Back to Journals » Clinical and Experimental Gastroenterology » Volume 9
Fecal calprotectin: a marker for clinical differentiation of microscopic colitis and irritable bowel syndrome
Authors von Arnim U, Wex T, Ganzert C, Schulz C, Malfertheiner P
Received 6 October 2015
Accepted for publication 14 December 2015
Published 21 April 2016 Volume 2016:9 Pages 97—103
DOI https://doi.org/10.2147/CEG.S97701
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Andreas M. Kaiser
Ulrike von Arnim, Thomas Wex, Christine Ganzert, Christian Schulz, Peter Malfertheiner
Department of Gastroenterology, Hepatology and Infectious Diseases, Otto-von-Guericke University, Magdeburg, Germany
Background: The aim of this study is to compare two methods for measuring fecal calprotectin (FC) concentration and to evaluate the possibility of differentiation between microscopic colitis (MC) and irritable bowel syndrome (IBS).
Methods: Twenty-three patients with MC (six patients with active disease and 17 patients retested in remission) and 20 patients with IBS were prospectively included in this study. Active disease state of MC was determined by clinical symptoms of >3 bowel movements per day and histological correlate. All patients underwent ileocolonoscopy, including segmental biopsy samples for histology. FC levels in stool samples were analyzed using a rapid test system (Quantum Blue®) and an enzyme-linked immunosorbent assay (ELISA).
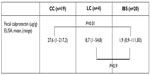
Results: FC levels were significantly higher in patients with active MC (median 48 µg/g [23–106]) compared to patients with IBS (median 2 µg/g [1–111.83]), P=0.0001 using an ELISA. FC level of patients with MC in remission was 22 µg/g (1–106.4), which is similar to those identified in patients with IBS. The difference of FC levels between active MC and IBS was not detected by the FC rapid test (P=0.635).
Discussion: FC levels might serve as parameter for differentiation between patients with active MC and IBS. Since there is no surrogate marker available at present for MC, FC appears to be a candidate for differentiating MC from IBS.
Conclusion: High FC levels, which were analyzed by ELISA, are a potential marker for patients with active MC compared to those with IBS. The FC rapid test was less suitable for this purpose.
Keywords: microscopic colitis, fecal calprotectin, irritable bowel syndrome, IBS, diarrhea, chronic diarrhea
Introduction
Chronic or intermittent watery, nonbloody diarrhea is the leading symptom of microscopic colitis (MC) but allows no discrimination from diarrhea-predominant irritable bowel syndrome (IBS) according to the Rome III criteria. IBS is a chronic functional disorder of the gastrointestinal tract dominated by abdominal discomfort and altered bowel habit1 in the absence of structural or anatomic disorders. Pathophysiology is not well understood, although several mechanisms such as visceral hypersensitivity, bacterial overgrowth, variation in fecal microbiota, and minimal mucosal inflammation have been proposed.2–5 MC represents an inflammatory bowel disease of still unknown causes/mechanisms and its diagnosis is based on the histological criteria, whereas the diagnosis of IBS is based on the symptom-based criteria. Recently, MC along with its two entities collagenous colitis (CC) and lymphocytic colitis (LC) have been considered as rare diseases when compared to diarrhea-predominant IBS. A recent study from Spain reported that MC was diagnosed in 13.7% of patients with chronic diarrhea6 and in 10.7% of patients with diagnostic criteria for IBS. Population-based studies in Europe reported the increasing incidence for CC from 1.1 to 5.2/100,000 persons per year and for LC from 3.1 to 4.0/100,000 persons per year,7–9 which are paralleled with incidence rates of MC from 1.1 to 19.6/100,000 persons per year in the United States.10
In order to detect MC in patients with chronic diarrhea, biopsies from normal or almost normal appearing colonic mucosa are required; however, this is often neglected in daily routine, because of insufficient awareness of MC. In fact, Limsui et al11 identified 33% of patients with MC who had previously been diagnosed with IBS, confirming the necessity for more alertness to search for MC.
Calprotectin is a calcium-binding protein found in neutrophilic granulocytes, monocytes, and macrophages; it represents the major constituent (>60%) of cytosolic protein in neutrophils12 and is also present in smaller amounts in mononuclear cells.13 Calprotectin remains stable against bacterial degradation14 during intestinal transit, and hence it can be measured in feces. Fecal calprotectin (FC) levels are significantly increased in patients with inflammatory bowel disease (IBD) and correlate with the clinical disease activity.15 It reliably differentiates between patients with IBD and IBS.16,17
Data on the use of FC in MC and their ability to distinguish MC and IBS are limited18,19 and also controversially discussed.20,21
Currently, FC is measured using commercially available enzyme-linked immunosorbent assay (ELISA), but it is only used occasionally in clinical laboratories. Thus, a simple rapid FC semiquantitative stool test was developed. This method provides a facile and practicable measurement of FC and can be used in an outpatient setting. We aimed to evaluate the possibility of differentiation between MC and IBS by comparing two methods for measuring FC concentration.
Methods
Study setting
This study was performed in the Department of Gastroenterology, Hepatology and Infectious Diseases, Otto-von-Guericke University, Magdeburg, Germany (tertiary center). We prospectively included patients presented between May 2010 and October 2010 with a histologically proven diagnosis of MC and patients with diarrhea-dominant IBS according to the Rome III criteria. This study was conducted in accordance with the approval of local ethical committee of Otto-von-Guericke University, Magdeburg, Germany. Clinical trial registry number 49/2010.
Patients with MC
Patients with a previously or newly diagnosed MC were included in the study. Every patient underwent a complete ileocolonoscopy with colonic biopsies after signing the patient’s informed consent form.
Inclusion criteria were as follows: age >18 years and histological diagnosis of CC or LC according to the diagnostic criteria.22–24
Active disease of MC was defined as chronic diarrhea for at least 4 weeks with >3 soft or liquid bowel movements per day. MC in remission was defined as <3 soft or solid stools per day.
Patients with a known history of IBD, history of previous gastrointestinal or colonic surgery (except appendectomy and cholecystectomy), malignancies, oral medication of nonsteroidal anti-inflammatory drugs, and signs of gastrointestinal infections (positive stool cultures for Clostridium difficile toxin, Salmonella spp., Shigella spp., Campylobacter or Yersinia, ova, and parasites) were excluded.
A medical history, including number of bowel movements, stool consistency according to Bristol stool scale,25 smoking habits, weight loss, and actual or former (eg, treatment with Budesonide) medical treatment, were assessed during inclusion. Every patient was asked to fill out a questionnaire. A fecal sample was obtained before performing ileocolonoscopy.
Patients with IBS
Patients with chronic diarrhea of unknown origin were referred to our outpatient department for further diagnosis. Every patient with diarrhea-predominant IBS was assessed according to the Rome III criteria. The control group was assessed in the same way as in patients with MC (see “Patients with MC” section).
Colonoscopy
A complete ileocolonoscopy was performed under sedation (midazolam ± disoprivan) in every patient and control. Two biopsy samples were obtained using a standard, one type endoscopic biopsy forceps from the cecum and/or colon ascendens, transverse colon, descending and sigmoid colon, and rectum. The biopsies were fixed separately in 4% formalin and were routinely processed.
Histopathology
Differentiation of CC and LC based on the histopathological criteria was assessed by an expert pathologist according to the established diagnostic criteria.22–24
Fecal calprotectin
Fecal samples (~5 g) were collected from patients with MC and IBS before performing ileocolonoscopy. Patients were asked to collect a stool sample 1 day before starting the preparation for colonoscopy, and the samples were kept at room temperature and were submitted within 48 hours. Stool samples were then frozen at −20°C, and after thawing, 5 mg aliquots of all samples (~100 mg) were subjected to the extraction protocol as described in the manufacturer’s protocol (Bühlmann Laboratories AG, Schönenbuch, Basel, Switzerland). FC levels were analyzed 1) semiquantitatively using the Quantum Blue® calprotectin lateral flow assays (LF-CAL20-RD; Bühlmann Laboratories AG) and 2) quantitatively using the commercial calprotectin ELISA (EK-CAL; Bühlmann Laboratories AG). Final data of ELISA are expressed as microgram of calprotectin per gram of stool. Semiquantitative assessment (Quantum Blue®) resulted in the qualitative stratification of “negative” (<30 μg/g) and two “positive” groups (either 30–60 μg/g or >60 μg/g).
Statistics
Data are expressed as number, relative proportion, median + range, or mean ± standard deviation, if not stated otherwise. Nonparametric Kruskal–Wallis test was applied if more than two groups were compared. Post hoc analyses for pairwise comparisons between groups were performed using Mann–Whitney U test. Age was analyzed by analysis of variance and Student’s t-test, and frequencies were analyzed by chi-square test. All tests were applied two-sided with a level of significance of P<0.05.
Results
We included 23 patients with MC (four male 17% and 19 female 83%, mean age 63.8 years ±11.7, range 42–86 years). Nineteen patients fulfilled the diagnostic criteria of CC, and four patients presented with LC. Figure 1 shows the stratification of patients with MC. Patients’ characteristics are listed in Table 1 according to the questionnaire.
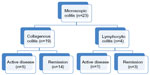  | Figure 1 Stratification of patients with microscopic colitis. |
The rapid test Quantum Blue® was performed in all patients with MC and IBS.
Comparing FC concentrations using Quantum Blue® rapid test, no significant difference was identified between patients with CC, LC, and IBS (Figure 2A). Median FC concentration in all three groups was 30 μg/g. Mean variation showed a tendency toward higher FC concentrations in CC (30–179 μg/g) than in LC (30–42 μg/g) and control group (30–80 μg/g). Classification into active and quiescent MC was not associated by different FC concentrations (Figure 2B).
Comparing all MC patients (CC + LC) with IBS patients, we could demonstrate a significant difference in FC concentrations (P=0.03) using an ELISA (Figure 3). FC measured by ELISA could show a significant difference between CC and IBS (P=0.01). FC in four patients with LC was similar to that in patients with IBS (Table 2). Number of active LC was n=1 (Figure 1).
After grouping patients with MC in active and in remission, a highly significant difference in median FC concentrations between active MC and IBS groups (P<0.0001) was identified (Table 3), whereas median FC concentrations of patients with MC in remission did not significantly differ from patients with IBS (Table 3).
Discussion
In our prospective study, patients with active MC (CC and LC) had significantly higher FC concentrations as compared to patients with IBS if analyzed by ELISA. We also found a significant difference in FC concentrations when comparing active and quiescent MC in respect to IBS.
Comparing ELISA and rapid FC test, the rapid test failed to detect active MC in almost every case. Rapid FC test showed a consistent value of 30 μg/g in all groups, which had been defined as the lowest cutoff level of the measurement range according to the methodology instructions (Bühlmann®). However, a study by Veestergaard et al26 showed a good association of a rapid test (PreventID® CalDetect®; Preventis GmbH, Bensheim, Germany) with calprotectin measured by ELISA. Calprotectin cutoff value in this specific setting was 15 μg/g, and sensitivity and specificity of the new rapid test were 96% (95% confidence interval, 87%–100%) and 70% (95% confidence interval, 55%–83%), respectively. The use of our FC rapid test with a standard range <30 μg/g to <60 μg/g for differentiation between MC from IBS requires readjustments and is not suitable in its present version.
The majority of previous studies examined FC as a fecal marker for differentiating inflammatory intestinal disorders (eg, IBD) from noninflammatory bowel diseases (eg, IBS)20,27 and reported a consistent and good correlation with histological findings, endoscopic disease activities, and clinical symptom scores.21,28,29
For MC, only two other studies have been reported as of to date.19,30 In these two studies discriminating CC and IBS patients with diarrhea, elevated FC levels and elevated inflammatory stool markers (fecal eosinophil cationic protein and eosinophil protein X) were detected in patients suffering from active CC. The results of elevated FC in active MC are consistent with our findings. Absolute FC levels vary among our and previous studies19 and may be explained since MC is histologically characterized by intraepithelial lymphocytosis (>20 intraepithelial lymphocytes/100 surface epithelial cells) and a variable infiltrate in the lamina propia dominated by chronic inflammatory cells,22 including neutrophils and active cryptitis, in 30%–40%.31 A recent study demonstrated that CC inflammation is mainly dominated by activated eosinophils, and neutrophil activity could not be detected; on the other hand, CD4+ and CD8+ T cells were increased in numbers but did not show a significant difference in activity patterns than in healthy controls.30
These conflicting results might be due to small sample sizes or different stages of the disease with different inflammatory activities, since there is no consensus definition existing for active or remission of MC neither clinically nor histologically in the literature.
The value of testing FC is best addressed by the fact that endoscopic appearance of the mucosa in patients with MC is macroscopically normal, MC still is considered to be a rare disease, and biopsies of the colonic mucosa are often not considered. Therefore, increased FC concentrations in active MC – in contrast to patients with IBS – will alert biopsy sampling for the confirmation of MC diagnosis. In a study from Denmark, time between first endoscopy and diagnostic endoscopy was 6 (0–84) months in CC and 3 (0–50) months in LC,32 which underlines the possible beneficial role of FC determination in identifying patients with MC.
Our results could show that there is no significant difference of FC levels in patients with MC in remission (after treatment) in comparison to patients with IBS. A further potential use of FC is to measure/monitor the outcome of the treatment of MC patients under Budesonide therapy. A significant decrease in FC levels in patients with active CC during treatment with Budesonide on day 28 was previously reported.29
Conclusion
In conclusion, we prospectively demonstrate that elevated FC levels measured by ELISA are a potential noninvasive surrogate marker in differentiating patients with active MC from patients with IBS. A multicenter study with robust numbers of patients with MC is needed for further confirmation. Furthermore, our data suggest that FC could become part of the clinical workup to determine which patients with chronic diarrhea should undergo biopsies for histological assessment of active MC.
Acknowledgment
No financial support was received to conduct this study.
Author contributions
Ulrike von Arnim: study concept and design, acquisition of data, analysis and interpretation of data, drafting and writing the article, and critical revision of the article for important intellectual content. Thomas Wex: analysis and interpretation of data, drafting the article, and critical revision of the article for important intellectual content. Christine Ganzert: acquisition of data, laboratory analysis of data, and data collection and analyzation, and this article is the basis to her medical thesis. Christian Schulz: data collection and analysis and interpretation of data. Peter Malfertheiner: critical revision of the article for important intellectual content and study supervision. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Ulrike von Arnim has received honoraria for clinical studies and lecture fees from Dr Falk Pharma GmbH, MSD SHARP and DOHME GmbH, Abbvie, and Almirall but has no competing interests with respect to the study to declare. Christine Ganzert and Thomas Wex report no financial conflicts of interest in this work. Christian Schulz received honoraria for lecture from Abbvie but has no competing interests with respect to the study to declare. Peter Malfertheiner has received honoraria for clinical studies and speakers’ fee from Astra Zeneca, Abbvie, and Aptalis but has no competing interests with respect to the study to declare. The authors have no other conflicts of interest in this work.
References
Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006; 130:1480–1491. | |
Kuiken SD, Lindeboom R, Tytgat GN, Boeckxstaens GE. Relationship between symptoms and hypersensitivity to rectal distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22(2):157–164. | |
Ford AC, Spiegel BMR, Talley NJ, Moayyedi P. Small bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1279–1286. | |
Kassinen A, Krogius- Kurikka L, Makivoukko H, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. | |
Ford AC, Talley NJ. Mucosal inflammation as a potential etiologic factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46:421–431. | |
Guagnozzi D, Lucendo AJ, Angueira-Lapeña T, González-Castillo S, Tenias Burillo JM. Prevalence and incidence of microscopic colitis in patients with diarrhoea of unknown aetiology in a region in central Spain. Dig Liver Dis. 2012;44:384–388. | |
Bohr J, Tysk C, Eriksson S, Järnerot G. Collagenous colitis in Orebro, Sweden, an epidemiological study. 1984–1993. Gut. 1995;37:394–397. | |
Olesen M, Eriksson S, Bohr J, Järnerot G, Tysk C. Microscopic colitis: a common diarrhoeal disease. An epidemiological study in Orebro, Sweden, 1993–1998. Gut. 2004;53:346–350. | |
Fernández-Bañares F, Salas A, Forné M, Esteve M, Espinós J, Viver JM. Incidence of collagenous and lymphocytic colitis: a 5-year population-based study. Am J Gastroenterol. 1999;94:418. | |
Pardi DS, Loftus EV Jr, Smyrk TC, et al. The epidemiology of microscopic colitis: a population based study in Olmsted County, Minnesota. Gut. 2007;56:504–508. | |
Limsui D, Pardi DS, Camilleri M, et al. Symptomatic overlap between irritable bowel syndrome and microscopic colitis. Inflamm Bowel Dis. 2007;13:175–181. | |
Fagerhol MK, Andersson KB, Naess-Andresen CF, Brandtzaeg P, Dale I. Calprotectin (the L1 leukocyte protein). In: Smith VL, Dedman JR, editors. Stimulus Response Coupling: The Role of Intracellular Calcium-Binding Proteins. Boca Raton, FL: CRC; 1990:187–210. | |
Johne B, Fagerhol MK, Lyberg T, et al. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol. 1997;50:113–123. | |
Tibble J, Sigthorsson G, Foster R, Sherwood R, Fagerhol M, Bjarnason I. Faecal calprotectin and faecal occult blood tests in the diagnosis of colorectal carcinoma and adenoma. Gut. 2001;49:402–408. | |
Tibble J, Teahon K, Thjodleifsson B, et al. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000;47:506–513. | |
Carroccio A, Iacono G, Cottone M, et al. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: a prospective study in adults and children. Clin Chem. 2003;49:861–867. | |
Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. | |
Lettesjö H, Hansson T, Peterson C, et al. Detection of inflammatory markers in stools from patients with irritable bowel syndrome and collagenous colitis. Scand J Gastroenterol. 2006;41:54–59. | |
Wildt S, Nordgaard-Lassen I, Bendtsen F, Rumessen JJ. Metabolic and inflammatory faecal markers in collagenous colitis. Eur J Gastroenterol Hepatol. 2007;19:567–574. | |
Tibble JA, Sigthorsson G, Foster R, Forgacs I, Bjarnason I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123:450–460. | |
Limburg PJ, Ahlquist DA, Sandborn WJ, et al. Fecal calprotectin levels predict colorectal inflammation among patients with chronic diarrhea referred for colonoscopy. Am J Gastroenterol. 2000;95:2831–2837. | |
Lazenby AJ, Yardley JH, Giardiello FM, Jessurun, J, Bayless TM. Lymphocytic (“microscopic”) colitis: a comparative histopathologicic study with particular reference to collagenous colitis. Hum Pathol. 1989;20:18–28. | |
Baert F, Wouters K, D`Haens G, et al. Lymphocytic colitis: a distinct clinical entitiy? A clinicopathological confrontation of lymphocytic and collagenous colitis. Gut. 1999;45:375–381. | |
Offnr FA, Jao RV, Lewin KJ, Havelec L, Weinstein WM. Collagenous colitis: a study of the distribution of morphological abnormalities and their histological detection. Hum Pathol. 1999;33:451–457. | |
Riegler G, Esposito I. Bristol scale stool form. A still valid step in medical practice and clinical research. Tech Coloproctol. 2001;5:163–164. | |
Veestergaard TA, Nielsen SL, Dahlerup JF, Hornung N. Fecal calprotectin. Assessment of a rapid test. Scand J Clin Lab Invest. 2008;68:343–347. | |
D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. | |
Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–169. | |
Wagner M, Peterson CGB, Stolt I, et al. Fecal eosinophil cationic protein as a marker of active disease and treatment outcome in collagenous colitis: a pilot study. Scand J Gastroenterol. 2011;46:849–854. | |
Wagner M, Lampinen M, Sangfelt P, Agnarsdottir M, Carlson M. Budesonide treatment of patients with collagenous colitis restores normal eosinophil and T-cell activity in the colon. Inflamm Bowel Dis. 2010;16:44–48. | |
Ayate G, Ithamukkala S, Sapp H, et al. Prevalence and significance of inflammatory bowel disease- like morphological features in collagenous and lymphocytic colitis. Am J Surg Pathol. 2002;26:1414–1423. | |
Bjørnbak C, Engel PJH, Nielsen PL, Munck LK. Microscopic colitis: clinical findings, topography and persistence of histopathological subgroups. Aliment Pharmacol Ther. 2011;34:1225–1234. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.