple nodular tumors were 1235 (79.9%) and 311 (20.1%) patients, respectively. The number of patients for each BCLC stages were 137 (stage 0), 1025 (stage A), 208 (stage B), 176 (stage C), respectively. The number of patients for each TNM stages were 744 (stage I), 486 (stage II), 301 (stage III), 15 (stage IV), respectively. Seven hundred nineteen patients were detected to have presence of MVI, and the constituent ratio was 46.5%. The numbers and percentages of M0, M1, M2a, M2b were 827 (53.5%), 447 (28.9%), 242 (15.7%), 30 (1.9%), respectively (Supplementary Figure 1). Baseline features among all the MVI groups are shown in Table 1.
 |
Table 1 Baseline Characteristics of All Patients |
Impact of MVI Grading on Prognosis
The median follow-up time for all the 1546 patients was 5 years, the median RFS time was 1.90 years [not reach, 0.33 years], and the median OS time was not reached [not reach, 2.46 years]. The median RFS time of the M0, M1, M2a, M2b groups was not reached [not reach, 0.95 years], 0.90 years [not reach, 0.23 years], 0.34 years [1.80 years, 0.12 years], and 0.15 years [0.44 years, 0.12 years], respectively. The corresponding median OS time was not reached [not reach, not reach], not reached [not reach, 1.73 years], 2.44 years [not reach, 0.73 years], and 0.56 years [2.35 years, 0.36 years], respectively. There were 23 patients who suffered from postoperative 30-day death (constituent percentage: 1.49%). The 1-, 2-, 3- and 5-year RFS rates for the patients in the M0, M1, M2a, and M2b groups were 74.7%, 63.4%, 57.1%, 50.2% vs 48.9%, 38.0%, 32.9%, 27.7% vs 32.5%, 24.0%, 19.9%, 17.1% vs 23.8%, 15.9%, 15.9%, 15.9%, respectively (Figure 2A, P<0.001). The corresponding OS rates were 95.2%, 90.1%, 84.9%, 76.0% vs 82.7%, 71.5%, 63.8%, 54.9% vs 70.8%, 54.3%, 46.7%, 36.7% vs 36.7%, 33.3%, 20.0%, 20.0%, respectively (Figure 2B, P<0.001). Early RFS was significantly different among the four groups (Figure 2C, P<0.001) but not for late RFS (Figure 2D, P=0.172).
 |
Figure 2 Survival analysis of HCC patients based on the MVI grading system. (A) RFS; (B) OS; (C) Early RFS; (D) Late RFS. |
Additional Analysis of the M2b Group
For the 30 patients in the M2b group who all had distal MVI (>1 cm away from tumor boundary), their proximal MVIs were further analyzed, of which 11 patients were detected to have 0~5 MVIs and the rest to have more than 5 MVIs. The prognosis of these two subgroups of patients was marginally significantly different in RFS (P=0.140, 5-year RFS rate: 27.3% vs 7.4%, Figure 3A) but significantly different in OS (P=0.015, 5-year OS rate: 45.5% vs 5.3%; Figure 3B).
 |
Figure 3 Survival analysis of HCC patients with different amounts of proximal MVI in the M2b group. (A) RFS; (B). OS. |
Comparison Between the MVI Grading System and the MVI Two-Tiered System
A comparison between this MVI grading system and the MVI two-tiered system by time-ROC showed that the AUC of 1-, 2-, 3-, 5-year RFS were 0.687, 0.671, 0.663, 0.653 vs 0.668, 0.655, 0.649, 0.641, respectively (Supplementary Figure 2A–D, P value for 1 year: <0.001, P value for 2 year: <0.001, P value for 3 year: <0.001, P value for 5 year: 0.001). The corresponding AUC of OS were 0.745, 0.725, 0.710, 0.681 vs 0.705, 0.694, 0.682, 0.657, respectively (Supplementary Figure 2E–H, P value for 1 year: <0.001, P value for 2 year: <0.001, P value for 3 year: <0.001, P value for 5 year: <0.001). The time-AUC of RFS and OS for these two systems are shown in Supplementary Figure 2I and J. Calibration curve revealed that this MVI grading system had a better predictive accuracy between the actual probability and the predicted probability of RFS and OS than the MVI two-tiered system (Supplementary Figure 3A–P, RFS C-index: 0.634 vs 0.619, OS C-index: 0.663 vs 0.639). In addition, decision curve analysis was used to evaluate the clinical usefulness of this grading system. As shown in Supplementary Figure 4A and B, the MVI grading system provided superior net benefits when compared to those of the MVI two-tiered system both in RFS and OS.
Risk Factors of RFS, OS, and Early RFS
The results of univariate Cox regression analysis of the crude cohort are shown in Supplementary Tables 1–4. Multivariable analysis suggested that sex, prealbumin (PA), lactate dehydrogenase (LDH), lymphocyte (Lym), hepatitis B surface antigen, surgical margin, tumor size, tumor number, extrahepatic metastasis, and MVI grading system were independent risk factors for RFS (Figure 4A, C-index=0.707, 95% confidence interval (CI): 0.691–0.723). PA, gamma glutamyltransferase, prothrombin time, Lym, surgical margin, tumor size, tumor number, portal invasion, tumor capsule, and MVI grading system were independent risk factors for OS (Figure 4B, C-index=0.766, 95% CI: 0.746–0.786). PA, LDH, Lym, red blood cell, AFP, surgical margin, tumor size, tumor number, extrahepatic metastasis, tumor capsule, and MVI grading system were independent risk factors for early RFS (Figure 4C, C-index=0.722, 95% CI: 0.704–0.740). Age, sex, thrombocytocrit, HBV DNA, and tumor number were independent risk factors for late RFS (Figure 4D, C-index=0.632, 95% CI: 0.587–0.677). The time-AUC and decision curve revealed that all four models were more powerful in assessing clinical outcomes than the BCLC staging and TNM classification (Supplementary Figure 5A–H).
Predictive Factors of MVI
Univariate analysis of the predictive factors of MVI is provided in Supplementary Table 5. Multivariate analysis verified that platelet, AFP, hepatitis B e antigen, ultrasonic tumor boundary, tumor size, tumor number, portal invasion were independent risk factors for presence of MVI (Table 2). The ROC curve of this multivariate model is shown in Supplementary Figure 6, and the value of AUC was 0.743.
 |
Table 2 Multivariate Logistic Analysis for the Related Factors of MVI |
Discussion
MVI has been widely recognized as a crucial pathological parameter related to adverse prognosis of HCC patients and was considered as the biological basis for the high early recurrence rate after liver surgery.8,29 Although it was generally believed that MVI was the precursor lesion of portal vein tumor thrombosis and/or hepatic vein tumor thrombosis,30 some studies showed that absence of MVI also could represent better prognosis even for these advanced stage HCC patients.31,32 Our study also showed that both MVI and macrovascular invasion were independent risk factors of prognosis. Therefore, an exclusive scheme is justified for the HCC patients with MVI. Unlike macrovascular invasion, MVI can only be definitively diagnosed by pathologists based on surgical specimens, thus the clinical trial of neoadjuvant therapy or surgical options cannot be conducted based on MVI, but postoperative adjuvant therapy is more appropriate. Numerous prospective and retrospective studies on postoperative adjuvant therapy of HCC with MVI have achieved positive results.33,34 The results of the IMbrave 050 study released in 2023 showed atezolizumab plus bevacizumab could potentially fulfill the anti-relapse effect on HCC patients with a high risk of recurrence. MVI was one of the inclusion criteria and accounted for 60.8% of patients in the experimental group.35 Thus, MVI has become an important factor in considering additional intervention in HCC patients, and identification of MVI should become increasingly important in the regular pathologic evaluation of HCC. Nevertheless, with advocacy of wide margin resection and anatomical hepatectomy,36,37 antiviral therapy was able to achieve good clinical outcomes for many HCC patients after R0 resection who were found to have MVIs,38 especially for those who had a small amount and range of MVIs. Furthermore, some adjuvant procedures could not improve survival outcomes in some patients with MVI, and could cause obvious hepatic dysfunctions.39,40 It is therefore not enough to interpret the impact of MVI on survival outcomes and try to develop therapeutic strategies based merely on “presence” or “absence” of MVI. A convenient and practical risk assessment system of MVI should be designed to guide individual treatment intervention and surveillance of HCC recurrence.
In the past two decades, several teams have established different MVI grading systems and confirmed that a stratified evaluation of MVI could predict the prognosis of HCC patients more accurately. The MVI-related grading by Roayaie et al included MVI with invasion of a vessel with a muscular wall and invasion of a vessel that was more than 1 cm from the tumor.41 Fujita et al42 and Iguchi et al43 proposed that presence of multiple invaded portal venous vessels (≥2) and more than 50 invading carcinoma cells to be necessary to determine in the histologic evaluation of MVI for HCC patients after liver resection or liver transplantation. Sumie et al demonstrated that the MVI classification as defined by the quantity of MVI could stratify HCC patients with different recurrence patterns and survival risks after hepatic resection.44 Zhao et al showed the number of invaded microvessels (≤5 vs >5) and invading tumor cells (≤50 vs >50), as well as the distance of invasion from tumor edge (≤1 cm vs >1 cm) could classify the risk of MVI in predicting prognosis of HCC patients.45 The classification system established by Feng et al included the number of MVI and histological characteristics of MVI in accurately predicting prognosis of HCC patients.46 Although, all the above mentioned studies analyzed MVI grading from different aspects, they have several demerits in common: 1) they did not elaborate on how sampling of the tumors was done, and sampling quantity and sampling sites would undoubtedly have an impact on detection of MVI;47 2) some MVI-related parameters used were quite complex and might not be suitable for daily pathologic practice; 3) all the above studies were conducted based on patients coming from a single center, hence there are questions in generalizability of their results. Our study has the following merits over the previous reported studies. First, all cases in this study were sampled with the standardized seven-point baseline sampling method, thus supplying the cornerstone for comparisons among different groups and individuals. Second, the number and location of MVI chosen in this MVI grading system were easy to use by most pathologists and they have great repeatability. Third, bias was less and conclusions were more reliable in this multicenter study with a reasonably good sample size.
Based on our results, this MVI grading system could distinguish the prognosis of HCC patients after liver resection with curative intent, and helped to select patients with high risk of HCC recurrence to receive adjuvant therapy. A series of statistical methods further showed this MVI grading system to be better in predicting HCC recurrence and survival than the MVI two-tiered system. As patients with more severe MVI grading were more likely to have residual tumors in the preserved livers, this MVI grading system could fundamentally influence the decision-making in postoperative adjuvant therapy and it should be routinely used in pathological report of HCC.
As detected number and distance from tumor boundary of MVI were found to have adverse effects on clinical outcomes. It would be valuable to analyze the weights of these two indexes on survival outcomes, which can be done in the M2b groups of HCC patients who all had distal MVIs. The results showed that the number of MVI in the tumor-adjacent tissues (proximal MVIs) also indicated different prognosis for these patients. Patients in the M2b group with 0~5 proximal MVIs had a prognosis similar to the patients in the M2a group whilst those M2b patients with extensive proximal MVIs (>5) had the worst survival outcomes. These results imply that these two indexes have independent and different effects on survival outcomes, leading to our proposal on the hypothesis of two patterns for presence of distal MVI: 1) Pattern I: the push-out mode. Evolving tumor cells gradually invade into multifocal vascular walls and MVIs were abundantly formed at the proximal peritumoral area initially and then moved to invade distal tissues from the tumor. For such patients, MVIs are abundant and extensive so that it would be more suitable to use adjuvant radiotherapy or immunotherapy to destroy residual tumor cells at the cutting edge (Figure 5A). 2) Pattern II: the rush-out pattern. One cluster of tumor cells with a highly metastatic ability starts to break into a blood vessel within or near the tumor, thus directly leading to distal dissemination of MVI. This type of tumor is commonly associated with a small amount of MVIs in the proximal peritumoral area and it may be more appropriate to be treated with transvascular postoperative interventions, such as TACE or HAIC (Figure 5B). In brief, the number of proximal MVI for M2b patients should also be adequately evaluated.
In view of the excellent capacity of this MVI grading system to predict prognosis of HCC patients after liver resection with curative intent, we constructed four multivariate prognostic models, and the models of RFS, OS, and early RFS to be included this MVI grading system. Different statistical analyses confirmed that these multivariate models might possess better prognostic ability than the BCLC staging and TNM classification based on this study cohort. In addition, an MVI predictive model was established based on preoperative parameters and it was confirmed to have good predictive ability. The above models can be used to help clinicians in their decision-making for personalized management of HCC patients.48
With the rapid development of precision medicine and molecular pathology, the genomic characteristics of HCC were better understood from bench to bedside.49,50 The application of multi-omics technology makes it possible to panoramically analyze the occurrence and progression of HCC. There have been some studies to explore the molecular features of HCC tumors with MVI. The abnormal expression of biomarkers such as microRNAs,51,52 circular RNAs,53 long noncoding RNAs,54,55 and proteins56,57 in HCC was strongly correlated with the presence of MVI. Deregulation of these markers affected the proliferation, invasion, metastasis, epithelial–mesenchymal transition, and anti-apoptotic processes of HCC.58 The application of single-cell sequencing combined with spatial transcriptomics made it possible to observe the molecular characteristics of MVI tumor cells.59 We suppose that a standardized flow that includes precise prediction of MVI,60 suitable surgical treatment, comprehensive gross assessment,61 homogenized sampling, pathological grading of MVI, targeted molecular detection of MVI, and reasonable postoperative surveillance62 should be well established to benefit the HCC patients with MVI.
Our study has limitations. First, the number of HCC patients in the M2b group is relatively small. Second, although it is a multicenter study, the results still need to be further validated in prospective studies with larger sample sizes. Third, the vast majority of patients in this study have HBV infection so that further validation of the conclusions on HCC patients who have hepatitis C infection, nonalcoholic or alcoholic steatohepatitis is necessary. Fourth, there have been no clinical trials to explore the value of the MVI grading system for the treatment options of HCC patients, so this MVI grading system can only be used prudently for the prognosis assessment of HCC patients after liver resection with curative intent at the present stage.
Conclusion
In this study, a novel four-tiered MVI grading system was proposed focusing on two essential parameters: detected number and distance from tumor boundary of MVI. This system could precisely predict the surgical outcomes of HCC patients after liver resection with curative intent, and it could practically and easily be incorporated into pathological reports on resected HCC specimens. We proposed that there might be two distinct occurrence patterns of distal MVI (>1 cm from tumor boundary) and these two patterns represented dissimilar prognoses leading to different adjuvant therapies which should be used. The severity of proximal and distal MVI should be adequately mentioned in pathologic reports.
Abbreviations
MVI, microvascular invasion; HCC, hepatocellular carcinoma; RFS, recurrence-free survival; OS, overall survival; TACE, transcatheter arterial chemoembolization; HAIC, hepatic arterial infusion chemotherapy; BCLC, Barcelona Clinic Liver Cancer; TNM, Tumor-Node-Metastasis; AFP, alpha-fetoprotein; HBV DNA, hepatitis B virus deoxyribonucleic acid; time-ROC, time-receiver operating characteristic; time-AUC, time-area under the curve; PA, prealbumin; LDH, lactate dehydrogenase; Lym, lymphocyte; CI, confidence interval.
Data Sharing Statement
All the data submitted is owned solely by us. The data that support the findings of this study are available from the corresponding author [Cong WM and Dong H], upon reasonable request.
Ethical Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (the institutional ethics board of Eastern Hepatobiliary Surgery Hospital, NO. EHBHKY2015-02-001) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Funding
This work was supported by the Shanghai Science and Technology Innovation Action Plan-Medical Innovation Research Project (No. 22Y11909100).
Disclosure
All authors declare that they have no conflict of interest.
References
1. Torbenson M, Ng I, Park Y, Roncalli M, Sakamato M. Hepatocellular carcinoma. In: WHO Classification of Tumours, Digestive System Tumours. Lyon: IARC Press; 2019:229–239.
2. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/s0140-6736(20)30925-9
3. Toh MR, Wong EYT, Wong SH, et al. Global epidemiology and genetics of hepatocellular carcinoma. Gastroenterology. 2023;164(5):766–782. doi:10.1053/j.gastro.2023.01.033
4. Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243(2):229–235. doi:10.1097/01.sla.0000197706.21803.a1
5. Wang H, Wu MC, Cong WM. Microvascular invasion predicts a poor prognosis of solitary hepatocellular carcinoma up to 2 cm based on propensity score matching analysis. Hepatol Res. 2019;49(3):344–354. doi:10.1111/hepr.13241
6. Wang H, Feng LH, Qian YW, Cao ZY, Wu MC, Cong WM. Does microvascular invasion in Barcelona clinic liver cancer stage A multinodular hepatocellular carcinoma indicate early-stage behavior? Ann Transl Med. 2019;7(18):428. doi:10.21037/atm.2019.08.114
7. Wang H, Qian YW, Wu MC, Cong WM. Liver resection is justified in patients with BCLC intermediate stage hepatocellular carcinoma without microvascular invasion. J Gastrointest Surg. 2020;24(12):2737–2747. doi:10.1007/s11605-019-04251-8
8. Erstad DJ, Tanabe KK. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann Surg Oncol. 2019;26(5):1474–1493. doi:10.1245/s10434-019-07227-9
9. Wang Z, Ren Z, Chen Y, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. 2018;24(9):2074–2081. doi:10.1158/1078-0432.Ccr-17-2899
10. Li SH, Mei J, Cheng Y, et al. Postoperative adjuvant hepatic arterial infusion chemotherapy with FOLFOX in hepatocellular carcinoma with microvascular invasion: a multicenter, phase III, randomized study. J Clin Oncol. 2023;41(10):1898–1908. doi:10.1200/jco.22.01142
11. Shi C, Li Y, Geng L, et al. Adjuvant stereotactic body radiotherapy after marginal resection for hepatocellular carcinoma with microvascular invasion: a randomised controlled trial. Eur J Cancer. 2022;166:176–184. doi:10.1016/j.ejca.2022.02.012
12. Hack SP, Spahn J, Chen M, et al. IMbrave 050: a Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020;16(15):975–989. doi:10.2217/fon-2020-0162
13. Sahin IH, Khalil L, Millett R, Kaseb A. Neoadjuvant and adjuvant treatment approaches for hepatocellular carcinoma: future outlook. Chin Clin Oncol. 2021;10(1):7. doi:10.21037/cco-20-248
14. Xu XF, Diao YK, Zeng YY, et al. Association of severity in the grading of microvascular invasion with long-term oncological prognosis after liver resection for early-stage hepatocellular carcinoma: a multicenter retrospective cohort study from a hepatitis B virus-endemic area. Int J Surg. 2023;109(4):841–849. doi:10.1097/js9.0000000000000325
15. Chen ZH, Zhang XP, Wang H, et al. Effect of microvascular invasion on the postoperative long-term prognosis of solitary small HCC: a systematic review and meta-analysis. HPB. 2019;21(8):935–944. doi:10.1016/j.hpb.2019.02.003
16. Cong WM, Bu H, Chen J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22(42):9279–9287. doi:10.3748/wjg.v22.i42.9279
17. Sheng X, Ji Y, Ren GP, et al. A standardized pathological proposal for evaluating microvascular invasion of hepatocellular carcinoma: a multicenter study by LCPGC. Hepatol Int. 2020;14(6):1034–1047. doi:10.1007/s12072-020-10111-4
18. Cheng S, Chen M, Cai J. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus: 2016 edition. Oncotarget. 2017;8(5):8867–8876. doi:10.18632/oncotarget.12817
19. Chua TC, Morris DL. Exploring the role of resection of extrahepatic metastases from hepatocellular carcinoma. Surg Oncol. 2012;21(2):95–101. doi:10.1016/j.suronc.2011.01.005
20. Zhong X, Peng J, Xie Y, et al. A nomogram based on multi-modal ultrasound for prediction of microvascular invasion and recurrence of hepatocellular carcinoma. Eur J Radiol. 2022;151:110281. doi:10.1016/j.ejrad.2022.110281
21. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503. doi:10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e
22. Pang YY, Belghiti J, Clavien P-A. The Brisbane 2000 terminology of liver anatomy and resections. HPB. 2000;2:333–339. doi:10.1080/136518202760378489
23. Wakabayashi G, Cherqui D, Geller DA, et al. The Tokyo 2020 terminology of liver anatomy and resections: updates of the Brisbane 2000 system. J Hepatobiliary Pancreat Sci. 2022;29(1):6–15. doi:10.1002/jhbp.1091
24. Chun YS, Pawlik TM, Vauthey JN. Edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 2018;25(4):845–847. doi:10.1245/s10434-017-6025-x
25. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
26. Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38(2):200–207. doi:10.1016/s0168-8278(02)00360-4
27. Nitta H, Allard MA, Sebagh M, et al. Prognostic value and prediction of extratumoral microvascular invasion for hepatocellular carcinoma. Ann Surg Oncol. 2019;26(8):2568–2576. doi:10.1245/s10434-019-07365-0
28. Heinze G, Wallisch C, Dunkler D. Variable selection - A review and recommendations for the practicing statistician. Biom J. 2018;60(3):431–449. doi:10.1002/bimj.201700067
29. Zhang X, Li J, Shen F, Lau WY. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2018;33(2):347–354. doi:10.1111/jgh.13843
30. Isik B, Gonultas F, Sahin T, Yilmaz S. Microvascular venous invasion in hepatocellular carcinoma: why do recurrences occur? J Gastrointest Cancer. 2020;51(4):1133–1136. doi:10.1007/s12029-020-00487-9
31. Kim SH, Moon DB, Park YH, et al. Favorable prognostic factors for survival outcomes of hepatocellular carcinoma with portal vein tumor thrombosis after hepatectomy. Ann Surg Oncol. 2023;30(7):4279–4289. doi:10.1245/s10434-023-13316-7
32. Pan Y, Yang L, Cao Y, et al. Factors influencing the prognosis patients with Barcelona clinic liver cancer stage C hepatocellular carcinoma undergoing salvage surgery after conversion therapy. Transl Cancer Res. 2023;12(7):1852–1862. doi:10.21037/tcr-23-70
33. Li J, Yang F, Li J, Huang ZY, Cheng Q, Zhang EL. Postoperative adjuvant therapy for hepatocellular carcinoma with microvascular invasion. World J Gastrointest Surg. 2023;15(1):19–31. doi:10.4240/wjgs.v15.i1.19
34. Feng X, Feng GY, Tao J, et al. Comparison of different adjuvant therapy regimen efficacies in patients with high risk of recurrence after radical resection of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2023;149(12):10505–10518. doi:10.1007/s00432-023-04874-0
35. Qin S, Chen M, Cheng AL, et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, Phase 3 trial. Lancet. 2023;402(10415):1835–1847. doi:10.1016/s0140-6736(23)01796-8
36. Zhang XP, Xu S, Lin ZY, et al. Significance of anatomical resection and resection margin status in patients with HBV-related hepatocellular carcinoma and microvascular invasion: a multicenter propensity score-matched study. Int J Surg. 2023;109(4):679–688. doi:10.1097/js9.0000000000000204
37. Liu J, Zhuang G, Bai S, et al. The comparison of surgical margins and type of hepatic resection for hepatocellular carcinoma with microvascular invasion. Oncologist. 2023. doi:10.1093/oncolo/oyad124
38. Kong J, Liang X, Zhang J, Zeng J, Liu J, Zeng J. Antiviral therapy improves survival in hepatocellular carcinoma with microvascular invasion: a propensity score analysis. Dig Dis Sci. 2022;67(8):4250–4257. doi:10.1007/s10620-021-07248-z
39. Feng LH, Zhu YY, Zhou JM, et al. Adjuvant TACE may not improve recurrence-free or overall survival in HCC patients with low risk of recurrence after hepatectomy. Front Oncol. 2023;13:1104492. doi:10.3389/fonc.2023.1104492
40. Liu S, Li H, Guo L, et al. Tumor size affects efficacy of adjuvant transarterial chemoembolization in patients with hepatocellular carcinoma and microvascular invasion. Oncologist. 2019;24(4):513–520. doi:10.1634/theoncologist.2018-0305
41. Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137(3):850–855. doi:10.1053/j.gastro.2009.06.003
42. Fujita N, Aishima S, Iguchi T, et al. Histologic classification of microscopic portal venous invasion to predict prognosis in hepatocellular carcinoma. Hum Pathol. 2011;42(10):1531–1538. doi:10.1016/j.humpath.2010.12.016
43. Iguchi T, Shirabe K, Aishima S, et al. New pathologic stratification of microvascular invasion in hepatocellular carcinoma: predicting prognosis after living-donor liver transplantation. Transplantation. 2015;99(6):1236–1242. doi:10.1097/tp.0000000000000489
44. Sumie S, Nakashima O, Okuda K, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21(3):1002–1009. doi:10.1245/s10434-013-3376-9
45. Zhao H, Chen C, Fu X, et al. Prognostic value of a novel risk classification of microvascular invasion in patients with hepatocellular carcinoma after resection. Oncotarget. 2017;8(3):5474–5486. doi:10.18632/oncotarget.12547
46. Feng LH, Dong H, Lau WY, et al. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2017;143(2):293–303. doi:10.1007/s00432-016-2286-1
47. Liao B, Liu L, Wei L, et al. Innovative synoptic reporting with seven-point sampling protocol to improve detection rate of microvascular invasion in hepatocellular carcinoma. Front Oncol. 2021;11:726239. doi:10.3389/fonc.2021.726239
48. Zhang EL, Cheng Q, Huang ZY, Dong W. Revisiting surgical strategies for hepatocellular carcinoma with microvascular invasion. Front Oncol. 2021;11:691354. doi:10.3389/fonc.2021.691354
49. Gao Q, Zhu H, Dong L, et al. Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma. Cell. 2019;179(2):561–577.e522. doi:10.1016/j.cell.2019.08.052
50. Amaddeo G, Cao Q, Ladeiro Y, et al. Integration of tumour and viral genomic characterizations in HBV-related hepatocellular carcinomas. Gut. 2015;64(5):820–829. doi:10.1136/gutjnl-2013-306228
51. Shi Y, Song Q, Yu S, Hu D, Zhuang X. Microvascular invasion in hepatocellular carcinoma overexpression promotes cell proliferation and inhibits cell apoptosis of hepatocellular carcinoma via inhibiting miR-199a expression. Onco Targets Ther. 2015;8:2303–2310. doi:10.2147/ott.S86807
52. Liu M, Wang L, Zhu H, et al. A preoperative measurement of serum microRNA-125b may predict the presence of microvascular invasion in hepatocellular carcinomas patients. Transl Oncol. 2016;9(3):167–172. doi:10.1016/j.tranon.2016.03.002
53. Xu L, Zhang M, Zheng X, Yi P, Lan C, Xu M. The circular RNA ciRS-7 (Cdr1as) acts as a risk factor of hepatic microvascular invasion in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2017;143(1):17–27. doi:10.1007/s00432-016-2256-7
54. Yuan SX, Yang F, Yang Y, et al. Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients’ poor recurrence-free survival after hepatectomy. Hepatology. 2012;56(6):2231–2241. doi:10.1002/hep.25895
55. Lu J, Li B, Xiong X, Cheng N. RNA sequencing reveals the long noncoding RNA and mRNA profiles and identifies long non-coding RNA TSPAN12 as a potential microvascular invasion-related biomarker in hepatocellular carcinoma. Biomed Pharmacother. 2020;126:110111. doi:10.1016/j.biopha.2020.110111
56. Cai Y, Fu Y, Liu C, et al. Stathmin 1 is a biomarker for diagnosis of microvascular invasion to predict prognosis of early hepatocellular carcinoma. Cell Death Dis. 2022;13(2):176. doi:10.1038/s41419-022-04625-y
57. Qi LN, Ma L, Wu FX, et al. S100P as a novel biomarker of microvascular invasion and portal vein tumor thrombus in hepatocellular carcinoma. Hepatol Int. 2021;15(1):114–126. doi:10.1007/s12072-020-10130-1
58. Zhao X, Wang Y, Xia H, et al. Roles and molecular mechanisms of biomarkers in hepatocellular carcinoma with microvascular invasion: a review. J Clin Transl Hepatol. 2023;11(5):1170–1183. doi:10.14218/jcth.2022.00013s
59. Ke L, Rui Z, Fukai W, et al. Single-cell dissection of the multicellular ecosystem and molecular features underlying microvascular invasion in hepatocellular carcinoma. Hepatology. 2023. doi:10.1097/hep.0000000000000673
60. Summers RM. Radiomics to predict microvascular invasion in hepatocellular carcinoma: a promising biomarker for tumor recurrence. Radiology. 2023;307(4):e230657. doi:10.1148/radiol.230657
61. Fan Z, Jin M, Zhang L, et al. From clinical variables to multiomics analysis: a margin morphology-based gross classification system for hepatocellular carcinoma stratification. Gut. 2023;72(11):2149–2163. doi:10.1136/gutjnl-2023-330461
62. Pelizzaro F, Trevisani F, Simeon V, et al. Predictors of non-transplantable recurrence in hepatocellular carcinoma patients treated with frontline liver resection. Liver Int. 2023;43(12):2762–2775. doi:10.1111/liv.15719
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


