Back to Journals » Patient Preference and Adherence » Volume 13
Factors Associated With Regorafenib Adherence With Metastatic Colorectal Cancer
Authors Kawakami K , Wakatsuki T, Soejima A, Kobayashi K , Yokokawa T, Aoyama T, Suzuki K, Suenaga M , Yamaguchi K , Inoue A, Machida Y , Hama T
Received 2 June 2019
Accepted for publication 21 September 2019
Published 15 October 2019 Volume 2019:13 Pages 1745—1750
DOI https://doi.org/10.2147/PPA.S217835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Kazuyoshi Kawakami,1 Takeru Wakatsuki,2 Azusa Soejima,1 Kazuo Kobayashi,1 Takashi Yokokawa,1 Takeshi Aoyama,1 Kenichi Suzuki,1 Mitsukuni Suenaga,2 Kensei Yamaguchi,2 Ayaka Inoue,3 Yoshiaki Machida,3 Toshihiro Hama1
1Department of Pharmacy, Cancer Institute Hospital, Japanese Foundation for Cancer Research, Koto-Ku, Tokyo 135-8550, Japan; 2Department of Gastroenterological Chemotherapy, Cancer Institute Hospital, Japanese Foundation for Cancer Research, Koto-Ku, Tokyo 135-8550, Japan; 3Section for Practical Education, Hoshi University School of Pharmacy and Pharmaceutical Sciences, Shinagawa-Ku, Tokyo 142-8501, Japan
Correspondence: Kazuyoshi Kawakami
The Japanese Foundation for Cancer Research, Cancer Institute Hospital, Depatment of Pharmacy, 3-8-31 Ariake Koto-ku, Tokyo 135-8550, Japan
Email [email protected]
Introduction: Regorafenib is an oral multikinase inhibitor for the treatment of metastatic colorectal cancer (mCRC). The clinical factors that may affect adherence to regorafenib remain unclear. The aim of this study was to evaluate adherence to regorafenib with mCRC and to identify factors that might affect adherence to regorafenib.
Methods: A total of 108 consecutively enrolled Japanese patients with mCRC received regorafenib. Adherence was measured by pharmacists using pill counts and a self-reported treatment diary for patients at a pharmaceutical outpatient clinic. The median relative dose intensities of regorafenib and the factors adversely affecting adherence were retrospectively surveyed. Logistic regression analysis was then performed using patient socio-demographic factors and clinical factors.
Results: A total of 96 patients were included in the analysis. The median adherence rate was 61.7% in the first cycle. The median relative dose intensity was 57.1%. The most common reason for non-adherence was a hand-foot-skin reaction (35.6%). On multivariate analysis, increased non-adherence to regorafenib was significantly associated with sex (female) [odds ratio (OR) = 4.36; 95% confidence interval (CI): 1.43–13.22, p = 0.01].
Discussion: Hand-foot-skin reactions and female sex were associated with lower adherence to regorafenib. Since these factors could be associated with lower adherence to regorafenib, it would be useful to consider these factors when assessing adherence.
Keywords: adherence, regorafenib, colorectal cancer, hand-foot-skin reactions
Introduction
Due to the availability of rapidly growing numbers of new oral anticancer agents directed towards specific tumor cell targets, medication adherence is becoming an increasingly important issue in oncology.1 In addition, over 80% of patients would prefer oral anticancer agents to intravenous administration.2 The development of new oral anticancer therapies has not only prolonged survival, but also improved cancer patients’ quality of life by decreasing the need for hospitalization.3
On the other hand, poor medication adherence could worsen treatment outcomes.4,5 Adherence rates to anticancer agents vary from 16% to 100%.6 Research on adherence to protein kinase inhibitor treatment in patients with chronic myeloid leukemia showed an association between the missing of only a small number of doses per month (5%) and a less favorable clinical outcome.7 According to published data from the World Health Organization, non-adherence to medication has become a global problem of striking magnitude.8 Therefore, maintaining good adherence is an important issue, particularly with oral anticancer agents.
Regorafenib is an oral multi-kinase inhibitor that blocks the activity of several protein kinases involved in the multiple biological processes of progression and development of cancer; these kinases include vascular endothelial growth factor receptor (VEGFR) 1–3 and tyrosine kinase with immunoglobulin and epidermal growth factor homology domain 2 (TIE2) involved in tumor angiogenesis.9 Treatment with regorafenib 160 mg once daily for the first 3 weeks of each 4-week cycle has shown significant benefits for overall survival and progression-free survival in patients with previously treated metastatic colorectal cancer (mCRC) in two placebo-controlled phase 3 trials, CORRECT10 and CONCUR 11
A previous study reported that the average regorafenib adherence was 82% in southern Italy, and patients’ level of education, other concomitant oral medications, and patients’ general clinical condition may affect regorafenib adherence.12 However, there are no available data regarding the regorafenib adherence rate in every cycle and the factors reducing regorafenib adherence. Additionally, very little has been published on adherence and persistence to oral molecular targeted therapies in solid malignancies.13 The purpose of this retrospective analysis was to evaluate adherence and patient profiles that can help predict poor adherence in mCRC patients receiving regorafenib.
Patients And Methods
Patients
This was a retrospective observational study to examine the clinical factors that may affect regorafenib adherence. Data from 108 consecutive Japanese patients with mCRC who received regorafenib between May 18, 2013 and March 31, 2015 at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research were enrolled. Patients received regorafenib (Bayer, Leverkusen, Germany) 160 mg once daily for the first three weeks of each 4-week cycle until disease progression or unacceptable toxicity. To prevent hand-foot-skin reactions, heparinoid ointment was applied to the hands and feet, and clobetasol propionate was applied at the time of symptom onset. Patients treated with regorafenib were followed-up on day 1, day 8, and day 15 until the end of the 3rd cycle.
Data Collection
Adherence to regorafenib was assessed using pill counts and patient-reported treatment diaries, and non-adherents were interviewed about the reasons for not taking regorafenib at a pharmaceutical outpatient clinic at every patient visit. The pill count was performed by counting the number of regorafenib pills remaining unused whenever the patient visited the outpatient clinic. All participants in this study visited a pharmaceutical outpatient clinic and completed the study. The adherence rate in a cycle was calculated as the number of times a patient took regorafenib/21.
Median relative dose intensities14 of regorafenib and factors associated with reduced adherence were retrospectively collected from electronic patient records. The study protocol was reviewed and approved by the clinical research ethics review committee at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research (approval no: 2015–1042). The all patients provided written informed consent in compliance with the Declaration of Helsinki.
Pharmaceutical Outpatient Clinic
In our hospital, proactive intervention by pharmacists to check adherence and side effects (which are one cause of non-adherence) is considered necessary, and a pharmaceutical outpatient clinic is helpful for patients undergoing oral chemotherapy treatment.15,16 The main tasks of the pharmaceutical outpatient clinic were: to check the regorafenib treatment diary (Bayer) for regorafenib adherence and leftover medication; to propose prescription support for regorafenib and supportive pharmacotherapy; and assessment of side effects such as the hand-foot-skin reaction and hypertension. In practice, prescription support for regorafenib consists of a pharmacist recording the regorafenib dose and administration period required for the next cycle in the electronic medical record. This is then checked and authorized by a physician to enable the prescription to be issued. If any regorafenib is left over from the previous cycle, the prescribed amount is adjusted accordingly.
Statistical Analysis
The primary endpoint was adherence, dichotomized according to success defined as an adherence rate ≥ 90% in the first cycle. Univariate analyses were then performed using socio-demographic factors (sex, age (≥ 70 years vs. < 70 years), living status (alone vs. with others), and marital status) and clinical factors (Eastern Cooperative Oncology Group (ECOG) performance status, adverse events (Grade ≥3), and number of systemic medications (≥ 6)). The factors that were significant on univariate analysis were entered into the multivariate analysis. Significance levels for uni- and multivariate analyses were set at p < 0.2 and p < 0.05, respectively. Odds ratios and p-values were computed for the variables in logistic regression models. All analyses were performed using SPSS version 24.0 (SPSS, Chicago, IL).
Results
Patients And Characteristics
A total of 108 patients were extracted from the outpatient pharmacy information systems in the Cancer Institute Hospital of the Japanese Foundation for Cancer Research. Among then, 12 patients were excluded. The reasons for exclusion were no data collected (n=10) and change in hospital during the first cycle (n=2) (Figure 1). Clinical characteristics and socio-demographic data for the 96 patients analyzed are reported in Table 1. The median age of the patients was 62.5 years (range, 34–83 years), and more than half of the patients were female. The majority of the patients had an ECOG performance status of 0. In terms of living status, 82 patients (85.4%) were living with family, and 14 patients (14.6%) were living alone. In almost 90% of patients, regorafenib was administered as 3rd-line or later. The total number of regorafenib treatments was 185 cycles.
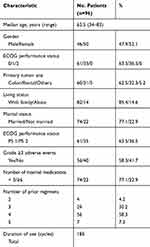 |
Table 1 Clinical And Socio-Demographic Characteristics Of The Study Population |
 |
Figure 1 Flow chart of patients and reasons for exclusion. |
Adherence Rate
Figure 2 shows the regorafenib adherence rate determined at the pharmaceutical outpatient clinic using the pill count and patient-reported treatment diaries. No cases of over-adherence (defined as adherence rate > 100%) were found. The rate of patients who completed regorafenib to the third cycle was 4.2% (4 patients). The median adherence rate was 61.7% (n=96) in the first cycle, 77.5% (n=68) in the second cycle, and 84.3% (n=34) in the third cycle. The median relative dose intensity of regorafenib in this study was 57.1%.
Factors Reducing Regorafenib Adherence
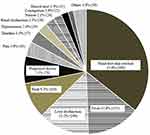
In total, non-adherence was seen in 92 patients (95.8%), with 1120 instances. The most common reasons for non-adherence were hand-foot-skin reactions (35.6%, 399 instances), fever (13.8%, 155 instances), liver dysfunction (13.2%, 148 instances), and rash (9.3%, 104 instances) (Figure 3).
Multivariate Model Of Factors Associated With Adherence
The results of the multivariate analysis are shown in Table 2. Univariate analyses identified sex, living status, and adverse events (Grade ≥3) as possible risk factors for predicting a < 90% adherence rate within the 1st cycle. A significant association was found for sex (female) [odds ratio (OR) = 4.36; 95% confidence interval (CI) = 1.43–13.22; p = 0.01] on logistic regression analysis.
 |
Table 2 Logistic Regression Analysis Of Socio-Demographic And Clinical Factors For An Adherence Rate < 90% In The First Cycle |
Discussion
In many previous studies, the dose intensity of oral anticancer agents was determined only by the amount of medicine prescribed.17 Thus, whether patients were adherent at home was not known. The present study examined not only the median relative dose intensity of regorafenib but also the median adherence rate determined by pharmacists by pill counts and patient-reported treatment diaries. Moreover, female sex was found to be a factor decreasing regorafenib adherence. To the best of our knowledge, this is the first report of a clinical factor that affects adherence to regorafenib with mCRC.
In the present study, the median regorafenib adherence rate was 61.7% in Japanese patients. However, it was reported that the average regorafenib adherence rate was 82.0% in a previous study that was conducted in Italy.12 These data suggest that the regorafenib adherence rate is lower in Japanese patients than in non- Japanese patients. Accumulated data from clinical trials have suggested that there are ethnic differences in the incidence of regorafenib treatment-related adverse events, with a higher incidence of serious adverse events in the Japanese subpopulation compared with the non-Japanese subpopulation.10,11,18 In the CORRECT trial, regorafenib treatment-related adverse events were clearly higher in the Japanese subpopulation than in the non-Japanese subpopulation, including hand-foot-skin reaction (80 vs 42%) and hypertension (60 vs 23%) of any grade.19 The median dose intensity of regorafenib was 71.4% (range 9.5–100.0%) in the Japanese subpopulation, while it was 83.3% (range 9.5–114.3%) in the non-Japanese subpopulation.19 The present data are consistent with the previous reports, and ethnic difference may cause lower adherence in Asians.
The present study showed that female sex was significantly associated with lower regorafenib adherence in mCRC patients. In general, female sex has been associated with lower adherence not only in cancer, but also in other diseases.20,21 It has been reported that female sex was associated with lower imatinib adherence in adult chronic myeloid leukemia.22 Adverse effects of imatinib include rash, edema, and weight gain. Because women are more concerned with their appearance, this issue may be related to the lower adherence in females. Interestingly, in the present study, Grade ≥2 rash/desquamation occurred in 5 patients, all of whom were female. All of them stopped taking regorafenib immediately. These data support the finding that females have lower regorafenib adherence than males.
The present study identified the factors for non-adherence to regorafenib. The major reasons for lower regorafenib adherence were subjective symptoms such as hand-foot-skin reaction, fever, rash, and pain. The relative dose intensity represents the ratio of the amount of drug prescribed to the amount planned for a fixed time period. On the other hand, adherence represents patients’ actual medication-taking behavior. This concept contains the actual amount of drug taken. Therefore, it is important to assess not only the relative dose intensity of regorafenib, but also the adherence.23
Several limitations of this paper should be taken into consideration. In this study, relevant patient-related factors that might be related to adherence, such as education, were not examined.24,25 In general, highly educated patients are more adherent.26,27 Another limitation of this study include its retrospective nature and small sample size from a single institute. These limitations make firm conclusions difficult. Nevertheless, the present data are important for demonstrating the clinical factors that may affect adherence to regorafenib in Japanese patients with mCRC.
In conclusion, the median regorafenib adherence rate was 61.7% in the first cycle, and the most common reason for non-adherence was a hand-foot-skin reaction. Female sex was associated with lower regorafenib adherence, careful dose reduction and interruption of regorafenib are required for female patients.
Availability Of Data And Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval And Consent To Participate
This study was approved by the Ethics Committee of the Cancer Institute Hospital, Japanese Foundation for Cancer Research. All the participants gave their written consent to be included in this study.
Disclosure
The abstract of this paper was presented at the 2017 Gastrointestinal Cancer Symposiumu (ASCO-GI) as poster presentation. The poster’s abstract was published in “Poster Abstracts” in J Clin Oncol, 35 (suppl 4S) (2017). K. Yamaguchi has received speaking honorariums from Taiho Pharmaceutical, Chugai Pharmaceutical, Merck Serono, Takeda Pharmaceutical, Yakult, Bayer, Ono Pharmaceutical, Eli Lilly, Sanofi and Bristol-Myers Squibb, and has received research grants from MSD, Ono Pharmaceutical, Sumitomo Dainippon Pharma, Taiho Pharmaceutical, Daiichi Sankyo, Eli Lilly, Gilead Sciences and Yakult, and has served on consulting or advisory role for Bristol-Myers Squibb. The other authors declare that they have no competing interests.
References
1. Timmers L, Beckeringh JJ, van Herk-Sukel MP, Boven E, Hugtenburg JG. Use and costs of oral anticancer agents in the Netherlands in the period 2000-2008. Pharmacoepidemiol Drug Saf.
2. Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for all oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;1:110–115. doi:10.1200/JCO.1997.15.1.110
3. Regnier Denois V, Poirson J, Nourissat A, Jacquin JP, Guastalla JP, Chauvin F. Adherence with oral chemotherapy: results from a qualitative study of the behaviour and representations of patients and oncologists. Eur J Cancer Care (Engl). 2011;4:520–527. doi:10.1111/j.1365-2354.2010.01212.x
4. DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40:794–811. doi:10.1097/00005650-200209000-00009
5. Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. doi:10.1136/bmj.38875.675486.55
6. Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi:10.3322/caac.20004
7. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–2388. doi:10.1200/JCO.2009.26.3087
8. World Health Organization (WHO). Adherence to Long-Term Therapies: Evidence for Action. Geneva: WHO; 2003.
9. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. doi:10.1002/ijc.25864
10. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi:10.1016/S0140-6736(12)61900-X
11. Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. doi:10.1016/S1470-2045(15)70156-7
12. Del Prete S, Cennamo G, Leo L, et al. Adherence and safety of regorafenib for patients with metastatic colorectal cancer: observational real-life study. Future Oncol. 2017;5:415–423. doi:10.2217/fon-2016-0421
13. Barillet M, Prevost V, Joly F, Clarisse B. Oral antineoplastic agents: how do we care about adherence? Br J Clin Pharmacol. 2015;80:1289–1302. doi:10.1111/bcp.12734
14. Hryniuk WM, Goodyear M. The calculation of received dose intensity. J Clin Oncol. 1990;8:1935–1937. doi:10.1200/JCO.1990.8.12.1935
15. Sugita K, Kawakami K, Yokokawa T, et al. Self-reported adherence to trifluridine and tipiracil hydrochloride for metastatic colorectal cancer: a retrospective cohort study. Oncology. 2016;91:224–230. doi:10.1159/000448717
16. Nonomiya Y, Yokokawa T, Kawakami K, et al. Regorafenib-induced hand-foot skin reaction is more severe on the feet than on the hands. Oncol Res. 2019;27:551–556. doi:10.3727/096504018X15291727589740
17. Yoon SE, Lee SJ, Lee J, et al. The use of regorafenib for patients with refractory metastatic colorectal cancer in clinical practice. Onco Targets Ther. 2018;12:225–231. doi:10.2147/OTT.S187621
18. Yamaguchi K, Komatsu Y, Satoh T, et al. Large-Scale, Prospective Observational Study of Regorafenib in Japanese patients with metastatic colorectal cancer in a real-world clinical setting. Oncologist. 2019;24:e450–e457. doi:10.1634/theoncologist.2018-0377
19. Yoshino T, Komatsu Y, Yamada Y, et al. Randomized phase III trial of regorafenib in metastatic colorectal cancer: analysis of the CORRECT Japanese and non-Japanese subpopulations. Invest New Drugs. 2015;33:740–750. doi:10.1007/s10637-014-0154-x
20. Perreault S, Blais L, Lamarre D, et al. Persistence and determinants of statin therapy among middle-aged patients for primary and secondary prevention. Br J Clin Pharmacol. 2005;59:564–573. doi:10.1111/j.1365-2125.2005.02355.x
21. Yang -C-C, Jick SS, Testa MA. Discontinuation and switching of therapy after initiation of lipid-lowering drugs: the effects of comorbidities and patient characteristics. Br J Clin Pharmacol. 2003;56:84–91. doi:10.1046/j.1365-2125.2003.01818.x
22. Darkow T, Henk HJ, Thomas SK, et al. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: a retrospective analysis among managed care patients with chronic myelogenous leukemia. Pharmacoeconomics. 2007;25:481–496. doi:10.2165/00019053-200725060-00004
23. Kimura M, Nakashima K, Usami E, et al. Adherence and awareness of the therapeutic intent of oral anticancer agents in an outpatient setting. Oncol Lett. 2015;9:2341–2346. doi:10.3892/ol.2015.3027
24. Timmers L, Boons CC, Kropff F, et al. Adherence and patients experiences with the use of oral anticancer agents. Acta Oncol. 2014;53:259–267. doi:10.3109/0284186X.2013.844353
25. Burkiewicz JS, Fit KE. Improving adherence–sharing experiences. Ann Pharmacother. 2007;12:2058–2060. doi:10.1345/aph.1K350
26. Mathes T, Pieper D, Antoine SL, Eikermann M. Adherence influencing factors in patients taking oral anticancer agents: a systematic review. Cancer Epidemiol. 2014;38:214–226. doi:10.1016/j.canep.2014.03.012
27. McCue DA, Lohr LK, Pick AM. Improving adherence to oral cancer therapy in clinical practice. Pharmacotherapy. 2014;34:481–494. doi:10.1002/phar.1399
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.