Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Facial skin revitalization with CPM®-HA20G: an effective and safe early intervention treatment
Authors Hertz-Kleptow D , Hanschmann A , Hofmann M, Reuther T, Kerscher M
Received 19 March 2019
Accepted for publication 27 June 2019
Published 13 August 2019 Volume 2019:12 Pages 563—572
DOI https://doi.org/10.2147/CCID.S209256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Dominique Hertz-Kleptow,1 Angelika Hanschmann,2 Matthias Hofmann,2 Tilmann Reuther,1 Martina Kerscher1
1University of Hamburg, Division of Biochemistry and Molecular Biology, Cosmetic Science, D-20146 Hamburg, Germany; 2Global Clinical Development, Merz Pharmaceuticals GmbH, Frankfurt, Germany
Background: Hyaluronic acid (HA) fillers are popular for the treatment of signs of facial skin aging.
Objective: The objective of this study was to confirm the performance and safety of a new cohesive polydensified matrix HA filler ([CPM®-HA20G, Belotero Revive,® lidocaine-free], Merz Pharmaceuticals GmbH, Frankfurt, Germany) for the treatment of early signs of facial skin aging by use of biophysical measurements as well as subject and investigator satisfaction.
Methods: Twenty-five healthy female subjects with signs of facial skin aging were enrolled in this open-label, rater-blinded, observational post-market clinical follow-up study, and received 20 micropuncture treatments of 50 μL CPM®-HA20G each into the lower cheek area at three injection visits 4 weeks apart. Objective biophysical assessments were conducted to demonstrate effects on viscoelastic properties of the skin, surface roughness, tone and radiance, and hydration, at baseline and at all follow-up visits up to 36 weeks.
Results: CPM®-HA20G significantly increased gross elasticity of the skin (at weeks 9 and 12), skin firmness (up to week 24), skin tone and radiance and skin hydration (all up to 36 weeks). Significant reduction of skin fatigue (up to 9 weeks), skin roughness (up to 28 weeks), and redness (up to 36 weeks) was also observed. Subjects and blinded investigator were highly satisfied with the treatment outcomes. The treating investigator reported a high level of satisfaction with the ease of injection and the clinical performance of the device. Moreover, data demonstrated a good safety profile of the device.
Conclusion: CPM®-HA20G is considered to be an effective and safe HA injectable for skin revitalization in patients suffering from signs of skin aging and loss of skin elasticity. It seems to be a perfect early intervention approach in patients that do not need volumizing treatment and a combination approach in older patients with more pronounced aging.
Keywords: hyaluronic acid, filler, cohesive polydensified matrix, skin revitalization
Introduction
The impacts of aging on the skin include wrinkling, laxity, pigment changes, coarseness, dryness, and loss of tensile strength.1 Molecular and histochemical features underlying age-dependent phenotypic alterations of human skin include decreased collagen production by dermal fibroblasts, leading to the fragmentation and disruption of the normal interaction of skin cells within the extracellular matrix (ECM).2,3 During the aging process, the concentration of endogenous hyaluronic acid (HA) concentration and hence skin hydration is decreased, contributing to an increased skin wrinkling amongst other effects.4
HA has shown its ability to rejuvenate skin in multiple studies. Based on the viscoelastic properties of HA a condensed network within the ECM is established to improve skin turgor and skin hydration.5 Moreover, it has been demonstrated recently that HA might increase collagen fiber production and promote the release of cellular growth factors.5
Furthermore, multiple and micro-dosed injections of HA into the mid to deep dermal layers of the skin demonstrate an increase in hydration and cellular activity, synthesis of collagen and elastin, and maintain and/or restore healthy, youthful skin texture with firm, bright, and moisturized skin.2,6–8 Clinical experience of skin revitalization with HA-based fillers suggests this technique is safe.6 For skin revitalization, the micropuncture injection technique by injecting small amounts of the product into the mid to deep dermis has been shown to be effective.9–11
A large variety of HA dermal fillers has been developed through recent years, including CPM®-HA20G, a polydensified filler containing a single phase of HA cross-linked continuously and manufactured with cohesive polydensified matrix (CPM) technology. This technology allows for homogeneous intradermal distribution.12–14
The purpose of this post-market follow-up study was to confirm the performance and safety of a new CPM HA filler [CPM®-HA20G, Belotero Revive®, lidocaine-free, containing glycerol, Merz Pharmaceuticals GmbH, Frankfurt, Germany] for the treatment of signs of facial skin aging and to collect data about subject and investigator satisfaction.
Materials and methods
Materials
CPM®-HA20G is a CE-marked sterile, resorbable, viscoelastic, transparent cross-linked sodium hyaluronate gel (20 mg/mL) of non-animal origin containing glycerol (17.5 mg/mL).
Study design & treatment
This study was an open-label, single center, rater-blinded, observational, post-market clinical follow-up study. It was conducted in accordance with the Declaration of Helsinki, and in compliance with the International Conference on Harmonization, EN ISO 14155 and Good Clinical Practice principles. The study was approved by the ethical committee of the Ärztekammer Hamburg. All study participants provided written informed consent.
Twenty-five Caucasian females (Fitzpatrick skin types I–IV) between the age of 31–44, showing early signs of facial skin aging in the lower cheeks area were enrolled in the study, to assess pre- and post-treatment changes using multiple objective biophysical skin parameters. Study duration was 36 weeks (baseline at day 0/first treatment to final follow-up visit). Subjects received three injections (3 ⨰ 2 mL) at day 0, week 4, and week 8.
Fifty microliters of CPM®-HA20G was injected according to 20 injection points on a grid on each lower cheek (Figure 1). A serial bolus micropuncture technique was used at an immediate subdermal level with 30 G ½ needles (0.3×13 mm). Subjects received a total volume of 1 mL CPM®-HA20G in each cheek per injection visit. The total volume of CPM®-HA20G injected during the study over all three injection visits was 6 mL (3×1 mL per cheek).
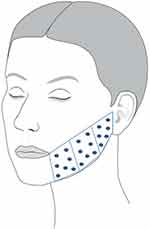 |
Figure 1 Treatment area and injection points for the left lower cheek. An identical injection pattern was used on the opposite cheek. |
Assessments
Demographic data included medical history, concomitant diseases, previous and concomitant therapies/procedures. Subject demographics and disposition are displayed in Figure 2. Baseline values for skin elasticity (R2), skin firmness (R0), skin fatigue (R3 and R9), skin roughness (Ra, Rq, Rz), skin tone and radiance (hemoglobin & melanin), and skin hydration were recorded.
 |
Figure 2 Overview of subject demographics and disposition.Abbreviation: FAS, full analysis set. |
Change of gross elasticity (R2) measured by use of a cutometer (Courage & Khazaka, Cologne, Germany) from baseline to 9 weeks after the first injection was defined to be the primary efficacy criterion.
The secondary endpoints were defined as changes in further viscoelastic parameters (R2, R0, and R3/R9 values) as determined by cutometer from baseline to all other time points along with improvement of skin surface roughness [determined using Phaseshift Rapid In-vivo Measurement Of Skin (PRIMOS, GFM, Berlin, Germany)], and skin radiance [glossymeter and mexameter (both Courage & Khazaka, Cologne, Germany)] and skin hydration [corneometer (Courage & Khazaka)].
Evaluations were conducted on the 7 points modified Global Aesthetic Improvement Scale (GAIS) by a blinded investigator at all visits. Treating investigator satisfaction regarding ease of injection and comparison to competitors was assessed at the end of the study. Subject Global Impression of Change Scale (GICS, 7 points) assessments were performed at all visits. In addition, subjects completed change in skin texture and treatment satisfaction questionnaires at week 4 and at all other time points. Adverse events were recorded during the whole study period. Visual improvements were assessed by a blinded evaluator (dermatologist) using photos (frontal full face, 45° and side view) taken prior to treatment at baseline, week 4 and week 8 as well as at all others visits (2D DermaViz, Quantificare, Sophia Antipolis, France).
Statistical analysis
A two-sided paired t-test, assuming a standard deviation (SD) of 0.1 (alpha =5%), based on data reported was used for the primary objective measures.8 The calculation was performed using SAS 9.4.
All performance analyses were based on the full analysis set comprised of 24 subjects who received all 3 treatments. Summaries (N, mean, SD, median, minimum and maximum) were calculated for the values and changes from baseline of the primary performance variable (gross elasticity). Secondary performance variables including skin gross elasticity, skin firmness, skin fatigue, skin hydration, skin radiance, and skin roughness were analyzed in a similar fashion. Explorative 95% confidence intervals and p-values pre- and post-comparison based on the t-distribution were presented Absolute and percent frequencies (N, %) based on values observed per facial side were calculated using blinded investigator´s GAIS, subject satisfaction (GICS) and for treating investigator´s satisfaction after injection completion and the categories of injection technique. Explorative 95% confidence intervals for the rate of answers with “Yes” for the treatment satisfaction and skin change were presented. Summaries were calculated for injected volume.
All safety analyses were performed based on the safety evaluation set containing the subset of all 25 subjects who were treated at least once. Categorical safety variables were analyzed using absolute and relative frequencies. AEs were coded according to the MedDRA version in effect at the time of database closure. Only treatment emergent adverse events (TEAEs) defined as AEs with onset or worsening during or after the first injection up to and including the final study visit were analyzed.
Results
Twenty-five Caucasian females, Fitzpatrick skin type I–IV, with a mean age of 36 years (±) years old were treated. Out of the 25 subjects enrolled in the study, 24 received all three injections, with 21 completing the study. None of the subjects who withdrew from the study did so due to adverse events.
Clinical performance
Viscoelastic properties of the skin (skin elasticity, skin firmness, and skin fatigue) were analyzed using cutometry measurements. As shown in Figure 3A, treatment with CPM®-HA20G improved skin elasticity (R2 value) significantly from baseline to week 9 and week 12. Gross elasticity remained increased up to week 28 with at least 25%. Following the last injection, skin firmness (R0 values) improved significantly up to 24 weeks, compared to the baseline value (Table 1). In addition, skin fatigue values (R9 values, Figure 3B) representing the tiring effects of the skin after repeated suction are reduced significantly until week 9 and stay below baseline levels up to week 24.
 |
Table 1 Summary of cutometer values for the assessment of skin firmness (R0 values) |
Subjects’ skin hydration was measured using a corneometer. Skin hydration values significantly increased after the first treatment (Figure 4). Figure 4A shows the overall increase in skin hydration from day 0 up to week 36. Figure 4B highlights the significant change in skin hydration values from week 4 to week 36 compared to baseline. Strong subject satisfaction of skin hydration of more than 70% from week 4 until week 28 corroborates the corneometer measurements (Table 2).
 |
Table 2 Subjects (n[%]) reporting satisfaction with various changes in skin texture over the study interval |
Figure 5A highlights that following the last injection at week 8, skin redness (hemoglobin/erythema values) decreased significantly until week 36. Melanin measurements of the skin showed a significant increase compared to baseline directly after the first injection session at week 4. This increase was observed in more than 75% of the subjects, across all Fitzpatrick skin types (type I–IV). Higher melanin measurement values stayed on a constant level until week 36.
Measurements with the PRIMOS device showed a significant decrease of all skin roughness values Ra, Rq, and Rz from baseline until week 28 (Figure 5B). Ra and Rq values showed more subtle value reductions compared to the Rz value, the arithmetic mean of single roughness depth, which decreased strongly between week 8 and week 28.
According to the blinded investigator´s rating (GAIS), at weeks 8 and 12 all subjects (100%) showed an improved aesthetic appearance. Eighty-six percent of the subjects were at least improved at week 24 and 28% and 71% still showed improvement at week 36 (Figure 6). Subject photographs indicate overall skin quality improvements after treatment with CPM®-HA20G at baseline and at weeks 9, 28, and 36 (Figure 7).
 |
Figure 6 Investigators Global Aesthetic Improvement Scale (GAIS) ratings. Improved = summary of ratings for improved, much improved, and very much improved scores. |
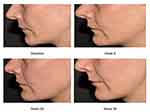 |
Figure 7 Subject’s photographs at baseline and at weeks 9, 28, and 36 exhibiting overall skin quality improvements after treatment with CPM®-HA20G. |
Subject satisfaction
The subject´s satisfaction rating (GICS) was consistently over 80% from week 8 to week 24 (with a satisfaction peak at week 12, 91% showing an improvement). By the end of the study at week 36, 57% of the subjects still rated an improvement. Furthermore, more than 70% of the subjects showed high satisfaction levels for skin hydration after the treatment finished at week 8 and up to week 28. In addition, more than 60% of all subjects were highly satisfied regarding skin softness and suppleness as well as the refreshing effect of the product up to week 28. Subject satisfaction was also strongly increased for skin tone improvements (Table 2).
Investigator satisfaction
The treating investigator reported a high level of satisfaction (all rated with 100% satisfaction) with clinical performance characteristics of CPM®-HA20G including gel distribution and positioning in the skin, skin moisturizing, ease of injection, skin texture in terms of elasticity and fine wrinkles smoothening in comparison to competitors (data not shown).
Safety assessments
All reported related adverse events were mild or moderate in severity. Table 3 shows the most common TEAEs. The most common adverse event was injection-site hematoma, occurring in 80% of the subjects, and was typically resolved within 3 days after injection.
Discussions
The present study demonstrated the clinical performance of CPM®-HA20G for facial skin revitalization as shown by markedly improved viscoelastic properties of the skin (skin elasticity, skin firmness, and skin fatigue), skin roughness, skin tone and radiance, and skin hydration for up to 36 weeks in 24 subjects. It was the first clinical study of CPM®-HA20G and subjects received micropuncture injections of 50 µL in up to 20 injection points across the lower cheek. Injections were performed at three consecutive visits (day 0, week 4, and week 8) each 4 weeks apart. Skin roughness and skin tone improved over 28 weeks, skin hydration values were increased even up to 36 weeks after the first treatment. In addition, CPM®-HA20G demonstrated an excellent safety profile.
Loss of dermal elasticity is the main feature of an early onset of photo-damaged skin. Treatment significantly improved dermal gross elasticity from baseline to week 9 and week 12. Gross elasticity remained increased up to week 28, although not significant. However, gross elasticity alone does not allow assessing all treatment-related underlying biophysical changes in the dermal microenvironment.15 Further assessments of dermal viscoelastic skin properties included the measurements of skin firmness and skin fatigue. Cutometer measurements highlighted improved skin firmness from week 12 onwards to week 28 (data not shown). In addition, a reduction in skin fatigue was assessed from week 9 to week 24. Treatment with CPM®-HA20G demonstrated an overall improvement of the viscoelastic skin properties by increased skin elasticity and firmness including a simultaneous reduction of skin “tiring” effects. As shown previously for NASHA products, the measured changes in the viscoelastic skin properties can be explained by short-term effects linked to the biophysical and hydrophilic character of the injected product and longer term effects eg, changes in the collagen disposition which are triggered by the application of the product.15 Compared to products based on the NASHA techniques, CPM®-HA20G highlighted a significant improvement in skin elasticity and also in reduced skin fatigue at earlier time points (week 9 compared to week 12) which could be linked to the stronger hydrophilic properties of the glycerol containing CPM®-HA20G.9,15
Besides the viscoelastic parameters, overall skin hydration constantly increased from baseline to week 36 following the last injection at week 8. Thus, the immediate subdermal injections function as dermal hydration “microreservoirs” by drawing interstitial fluid to the ECM into the dermis or might also trigger body´s own long-term rehydrating effect through remodeling of the ECM. In addition, the increased skin hydration hampers distending of the skin – increasing the skin firmness after week 12 up to week 24. The strong and long-lasting improvement in skin hydration as shown in the clinical study can also be related to the glycerol content of CPM®-HA20G. Glycerol is one of the smallest polyols, which is miscible with water in all proportions and is known to have a very strong ability to attract and absorb water.16 In addition, in vitro data show that glycerol also stabilizes the triple-helical structure of collagen and protects bacterial cells against ultraviolet light.16
A significant decrease of hemoglobin/erythema values up to week 36 was indicative of the reduction of intrinsic redness/erythema, influencing and improving the overall skin tone and “glow” possibly due to the unique combination of glycerol and HA in the product used.17 The measured effect of reduced skin erythema is intensified by increased melanin values after the first treatment. The increase is kept on a significant level up to week 36. Besides the skin tone, the skin softness is remarkably improved by the significant decrease of skin roughness from baseline until week 28 indicating a significant decrease in the depth of fine lines and smoothening the skin surface.15 The measured improvements in biophysical skin properties were corroborated by the blinded investigator´s ratings (GAIS) and the subject´s satisfaction ratings (GICS). One hundred percent of the subjects between week 8 and week 12 were rated as improved on the GAIS while over 80% of the subjects from week 8 to week 24 rated them as improved on the GICS. GICS rating at week 36 showed that still 57% of the subjects continued to see treatment benefits.
GICS and GAIS assessments further confirmed the favorable findings of the study outcomes, supporting the ability of CPM®-HA20G to revitalize facial skin, with the subjects highlighting in the questionnaire that they have the feeling of “looking fresher”. Furthermore, 90% of the subjects stated that they would recommend the product to their friends (data not shown).
The treating investigator´s experience demonstrated very good product performance characteristics including gel distribution and positioning in the skin, skin moisturizing effect, ease of injection, skin texture improvement in terms of elasticity and fine wrinkles smoothening.
CPM®-HA20G was well tolerated when injected into the lower face. The majority of treatment-emergent adverse events were related to the injection as injection-site hematoma, injection-site pain, or injection-site swelling (Table 3).
Although CPM®-HA20G is lidocaine-free, only a few subjects indicated injection-site pain, thus emphasizing that lidocaine might not have an additional benefit in skin quality enhancing products. Lidocaine, however, bears the potential of allergic reactions.18 In addition, with this particular type of injection technique (mid-deep dermal injections with multiple punctures), lidocaine is questionable as every needle entry can be felt by the subjects prior to the numbing effect of the onset of numbing effect. Therefore, it could make more sense to use a topical numbing agent prior to the treatment to enhance subject comfort. Furthermore, the injected lidocaine, a vasodilator, may lead to additional redness due to the dilation of blood vessels. In this clinical study, no case of post-injection redness was recorded. In addition, CPM®-HA20G lidocaine-free used here offers a good treatment option to subjects with known lidocaine hypersensitivity or allergy.
Summarizing the shown biophysical parameters, physician and subject’s satisfaction data obtained from the clinical study it can be concluded that CPM®-HA20G treatment improves the subjects overall skin quality and skin attractiveness. Respectively, it might be used as a preventive and early aesthetic intervention treatment to slow the progression of facial changes over time, especially reducing the risk of cutaneous loss of skin elasticity and skin smoothness in later years, and improving the well-being of subjects. For patients with a more pronounced loss of skin elasticity, CPM®-HA20G could be introduced in combination with microfocused ultrasound.19
Conclusions & future implications
The study results provide evidence on the clinical performance and safety of CPM®-HA20G to revitalize the facial skin. Although this study was conducted in the lower face, the results are translatable to other areas. Increased skin elasticity and skin hydration as well as decreased redness and surface roughness are characteristic outcomes after treatment with the device. Subjects and investigators were highly satisfied with the treatment effects as well as the safety profile.
Acknowledgments
The authors would like to thank all members of the BELOVE Study Group. This study was sponsored by Merz Pharmaceuticals GmbH.
Disclosure
Angelika Hanschmann and Matthias Hofmann are employees of Merz Pharmaceuticals GmbH. Martina Kerscher has conducted clinical trials and acted as a speaker for Merz Pharmaceuticals GmbH. Martina Kerscher also reports educational grants and products from Merz Pharmaceuticals, during the conduct of the study and received educational grants from Galderma/ Q Med as the Advisory Board Member. Dominique Hertz-Kleptow reports grants from Merz Pharmaceuticals, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Savoia A, Landi S, Baldi A. A new minimally invasive mesotherapy technique for facial rejuvenation. Dermatol Ther (Heidelb). 2013;3(1):83–93. doi:10.1007/s13555-012-0018-2
2. Mammucari M, Gatti A, Maggiori S, Bartoletti CA, Sabato AF. Mesotherapy, definition, rationale and clinical role: a consensus report from the Italian society of mesotherapy. Eur Rev Med Pharmacol Sci. 2011;15(6):682–694.
3. Succi IB, Da Silva RT, Orofino-costa R. Rejuvenation of periorbital area: treatment with an injectable nonanimal non-crosslinked glycerol added hyaluronic acid preparation. Dermatol Surg. 2012;38(2):192–198. doi:10.1111/j.1524-4725.2011.02182.x
4. Baspeyras M, Rouvrais C, Liegard L, et al. Clinical and biometrological efficacy of a hyaluronic acid-based mesotherapy product: a randomised controlled study. Arch Dermatol Res. 2013;305(8):673–682. doi:10.1007/s00403-013-1360-7
5. Belmontesi M, De Angelis F, Di Gregorio C, et al. Injectable non-animal stabilized hyaluronic acid as a skin quality booster: an expert panel consensus. J Drugs Dermatol. 2018;17(1):83–88.
6. Galadari H, Al Faresi F. Mesotherapy. Skinmed. 2011;9(6):342–343.
7. O’Mahony M. Skin rejuvenation using mesotherapy: indications, techniques and ingredients. J Anesth Nurs. 2012;1(6):292–297. doi:10.12968/joan.2012.1.6.292
8. Fabi SG, Goldman MP. Hand rejuvenation: a review and our experience. Dermatol Surg. 2012;38(7p2):1112–1127. doi:10.1111/j.1524-4725.2011.02291.x
9. Kerscher M, Bayrhammer J, Reuther T. Rejuvenating influence of a stabilized hyaluronic acid-based gel of nonanimal origin on facial skin aging. Dermatol Surg. 2008;34(5):720–726. doi:10.1111/j.1524-4725.2008.34176.x
10. Streker M, Reuther T, Krueger N, Kerscher M. Stabilized hyaluronic acid-based gel of non-animal origin for skin rejuvenation: face, hand, and decolletage. J Drugs Dermatol. 2013;12(9):990–994.
11. Ribe A, Ribe N. Neck skin rejuvenation: histological and clinical changes after combined therapy with a fractional non-ablative laser and stabilized hyaluronic acid-based gel of non-animal origin. J Cosmet Laser Ther. 2011;13(4):154–161. doi:10.3109/14764172.2011.594060
12. Philipp-Dormston WG, Hilton S, Nathan M. A prospective, open-label, multicenter, observational, postmarket study of the use of a 15 mg/ml hyaluronic acid dermal filler in the lips. J Cosmet Dermatol. 2014;13(2):125–134. doi:10.1111/jocd.12085
13. Flynn TC, Thompson DH, Hyun SH. Molecular weight analyses and enzymatic degradation profiles of the soft-tissue fillers Belotero Balance, Restylane, and Juvederm Ultra. Plast Reconstr Surg. 2013;132(4suppl2):22s–32s. doi:10.1097/PRS.0b013e31829e88a3
14. Santoro S, Russo L, Argenzio V, et al. Rheological properties of cross-linked hyaluronic acid dermal fillers. J Appl Biomater Biomech. 2011;9(2):127–136.
15. Reuther T, Bayrhammer J, Kerscher M. Effects of a three-session skin rejuvenation treatment using stabilized hyaluronic acid-based gel of non-animal origin on skin elasticity: a pilot study. Arch Dermatol Res. 2010;302:37–45. doi:10.1007/s00403-009-0988-9
16. Fluhr JW, Bornkessel A, Berardesca E. Glycerol – just a moisturizer? Biological and biophysical effects. In: Dry Skin and Moisturizers, Chemistry and Function. Taylor & Francis. 2005. Available from: http://www.scientificspectator.com/documents/personal%20care%20spectator/Glycerol%20a%20Moisturizer.pdf. Accessed July 08, 2019.
17. Lim HK, Suh DH, Lee SJ, Shin MK. Rejuvenation effects of hyaluronic acid injection on nasojugal groove: prospective randomized split face clinical controlled study. J Cosmet Laser Ther. 2014;16:32–36. doi:10.3109/14764172.2013.854620
18. Lafaille P, Benedetto A. Fillers: contraindications, side effects and precautions. J Cutan Aesthet Surg. 2010;3:16–19. doi:10.4103/0974-2077.63222
19. Landau M, Anand CV, Besins T, et al. First consensus on primary prevention and early intervention in aesthetic medicine. J Drugs Dermatol. 2017;16:846–854.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




