Back to Journals » Clinical Ophthalmology » Volume 14
Evaluation of Intraocular Pressure After Water Drinking Test in Patients with Unilateral Hemifacial Spasm
Authors Low JR, Wong CW, Loo JL, Milea D, Perera SA, Lee YF, Ng SR, Baskaran M, Nongpiur ME, Tow SLC
Received 15 February 2020
Accepted for publication 15 April 2020
Published 18 June 2020 Volume 2020:14 Pages 1675—1680
DOI https://doi.org/10.2147/OPTH.S249943
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jin Rong Low,1– 3 Chee Wai Wong,1,3– 6 Jing Liang Loo,1,3,5– 8 Dan Milea,3,5,7,8 Shamira A Perera,1– 3,5 Yi Fang Lee,3,9 Si Rui Ng,1– 3 Mani Baskaran,2,3,5 Monisha Esther Nongpiur,2,3,5 Sharon Lee Choon Tow1,3,5– 8
1Department of Cataract and Comprehensive Ophthalmology, Singapore National Eye Centre, Singapore; 2Department of Glaucoma, Singapore National Eye Centre, Singapore; 3Singapore Eye Research Institute, Singapore; 4Department of Surgical Retina, Singapore National Eye Centre, Singapore; 5Ophthalmology and Visual Sciences Academic Clinical Program, Duke-NUS Medical School, Singapore; 6Department of Ophthalmology, National University of Singapore, Singapore; 7Department of Neuro-Ophthalmology, Singapore National Eye Centre, Singapore; 8Neuroscience and Behavioral Disorders Program, Duke-NUS Medical School, Singapore; 9Department of Training and Education, Singapore National Eye Centre, Singapore
Correspondence: Sharon Lee Choon Tow Tel +65 6322 7443
Fax +65 6333 8339
Email [email protected]
Purpose: The aim of the study is to examine the baseline intraocular pressure (IOP) and its changes after performing a water drinking test (WDT) in patients with unilateral hemifacial spasm (HFS).
Patients and Methods: In this prospective observational study, patients aged 21 years and above diagnosed with unilateral HFS were recruited from the Singapore National Eye Centre between January 2015 and August 2016. The unaffected eye of each patient served as a matched control. An interviewer-administered standardized questionnaire on HFS symptoms and ophthalmic examination was performed. Automated perimetry, optical coherence tomography (OCT) of the optic nerve head, color disc stereophotography and water drinking test (WDT) were done. The primary outcome measure was the difference in IOP between eyes affected by HFS and fellow eyes at baseline and at 15, 30 and 45 minutes of the WDT.
Results: Fifty-four patients with unilateral HFS were included. Mean age was 59.8± 9.9 years (range, 37.0– 84.0). Of these, 54% were female and 94% were Chinese. Mean baseline IOP was significantly higher in eyes with HFS (13.9± 3.1mmHg) compared to fellow eyes (13.3± 2.8mmHg) (p=0.008). There was no significant difference in absolute or percentage change in IOP from baseline between the 2 groups at 15, 30 and 45 minutes of the WDT. Mean vertical cup–disc ratio (VCDR) on clinical examination was significantly higher in eyes with HFS (0.5± 0.2) compared to fellow eyes (0.4± 0.2) (p=0.02). There was no significant difference between the groups for visual field parameters and mean retinal nerve fiber layer thickness on OCT.
Conclusion: Hemifacial spasm is associated with a small but significant difference in mean baseline IOP and VCDR between affected and fellow eyes. However, when eyes affected by HFS and fellow eyes were challenged with the WDT, both responded in similar ways.
Keywords: glaucoma, hemifacial spasm, intraocular pressure, water drinking test
Plain Language Summary
Spasms of the orbicularis oculi have been hypothesized to cause glaucomatous optic nerve head damage with studies reporting intraocular pressures of up to 50-100 mmHg with forced eyelid closure. The water drinking test is a provocative test that evaluates the ability of the eye to manage a transient rise in intraocular pressure. It stresses the trabecular meshwork by increasing the episcleral venous pressure, hence allowing the outflow facility and peak diurnal intraocular pressure to be evaluated. There are limited studies in the literature on the association of hemifacial spasm and the risk of glaucoma. To our knowledge, our study is one of the first to compare the intraocular pressure of eyes affected by unilateral hemifacial spasm and their response to the water drinking test to fellow unaffected eyes. In this prospective observational study, we found that eyes affected by hemifacial spasm have higher mean baseline intraocular pressure and mean vertical cup-disc ratio, compared to fellow unaffected eyes. However, eyes affected by hemifacial spasm and fellow unaffected eyes both responded in similar ways to the water drinking test.
Introduction
Hemifacial spasm (HFS) is a movement disorder of the muscles innervated by the facial nerve, characterized by progressive clonic or tonic movements.1 It is typically unilateral and begins with involuntary spasms of the orbicularis oculi, extending gradually to other facial muscles, with symptoms persisting during sleep.1 In most cases, an ectatic or aberrant blood vessel, usually an artery, compressing the facial nerve at the root exit zone, is the cause of the disease.2 Rarer causes include space-occupying lesions in the cerebellopontine angle or within the brainstem, demyelinating disease and brainstem infarction.3 In contrast, benign essential blepharospasm (BEB) is a facial dystonia that affects the orbicularis oculi but not the other muscles supplied by the facial nerve, and typically progresses to bilateral involvement.4
The main morbidities associated with HFS are psychosocial stress and impaired vision during episodes of eyelid spasms.5 Botulinum toxin (BTX) is an effective and minimally invasive treatment option for HFS but has the disadvantage that multiple injections are required at 3–4 monthly intervals and there are dampening effects after many years of treatment in some patients.1,6 Microvascular decompression of the facial nerve is a definitive treatment modality but only in patients with vascular compression of the facial nerve at the root exit zone.7 Little is known about the long-term sequelae of untreated HFS. Some patients may develop low-grade paralysis of the facial nerve after many years.
Spasms of the orbicularis oculi have been hypothesized to cause glaucomatous optic nerve head damage.8 Many reports have described increases of 5–10mmHg in intraocular pressure (IOP) with blinking and up to 50–100mmHg with forced eyelid closure.9–11 Furthermore, high IOP fluctuations have been implicated in glaucoma progression, most notably in the Advanced Glaucoma Intervention Study.12 There are few reports in the literature that examine the risk of glaucoma in persons with HFS or BEB.8,13–15 Killer et al reported a case of HFS associated with unilateral glaucoma.8 Erdogan et al compared 24 consecutive patients with HFS and 25 age- and gender-matched randomly selected subjects and found no statistically significant difference in IOP measurements between the 2 groups, as well as between the involved eye-side and uninvolved eye-side of HFS patients.15
The water drinking test (WDT) is a provocative test that evaluates the ability of the eye to manage a transient rise in IOP. It stresses the trabecular meshwork by increasing the episcleral venous pressure, hence allowing the outflow facility and peak diurnal IOP to be evaluated.16,17 A case series of 4 patients with BEB and glaucoma in the affected eye found that 3 of the patients have normal IOP measurements that became elevated to abnormal levels after a WDT, suggesting that patients with low trabecular outflow facility and BEB are at an increased risk of glaucomatous damage.14
We aimed to address some of the gaps in the literature by performing an observational study to assess the association of unilateral HFS with changes in IOP using the WDT, which to our knowledge, has not been evaluated before. We hypothesized that the eyes with HFS are associated with a greater rise in IOP after the WDT.
Patients and Methods
This observational study was conducted in accordance with the ethical principles described in the Declaration of Helsinki and that were consistent with the Singapore Good Clinical Practice and the applicable regulatory requirements. The study obtained ethics approval from the SingHealth Institutional Review Board and written informed consent was obtained from all study participants. Patients above the age of 21 years with a prior or new diagnosis of unilateral HFS were recruited from the Singapore National Eye Centre between January 2015 and August 2016. The unaffected eye of each patient served as a matched control.
Patients were excluded if there were any coexisting or previous ocular disease in either eye that may confound the assessment of the optic disc, measurement of retinal nerve fiber layer thickness and/or visual field assessment. These include corneal opacities, uveitis, dense cataracts, vitreous haemorrhage, diabetic retinopathy or diabetic macular edema, previous retinal laser photocoagulation, previous and coexisting retinal detachment, retinal dystrophies, retinopathy from any cause, macular scarring from any cause, optic neuropathy from any cause, anomalous optic discs or severely tilted optic discs, and peripapillary atrophy. Patients with pre-existing glaucoma or conditions that increase the risk of glaucoma including ocular hypertension, primary angle closure or suspect, topical steroid use, angle recession, pseudoexfoliation syndrome, pigment dispersion syndrome, Sturge-Weber syndrome, thyroid eye disease, carotid-cavernous sinus fistula were excluded. We also excluded patients who were pregnant, cognitively impaired, prisoners, or on a fluid-restricted diet.
The diagnoses of HFS were made clinically, based on signs and symptoms stated above, by a trained neuro-ophthalmologist. Baseline characteristics of study participants including age, gender, duration and frequency of facial or eyelid spasms, medical history and prior treatment for HFS, including time since last BTX injection, were recorded. An interviewer-administered questionnaire was performed to assess subjective improvement in symptoms after treatment of HFS.
Before pupil dilation, all subjects underwent a standardized ophthalmic examination including assessment of visual acuity, auto-refraction, IOP measurement with the Goldmann applanation tonometry (GAT) (Haag-Streit, Konig, Switzerland), blood pressure measurements and slit-lamp biomicroscopy. IOP was measured by an unmasked examiner with the average of 3 consecutive measurements obtained. Gonioscopy was performed with a Goldmann 2-mirror lens. Dynamic indentation gonioscopy with a four-mirror Sussman lens was performed to identify any peripheral anterior synechiae. After pupil dilation, the optic disc was examined for vertical cup–disc ratio (VCDR) and/or focal notching, and any maculopathy or retinopathy was excluded.
Automated perimetry was performed with near refractive correction (SITA 24-2 Fast program, Humphrey visual field (HVF) analyzer II; Carl Zeiss Meditec, Dublin, CA) before pupil dilation. Imaging of the optic nerve head (ONH) was performed with Cirrus (Carl Zeiss Meditec Inc, Dublin, CA) High Definition Optical Coherence Tomography (HDOCT). During a single scan, the retinal nerve fibre layer (RNFL) thickness was determined at 256 points around a set circular diameter of 3.4mm. This circle was then divided into 12 clock hours and also into four quadrants (temporal, superior, nasal and inferior). The RNFL values were then averaged to yield measurements for RNFL thickness in each clock hour, in each quadrant, and into a global average (360-degree measure). Color disc stereophotography was acquired using a digital fundus camera (Topcon 50-DX, Topcon Corporation, Tokyo, Japan).
Four hours prior to starting the WDT, all subjects were instructed not to ingest any fluids. The subjects were then asked to drink water at 10mL/kg body weight within 5 minutes. IOP was taken at the following time points: baseline, 15, 30 and 45 minutes after the end of water drinking, using the GAT in the sitting position, before pupillary dilatation. If the IOP at 45 minutes was high (>30 mmHg), it was re-checked again after half an hour. If it was still high, glaucoma medications might be prescribed.
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were taken during the WDT at the following time points: baseline, 15, 30 and 45 minutes after the end of water drinking. If SBP is more than 180 mmHg or DBP is more than 100 mmHg at 45 minutes, they were re-checked. If blood pressure was consistently high, oral Furosemide 40mg might be prescribed. We would also refer the patient to a primary care physician or the Accident and Emergency Department for evaluation of high blood pressure.
The primary outcome measure was the difference in IOP between eyes affected by HFS and fellow eyes at baseline and at 15, 30 and 45 minutes after the start of the WDT. We defined a positive WDT as a rise in IOP of 6 mmHg or more, or at least a 30% increase from baseline at any time after initiation of water consumption.18,19
Data were analyzed using Statistical Package for Social Sciences (SPSS) Version 20.0 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp., 2011). IOP, VCDR, RNFL thickness and HVF parameters were analysed with the paired samples T-test. All tests were 2 sided with statistical significance set at p<0.05.
Results
Fifty-four patients with unilateral HFS were included in the analysis. The baseline patient characteristics are shown in Table 1. Half of the patients had right-sided HFS. Mean duration of HFS, calculated from the difference between study visit date and date of diagnosis of HFS, was 57.5 ± 50.3 months (range, 0–222.0). Twenty-seven (50.0%) patients had HFS for less than 50% of waking hours. Forty-eight (88.9%) patients were symptomatic from HFS. Four (7.4%) patients reported upper eyelid fluttering, 10 (18.5%) reported lower eyelid fluttering, and 30 (55.6%) reported upper and lower eyelid fluttering. Eight (14.8%) patients reported lower eyelid spasms, and 31 (57.4%) reported upper and lower eyelid spasms. Eighteen (33.3%) patients reported complete eyelid closure. Seven (13.0%) patients were unaware of the symptoms.
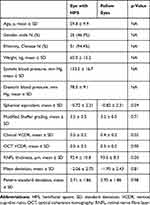 |
Table 1 Baseline Characteristics of Study Subjects and the Examination, Optical Coherence Tomography and Visual Field Parameters of Eyes Affected by Hemifacial Spasm and Fellow Eyes |
The Vladimir Theodor Thaller (VTT) grading was used to grade the severity of HFS during the study visit.20 Two (3.7%) patients had no observable signs of HFS. Fifteen (27.8%) patients were grade 0 (incomplete eyelid closure), 17 (31.5%) were grade 1 (lids just closing, minimal resistance to overcome), 13 (24.1%) were grade 2 (closing well, some resistance, easily overcome), and 3 (5.6%) were grade 3 (strong closure, can be overcome with difficulty). None were grade 4 (very strong closure, cannot be overcome or overcome with extreme difficulty).
Forty-three (79.6%) were treated with BTX injections, 10 (18.5%) had acupuncture, 6 (11.1%) had clonazepam therapy, and 3 (5.6%) had microvascular decompression. Eleven (20.4%) patients had more than 1 type of treatment, which included BTX injections and another modality. The mean duration from last BTX treatment was 15.8 ± 27.8 months (range, 0–138.0). Seven (13.0%) patients received no treatment.
Mean baseline IOP was statistically significantly higher in eyes with HFS (13.9 ± 3.1 mmHg, range 7.7–21.3) compared to fellow eyes (13.3 ± 2.8 mmHg, range 7.3–18.3) (p=0.008) (Table 2). However, there was no significant difference in absolute change in IOP or percentage change in IOP from baseline between the 2 groups at 15, 30 and 45 minutes from the start of the WDT. There were 17 (31.5%) and 13 (24.1%) eyes with positive WDT in eyes with HFS and fellow eyes, respectively (p=0.44). There was no significant difference between patients with and without a positive WDT in terms of clinical VCDR, Mean Deviation (MD), Pattern Standard Deviation (PSD) and RNFL thickness.
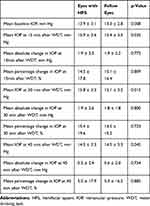 |
Table 2 Intraocular Pressures of Eyes Affected by Hemifacial Spasm and Fellow Eyes at Baseline, 15 Minutes, 30 Minutes and 45 Minutes After the Start of Water Drinking Test |
Mean VCDR on clinical examination was significantly higher in eyes with HFS (0.5 ± 0.2, range 0.1–0.8) compared to fellow eyes (0.4 ± 0.2, range 0.2–0.8) (p=0.02). Three eyes with HFS and 1 fellow eye were graded as suspicious disc cupping on examination; however, they had normal OCT and HVF results.
There was no statistically significant difference between study and fellow eyes in terms of MD (p=0.81), PSD (p=0.98), and Glaucoma Hemifield Test (GHT) (p=0.47). 1 eye with HFS was graded as having an abnormal visual field; however, it had normal disc appearance on examination and normal OCT results.
There was no statistically significant difference between the 2 groups in terms of average RNFL thickness (p=0.50). When the individual quadrants of the RNFL were compared, there was also no statistically significant difference between the 2 groups (p>0.05 in all quadrants). However, when we analysed the mean RNFL thickness in terms of clock hours, there was a statistically significant difference between the 2 groups at 3 O’clock (p=0.016) with the eyes affected by HFS having greater mean RNFL thickness (60.7 ± 13.5 µm) compared to fellow eyes (56.8 ± 10.5 µm). Mean RNFL thickness for the other clock hours showed no significant difference between the groups. Both the eyes affected by HFS and the fellow eyes have RNFL thickness within the normal range compared to age-matched subjects in the normative database. Rim area (p=0.79), disc area (p=0.39), average cup–disc ratio (p=0.91) and VCDR (p=0.90) on OCT were not significantly different between the 2 groups. Stratification by VTT grading did not show any significant results. 1 eye with HFS was graded to have superior RNFL thinning on OCT; however, it had normal disc appearance on examination and normal HVF results.
Discussion
We have found that eyes affected by HFS have a greater mean baseline IOP and mean VCDR compared to fellow unaffected eyes. However, when challenged with the WDT, the eyes affected by HFS and fellow eyes responded in similar ways with no significant difference in absolute change or percentage change in IOP from baseline between the 2 groups.
There are limited studies in the literature on the association of HFS with risk of glaucoma and no study, to our knowledge, which examines their causal relationship. Killer et al reported the only case in the literature of HFS associated with unilateral glaucoma.8 It is interesting that in this case, the optic disc appearance and visual field defect remained stable for 4 years since the time the patient was started on BTX treatment, with stable IOPs and not requiring any ocular hypotensive agents. This suggested a possible role of HFS treatment in halting glaucoma progression in such cases.
While our study found a significantly higher mean baseline IOP in eyes with HFS compared to fellow eyes, Erdogan et al found no significant difference in IOP measurements between the involved eye-side and uninvolved eye-side of HFS patients.15 They also noted that IOP was similar before and 2 weeks after BTX injections. However, in their study, Erdogan et al did not analyze cup–disc ratio or visual field parameters to look for possible signs of glaucomatous optic neuropathy and it was also limited by a smaller sample size. Although we found a significant difference in mean baseline IOP between eyes with HFS and fellow eyes, the WDT revealed no significant difference in absolute change in IOP or percentage change in IOP from baseline between the 2 groups at 15, 30 and 45 minutes from the start of the WDT. This suggested that the outflow facility and peak diurnal IOP of eyes affected by HFS and fellow eyes in this study were similar. Other methods to assess the underlying mechanisms of how HFS may affect IOP and glaucoma needs to be investigated.
Nicoletti et al’s case series of 4 patients with BEB and glaucoma in the affected eye found that 75% of them had normal IOP which became elevated to abnormal levels after WDT.14 In comparison, our study of non-glaucomatous eyes showed 17 (31.5%) HFS affected eyes with a positive WDT but none had any significant difference in terms of VCDR, MD, PSD and RNFL thickness, compared to eyes with a negative WDT.
Lee et al assessed the risk of incident glaucoma in a cohort of 1350 medicare beneficiaries receiving a diagnosis of BEB compared to a matched control group.13 They found no increase in risk of glaucoma and concluded that the cumulative effects of frequent, long term, intermittent and ultra-short-term IOP elevations from BEB do not result in glaucomatous damage. This study was limited by a lack of standardized diagnostic criteria, use of a propensity score matched cohort as a control group that does not reduce confounding, and did not address the confounding effect of BEB treatment on glaucoma risk.
Limitations of the present study include a relatively small sample size, lack of masking of the study examiner, and it is an interocular comparative study with no healthy control group. The difference in measurements of 0.6 mmHg of IOP and 0.1 of vertical cup–disc ratio between eyes with HFS and fellow eyes may not be clinically significant. Yet, the Early Manifest Glaucoma Trial has shown that even a 1 mmHg rise in IOP was associated with an 11% increase in the hazard ratio for glaucoma progression.21 Most of the patients included in our study had well-controlled HFS (83.4% had VTT grade 2 or less) and may have contributed to the small differences that we found. We also excluded eyes with pre-existing glaucoma. It is possible that among those excluded were eyes that developed glaucoma as a result of long-term poor control of HFS. However, by excluding eyes with pre-existing glaucoma, our cohort was free from confounding factor of non-HFS-related glaucoma.
In conclusion, we found significant differences in the mean baseline IOP and clinical VCDR measurements in eyes affected by HFS in our study, compared to the fellow eye. These differences were small and could be related to the relatively good control of HFS in our cohort. When challenged with the WDT, the eyes affected by HFS and fellow eyes responded in similar ways.
Disclosure
Dr Shamira A Perera reports personal fees from Santen, Glaukos, Allergan, Mundipharma, and Alcon Novartis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Wang A, Jankovic J. Hemifacial spasm: clinical findings and treatment. Muscle Nerve. 1998;21(12):1740–1747. doi:10.1002/(SICI)1097-4598(199812)21:12<1740::AID-MUS17>3.0.CO;2-V
2. Girard N, Poncet M, Caces F, et al. Three-dimensional MRI of hemifacial spasm with surgical correlation. Neuroradiology. 1997;39(1):46–51. doi:10.1007/s002340050366
3. Yaltho TC, Jankovic J. The many faces of hemifacial spasm: differential diagnosis of unilateral facial spasms. Mov Disord. 2011;26(9):1582–1592. doi:10.1002/mds.23692
4. Ben Simon GJ, McCann JD. Benign essential blepharospasm. Int Ophthalmol Clin. 2005;45(3):49–75. doi:10.1097/01.iio.0000167238.26526.a8
5. Wilkins RH. Hemifacial spasm: a review. Surg Neurol. 1991;36(4):251–277. doi:10.1016/0090-3019(91)90087-P
6. Simpson DM, Blitzer A, Brashear A, et al. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699–1706. doi:10.1212/01.wnl.0000311389.26145.95
7. Rosenstengel C, Matthes M, Baldauf J, et al. Hemifacial spasm: conservative and surgical treatment options. Dtsch Arztebl Int. 2012;109(41):667–673. doi:10.3238/arztebl.2012.0667
8. Killer HE, Rust O, Muller O, et al. Unilateral glaucomatous damage in a patient with hemifacial spasm. Ophthalmologica. 1999;213(4):273–275. doi:10.1159/000027436
9. Miller D. Pressure of the lid on the eye. Arch Ophthalmol. 1967;78(3):328–330. doi:10.1001/archopht.1967.00980030330011
10. Comberg W, Stoewer E. Die augendrucksteigernde wirkung verschiedener muskelaktionen und ihre bedeutung für klinische verhältnisse. Z Augenheilkd. 1926;58:92–105.
11. Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969;82(5):637–640. doi:10.1001/archopht.1969.00990020633011
12. Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111(9):1627–1635. doi:10.1016/j.ophtha.2004.02.017
13. Lee MS, Harrison AR, Grossman DS, et al. Risk of glaucoma among patients with benign essential blepharospasm. Ophthal Plast Reconstr Surg. 2010;26(6):434–437. doi:10.1097/IOP.0b013e3181d3da43
14. Nicoletti AG, Zacharias LC, Susanna R
15. Erdogan C, Rengin Y, Ceyhun A, et al. Effect of hemifacial spasm on intraocular pressure measurement. J Ophthalmol. 2018. Article ID 3621215.
16. Miller D. The relationship between diurnal tension variation and the water-drinking test. Am J Ophthalmol. 1964;58(2):243–246. doi:10.1016/0002-9394(64)91571-5
17. Susanna R
18. Leydhecker W. The water-drinking test. Br J Ophthalmol. 1950;34(8):457–479. doi:10.1136/bjo.34.8.457
19. Spaeth GL, Vacharat N. Provocative tests and chronic simple glaucoma. I. Effect of atropine on the water-drinking test: intimations of central regulatory control. II. Fluorescein angiography provocative test: a new approach to separation of the normal from the pathological. Br J Ophthalmol. 1972;56(3):205–216. doi:10.1136/bjo.56.3.205
20. Rahman R, Berry-Brincat A, Thaller VT. A new grading system for assessing orbicularis muscle function. Eye (Lond). 2003;17(5):610–612. doi:10.1038/sj.eye.6700444
21. Bengtsson B, Leske MC, Hyman L, et al.; Early Manifest Glaucoma Trial Group. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology. 114;2007:205–209. doi:10.1016/j.ophtha.2006.07.060
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
