Back to Journals » International Journal of Women's Health » Volume 16
A Case Report of Meigs’ Syndrome Caused by Ovarian Fibrothecoma with High Levels of CA125
Authors Yuan L , Cui L, Wang J, Gong L
Received 1 December 2023
Accepted for publication 12 March 2024
Published 23 March 2024 Volume 2024:16 Pages 519—525
DOI https://doi.org/10.2147/IJWH.S450833
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Marleen van Gelder
Liqin Yuan, Lin Cui, Jie Wang, Li Gong
Department of Gynecology, Rizhao People’s Hospital, Rizhao, Shandong Province, 276800, People’s Republic of China
Correspondence: Li Gong, Rizhao People’s Hospital, Rizhao, Shandong Province, 276800, People’s Republic of China, Tel +86-18663351821, Email [email protected]
Purpose: Meigs’ syndrome is a rare gynecological disease characterized by the triad of benign ovarian tumor, ascites, and pleural effusion. Ovarian malignancies should be highly suspected in a postmenopausal woman with a pelvic mass, ascites, hydrothorax, and an elevated carbohydrate antigen 125 (CA125) level. It can be challenging to make a preoperative diagnosis of Meigs’ syndrome. In this report, we present a case of Meigs’ syndrome caused by an ovarian fibrothecoma and review the relevant literature to raise awareness and avoid misdiagnosis.
Case Presentation: An 82-year-old woman with a 2-week history of abdominal distension was admitted to the Department of Gynecology. Ultrasound and thoracoabdominal computed tomography scans showed a left-sided hypoechoic mass in the pelvic cavity with bilateral pleural effusion and massive ascites. The CA125 concentration was 1040 U/mL (normal, 0– 35 U/mL). With a working diagnosis of ovarian malignancy, the patient underwent ultrasound-guided fine-needle puncture of the pelvic mass and paracentesis to drain the ascites. The fine-needle puncture and paracentesis fluid analysis results revealed that the ascites did not contain any tumor cells, and the pelvic mass was identified as a spindle cell tumor. Immunohistochemistry confirmed that it was a sex-cord stromal tumor. Total abdominal hysterectomy and bilateral adnexectomy were performed under general anesthesia. The pathology results confirmed the mass to have been an ovarian fibrothecoma. At the 2-month postoperative follow-up, the ascites and hydrothorax had resolved and not recurred, and the CA125 level was normal.
Conclusion: Despite the high suspicion of ovarian carcinoma in postmenopausal women presenting with pelvic mass, ascites, pleural effusion, and elevated CA125, Meigs’ syndrome should be considered.
Keywords: Meigs’ syndrome, ascites, pleural effusion, CA125, ovarian fibrothecoma, ovarian cancer
Introduction
Meigs’ syndrome is the triad of benign ovarian tumor, ascites, and pleural effusion, which gradually resolve after surgical resection of the tumor.1 It is more common in postmenopausal women around the age of 50 years and is rarely associated with high levels of carbohydrate antigen 125 (CA125).2 Although Meigs’ syndrome is a benign disease, it mimics malignant conditions and is easily misdiagnosed; confirmation is often necessary after surgery. Here, we report a case of Meigs’ syndrome in a postmenopausal woman who presented with a pelvic mass, ascites, pleural effusion, and an elevated CA125 level of 1040 U/mL.
Case Presentation
Chief Complaints
On March 8, 2023, an 82-year-old postmenopausal woman was admitted to our Department of Gynecology with abdominal distention. She had no other physical complaints.
History of Present Illness
The patient’s symptoms started about 2 weeks before admission.
History of Past Illness
The patient’s past medical history included tonsillectomy and minilaparotomy tubal ligation about 40 years before this presentation.
Personal and Family History
There was no relevant family history of the disease.
Physical Examination
General examination of the patient revealed a body temperature of 36.1°C, pulse rate of 100 beats per minute, respiratory rate of 20 cycles per minute, and a blood pressure of 159/96 mmHg. Auscultation revealed slightly diminished breath sounds in both lungs, which were dull to percussion. The cervix was atrophic; neither the uterus nor the adnexae were palpable.
Laboratory Examinations
The patient’s hematology, urinalysis, and biochemistry results were normal. The serum CA125 concentration was 1040 U/mL (normal, 0–35 U/mL); the results of carcinoembryonic antigen, alpha-fetoprotein, CA153, CA199, and HE4 were unremarkable. A drainage tube was placed in the left pelvic cavity, and a total of 3650 mL of ascites was withdrawn. The ascites was yellow and transparent, with no blood detected and a white blood cell count of 180 × 106/L (95% lymphocytes and 5% neutrophilic granulocytes). The biochemical analysis of the ascites revealed the following findings: total proteins 41.2 g/L, alkaline phosphatase 23.0 U/L, sialic acid 34.9 mg/dL, lactate dehydrogenase 95.0 U/L, adenosine deaminase 4.0 U/L, and amylase 35 U/L.
Imaging Examinations
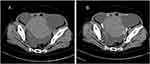
Combined transvaginal and transabdominal ultrasonography showed massive pelvic ascites and a large irregular mass (13.1 cm × 8.2 cm) with hypoechoic features on the left side of the pelvic cavity (Figure 1A and B). Chest computed tomography (CT) confirmed the presence of bilateral pleural effusions (Figure 2). Abdominal CT revealed a pelvic soft tissue mass (10.9 × 8.2 × 14.3 cm) with clear boundaries, heterogeneous density, no obvious enhancement, and an artery originating from the left ovarian artery (Figure 3A and B).
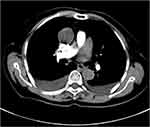 |
Figure 2 Chest computed tomography showing pleural fluid in both lungs. |
Further Diagnostic Examination
The patient underwent pelvic mass fine-needle puncture and drainage of ascites guided by abdominal ultrasound; cytologic analysis of the fluid samples from these procedures found no tumor cells present in the ascites and identified the pelvic mass as a spindle-cell, sex-cord stromal tumor. Immunohistochemical analysis revealed that vimentin, α-Inhibin, CD56, CR, and WT-1 were all positive; SF-1, Melan-A, CK, S-100, CD34, P53, and SMA were all negative; and the positivity rate of Ki-67 was approximately 3%.
Differential Diagnosis
A postmenopausal woman with a pelvic mass, ascites, pleural effusion, and a high level of CA125 could easily be misdiagnosed as having an ovarian malignancy. The differential diagnoses for our patient included ovarian cancer, ovarian fibroma, and ovarian thecoma. Clinicians should have a high index of suspicion of malignancy, but they should be fully aware of the range of differential diagnoses and assess adequately before operating. To further clarify the diagnosis, in view of a comprehensive preoperative evaluation, our patient’s diagnosis had to be confirmed histopathologically. Furthermore, coronary angiography revealed multiple coronary artery lesions with varying degrees of stenosis, as well as mixed plaques in the proximal tube wall of the left anterior descending branch with moderate to severe luminal stenosis. So, we invited cardiologist to assist us in the preoperative evaluation. After the exclusion of absolute surgical contraindications, the patient was scheduled for an exploratory laparotomy.
Treatment
Regardless of whether the patient’s pelvic mass is benign or malignant, surgical removal of the pelvic mass is the mainstay of treatment. Therefore, an exploratory laparotomy—including total abdominal hysterectomy, bilateral adnexectomy, and pelvic drainage—was performed under general anesthesia. The surgical findings were as follows: The volume of faint yellow ascites was approximately 200 mL; the left ovary was enlarged to approximately 13 cm × 11 cm with an irregular surface, hard texture, and complete capsule; the left fallopian tube, the right adnexa, and the uterus did have any obvious morphological abnormalities. There were no other obvious findings in the comprehensive surgical exploration. Histopathologic examination of the intraoperative frozen tissue section of the suspected left adnexal ovarian fibrothecoma later confirmed a final diagnosis of left ovarian fibrothecoma (Figure 4).
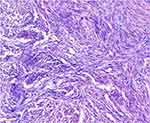 |
Figure 4 Histopathologic photomicrograph showing fibro cells and theca cells (hematoxylin and eosin staining, ×200). |
Outcome and Follow-Up
The patient recovered well postoperatively and was discharged on postoperative day 7. Outpatient follow-up evaluation 2 months after the surgery included chest CT revealing that the pleural effusion had resolved (Figure 5). The ascites had also resolved, the CA125 level returned to normal, and the full workup and physical examination detected no obvious abnormalities.
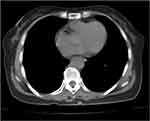 |
Figure 5 A follow-up chest computed tomography image 2 months after surgery showing resolution of the bilateral pleural effusion. |
Discussion
Fibrothecomas are rare ovarian tumors that originate from sex-cord stromal cells.3 They are usually benign, and their incidence among all ovarian tumors is estimated to be 1–4%.4 Fibrothecomas are more common in perimenopausal and postmenopausal women. According to the number of follicular membrane cells and fibroblasts, these tumors are histopathologically divided into three subtypes, including thecomas, fibrothecomas, and fibroma.5 The histologic features of these tumors overlap, making precise classification difficult. The World Health Organization’s classification denotes these tumors as the thecoma-fibroma group.6 Ovarian fibrothecomas can be accompanied by hydrothorax and ascites; this is known as Meigs’ syndrome. It was first reported by Meigs and Cass in 1937, who reported seven patients presenting with the triad of ascites, pleural effusion, and fibroma of the ovary.7 In 1954, Meigs eventually refined the syndrome’s definition; four criteria should be met to diagnose classic Meigs’ syndrome: (1) the presence of a benign ovarian tumor (fibroma, thecoma, granulosa cell tumor, or Brenner tumor), (2) ascites, (3) pleural effusion, and (4) resolution of ascites and pleural effusion after removal of the tumor.1 Meigs’ syndrome stands in contrast to pseudo-Meigs’ syndrome, which also presents similar clinical symptoms but with ascites, pleural effusion, and ovarian or primary pelvic tumors other than fibromas, such as teratomas, struma ovarii, and leiomyomas.8,9 However, in Meigs’ syndrome, ascites and pleural effusion should resolve after tumor removal.
The mechanism underlying the production of ascites and pleural effusion in Meigs’ syndrome remains unclear. The main causes of ascites are as follows: tumor penetration itself; pressure in pelvic and abdominal lymphatic vessels caused by the tumor; the increase in vascular permeability and capillary leakage caused by the release of VEGF, IL-1, IL-6, IL-8, and TNF-α.10,11 Studies have shown high concentrations of VEGF, IL-6, and FGF in plasma, pleural fluid, and ascitic fluid samples from patients with Meigs’ syndrome and reported that after the removal of pelvic masses, the plasma levels of VEGF, IL-6, and FGF decreased, and the ascites and hydrothorax disappeared.12 Other studies have suggested that the VEGF signal pathways could leaded to increased proliferation, migration, and survial of blood endothelial cells required for angiogenesis, and the VEGF was overexpressed in the leiomyoma and leaded to tumor growth.13 The VEGF raises capillary permeability, contributing to formation of peritoneal and pleural fluid. The generation of hydrothorax may arise from the passage of ascitic fluid into the pleural space through diaphragmatic lymphatic vessels or the diaphragmatic hiatus.14 Therefore, after removal of the primary tumor, the pressure of the tumor disappears, and compressed or blocked lymphatic vessels return to normal, thereby clearing ascites and pleural effusion. In our patient, the symptoms were typical, the ascites resolved, and little pleural fluid remained at the 2-month postoperative follow-up visit.
Notably, the patient had a significant increase in her serum CA125 level. CA125 is a glycoprotein with a high molecular weight. It is the preferred tumor marker for ovarian cancer but is not a specific diagnostic indicator; it is also expressed in many other clinical tissues, such as the epithelium of the Fallopian tubes, endometrium, endocervix, and breast cancer.15 Additionally, CA125 is significantly elevated in patients with pseudo-Meigs’ syndrome.16,17 Therefore, CA125 results cannot be used as the basis for the diagnosis of Meigs’ syndrome. Meigs’ syndrome associated with a high CA125 level is rare. Over the past of 20 years, less than 40 case reports have been published citing high levels CA125 in patients with Meigs’ syndrome, with only 9 reported cases citing CA125 levels over 1000IU/mL. The source of CA125 elevation remains unclear. Studies have suggested that the main causes of elevated CA125 are as follows:18 intraperitoneal pressure from a large tumor volume or ascites, mechanical irritation of the peritoneum, and increased expression of CA125 in mesothelial cells (rather than being directly related to the mass). In menopausal women, elevated CA125 with a pelvic mass, ascites, and hydrothorax is highly suggestive of a malignant ovarian tumor. Therefore, Meigs’ syndrome is easily misdiagnosed because it is rare in clinical practice and lacks a specific diagnostic marker and typical clinical symptoms. Except for metastatic tumors of the ovary, other diseases—such as Meigs’ syndrome, tuberculous peritonitis, and pelvic abscess—should be considered in the differential diagnosis. Intraoperative and postoperative pathological examination is the best diagnostic method for Meigs’ syndrome. The diagnosis of Meigs’ syndrome can only be confirmed when histopathologic examination suggests a solid benign ovarian tumor and resolved ascites or hydrothorax does not recur following surgical tumor removal.
Although Meigs’ syndrome manifests with a similar clinical presentation as malignant tumors, it is a benign disease. The treatment of Meigs’ syndrome can be divided into symptomatic treatment and surgical treatment. Symptomatic treatment involves therapeutic drainage of ascites and hydrothorax to relieve symptoms. However, surgical treatment is the mainstay of treatment owing to its favorable effects and associated low recurrence rates. For young women who have given birth, ipsilateral adnexectomy or tumor removal is the primary treatment method, and for postmenopausal women, hysterectomy plus bilateral adnexectomy is the main strategy.11 After resection of the tumor, ascites and hydrothorax will resolve and not recur.
Conclusion
We excised the primary lesion in a timely manner during the exploratory laparotomy. The patient’s ascites and hydrothorax resolved, leading to rapid recovery and a good prognosis. Clinicians should consider the rare Meigs’ syndrome in all women with pelvic masses, ascites, and hydrothorax, especially in the context of high CA125 levels. An enhanced understanding of this benign disease is necessary to avoid misdiagnosis and facilitate the implementation of effective measures to improve prognosis.
Abbreviations
CA125, carbohydrate antigen 125; CT, computed tomography.
Ethical Statement
All procedures of the research conformed to the Declaration of Helsinki and the Institutional and/or National Research Council ethical standards. Written informed consent was obtained from the patient for publication of this case report and the accompanying images. A copy of the written consent is available upon request.
Ethics Approval and Informed Consent
Writing and publishing this case report was approved by Rizhao People’s Hospital.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and the accompanying images. A copy of the written consent is available upon request.
Acknowledgments
The authors would like to express gratitude to the doctors in the Imaging Department and Pathology Department of Rizhao People’s Hospital for their assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This case report involved no source of funding for any of the authors.
Disclosure
The author(s) report no conflicts of interest in this work.
References
1. Meigs JV. Fibroma of the ovary with ascites and hydrothorax; Meigs’ syndrome. Am J Clin Exp Obstet Gynecol. 1954;67(5):962–985. doi:10.1016/0002-9378(54)90258-6
2. Stabile G, Zinicola G, Romano F, Laganà AS, Pozzolo CD, Ricci G. Pelvic mass, ascites, hydrothorax: a malignant or benign condition? Meigs syndrome with high levels of CA 125. Przeglad menopauzalny. 2021;20(2):103–107. doi:10.5114/pm.2021.106100
3. Chen J, Gu H, Zhang Y, et al. MRI-based nomogram for differentiation of ovarian fibrothecoma and broad ligament myoma. Sci Rep. 2022;12(1):8122. doi:10.1038/s41598-022-12218-0
4. Iyer R, Chow J, El-Bahrawy M, Savage P. Meigs syndrome presenting with axillary vein thrombosis and lymphadenopathy: a case report. J Med Case Rep. 2013;7(1):182. doi:10.1186/1752-1947-7-182
5. Zhang Z, Wu Y, Gao J. CT diagnosis in the thecoma-fibroma group of the ovarian stromal tumors. Cell Biochem Biophys. 2015;71(2):937–943. doi:10.1007/s12013-014-0288-7
6. Danilos J, Michał Kwaśniewski W, Mazurek D, Bednarek W, Kotarski J. Meigs’ syndrome with elevated CA-125 and HE-4: a case of luteinized fibrothecoma. Przeglad Menopauzalny. 2015;14(2):152–154. doi:10.5114/pm.2015.52157
7. Hou YY, Peng L, Zhou M. Meigs syndrome with pleural effusion as initial manifestation: a case report. World J Clin Cases. 2021;9(21):5972–5979. doi:10.12998/wjcc.v9.i21.5972
8. Nguyen P, Yazdanpanah O, Schumaker B. Meigs’ versus pseudo-meigs’ syndrome: a case of pleural effusion, ascites, and ovarian mass. Cureus. 2020;12(8):e9704. doi:10.7759/cureus.9704
9. Navarro-Esteva J, Laseca-Modrago M, Arencibia-Sánchez O. Two patients with meigs’ syndrome and elevated serum CA-125: a case report. Cureus. 2020;12(6):e8927. doi:10.7759/cureus.8927
10. Wu XJ, Xia HB, Jia BL, et al. Meigs’ syndrome caused by granulosa cell tumor accompanied with intrathoracic lesions: a case report. World J Clin Cases. 2021;9(18):4734–4740. doi:10.12998/wjcc.v9.i18.4734
11. Miyoshi A, Miyatake T, Hara T, et al. Etiology of ascites and pleural effusion associated with ovarian tumors: literature review and case reports of three ovarian tumors presenting with massive ascites, but without peritoneal dissemination. Case Pep Obstetr Gynecol. 2015;2015:414019. doi:10.1155/2015/414019
12. Abramov Y, Anteby SO, Fasouliotis SJ, et al. Markedly elevated levels of vascular endothelial growth factor, fibroblast growth factor, and interleukin 6 in Meigs syndrome. Am J Obstet Gynecol. 2001;184(3):3. doi:10.1067/mob.2001.110028
13. Ura B, Monasta L, Arrigoni G, et al. Phosphoproteins involved in the inhibition of apoptosis and in cell survival in the leiomyoma. J Clin Med. 2019;8(5):5. doi:10.3390/jcm8050691
14. Palmieri A, ElSahwi K, Hicks V. Meigs syndrome presenting with severely elevated CA-125 level. BMJ Case Rep. 2021;14(3):3. doi:10.1136/bcr-2020-238931
15. Miyawaki E, Naito T, Kasamatsu Y. Pseudo-Meigs’s syndrome. BMJ Case Rep. 2021;14(2):2. doi:10.1136/bcr-2020-241337
16. Abe T, Saida T, Fujieda K, Inoue K, Satoh T, Nakajima T. A case of Pseudo-Meigs’ syndrome due to Brenner tumor. Radiol Case Rep. 2023;18(3):1349–1352. doi:10.1016/j.radcr.2023.01.003
17. Liou JH, Su TC, Hsu JC. Meigs’ syndrome with elevated serum cancer antigen 125 levels in a case of ovarian sclerosing stromal tumor. Taiwanese J Obstetrics Gynecol. 2011;50(2):196–200. doi:10.1016/j.tjog.2011.01.011
18. Shen Y, Liang Y, Cheng X, Lu W, Xie X, Wan X. Ovarian fibroma/fibrothecoma with elevated serum CA125 level: a cohort of 66 cases. Medicine. 2018;97(34):e11926. doi:10.1097/MD.0000000000011926
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.