Back to Journals » Clinical and Experimental Gastroenterology » Volume 12
Evaluating the clinical and economic consequences of using video capsule endoscopy to monitor Crohn’s disease
Authors Saunders R , Torrejon Torres R , Konsinski L
Received 20 December 2018
Accepted for publication 9 July 2019
Published 12 August 2019 Volume 2019:12 Pages 375—384
DOI https://doi.org/10.2147/CEG.S198958
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Wing-Kin Syn
Rhodri Saunders,1 Rafael Torrejon Torres,1 Lawrence Konsinski2
1Coreva Scientific, Freiburg, Germany; 2Illinois Gastroenterology Group, Elgin, IL, USA
Background: To assess the cost and patient impact of using small bowel and colon video capsule endoscopy (SBC) for scheduled monitoring of Crohn’s disease (CD).
Methods: An individual-patient, decision-analytic model of the CD care pathway was developed given current practice and expert input. A literature review informed clinical endpoints with data from peer-reviewed literature. Four thousand simulated CD patients were extrapolated from summary patient data from the Project Sonar Database. Two monitoring scenarios were assessed in this population. The first scenario represented common monitoring practice (CMP) for CD (ileocolonoscopy plus imaging), while in the second scenario patients were converted to disease monitoring using SBC. The cost-effectiveness of using SBC was assessed over 20 years. The cost of switching 50% of patients to SBC was assessed over 5 years for a health-plan including 12,000 patients with CD. Uncertainty of results was assessed using probabilistic sensitivity analysis.
Results: All patient groups showed increased quality of life with SBC versus CMP, with the highest gain in active symptomatic patients. Over 20 years, SBC reduced costs ($313,367 versus $320,015), increased life expectancy (18.15 versus 17.9 years) and increased quality of life (8.7 versus 8.0 QALY), making it a cost-effective option. SBC was cost-effective in 71% of individuals and 78% of populations including 50 patients. A payer implementing SBC in 50% of patients over 5 years could expect a decreased cost of monitoring (–$469 mean per patient) and surgery (–$698), but increased costs for active treatments (+$717). The discounted mean annual cost of care using CMP was $22,681 per patient over 5 years. The annual savings were $1135 per SBC-patient. The total savings for the payer over 5 years were $36.5 million.
Conclusion: SBC is likely to be a cost-effective and cost-saving strategy for monitoring CD in the US.
Keywords: video capsule, Crohn’s disease, budget impact, cost-effectiveness, inflammatory bowel disease, United States
Background
Crohn’s disease (CD) is a chronic inflammatory bowel disease which induces ulcers, strictures, fistulas, and abscesses within the gastrointestinal tract.1 Every year in the US, about 11 new CD cases per 100,000 person-years are diagnosed;2 with estimates putting the US prevalence of CD at 780,000 patients or about 246.7 cases per 100,000 persons.2,3 The economic burden of CD is also high.3 Median (interquartile) cost of care over 5 years has been reported at $116,838 ($45,643 to $240,398).4
 |
Figure 1 Comparison of major surgical outcomes between the model and published literature on Crohn’s disease. Bowel resection after 6 years using CMP (black crosses) is closely aligned to data published by Froslie et al,30 in initial years showing similarity to patients with mucosal healing and in later years transitioning to data for patients with active disease (A). Over 20 years (B), model data for bowel resection when using CMP (black crosses) and SBC (blue circles) is in line with data presented by Bernstein et al.31 To 10 years, SBC data most closely resemble that of the most recent clinical data. In both cases, model data are overlaid on figures extracted from the original, referenced publications.Abbreviations: SBC, Small bowel and colon video capsule endoscopy; CMP, Common monitoring practice. |
The course of CD is defined by periods of active and inactive disease (remission). In remission, patient quality of life and productivity increase,5 while health care resource use and costs of care decrease.6,7 The most efficient way to achieve and maintain remission usually involves the use of biologics, which are associated with a more positive disease course, but higher treatment costs.8,9 Optimized allocation of therapy in CD therefore relies on a precise diagnosis of active disease. Activity is assessed by clinical presentation and/or endoscopic activity.1 Clinical presentation is commonly measured using the Crohn’s Disease activity index (CDAI), while ileocolonoscopy is the current standard for measurement of endoscopic activity. The use of the CDAI alone to characterize disease activity is under debate, as recent work has determined that its ability to accurately differentiate between active and non-active disease is relatively low.1,9
Increasingly, the use of endoscopic remission (or mucosal healing) is advocated for stratifying patients into active and non-active disease as it has been associated with better long-term outcomes, lower need for surgery, and lower health care resource usage.8,10 Determination of mucosal healing requires visualization of the intestinal epithelium, generally achieved using ileocolonoscopy. However, this form of endoscopy cannot assess the proximal small intestine and small bowel, a CD location that affects between 16% and 65% of patients and is associated with a higher risk of severe complications.11–14 As such, further imaging such as magnetic resonance enterography (MRE) or computed tomographic enterography (CTE) may be required.15,16
A novel device in video capsule endoscopy (VCE) designed to visualize both the small bowel and colon (the SBC capsule, now PillCam Crohn’s, Medtronic Inc) allows for pan-enteric assessment of the small bowel and colon concurrently. Trials have reported promising sensitivity and specificity in identifying active CD alongside a low complication rate.13,17,18 Capsule retention may be of concern during VCE, though its incidence can be reduced with the use of a patency capsule to screen for contraindicated patients. VCE studies in patients with active CD have reported capsule retention rates of around 8% of procedures,19 but patient screening has been shown to reduce the rate to between 0% and 4% of procedures, mean 0.9%.20 Given its safety and potential benefits over MRE and CTE,21 SBC could reduce the number of monitoring procedures patients require to provide accurate information on mucosal healing.
To date, there have been no assessments of how changing to CD monitoring with SBC may impact costs or patient health. In the absence of extended clinical trials, computational modeling is a method to estimate this impact given relevant data from multiple studies. Models are growing in importance for assessing the impact of potential new therapies or changes in treatment practice. Patients are not exposed to potential harm, larger populations can be readily generated, and the effect of changes in practice can be quantified over time horizons beyond the limit of real-world studies. With this approach, the cost differential between interventions, so-called budget-impact analysis, or the balance between cost and health outcomes (cost-effectiveness) can be assessed.
A patient-level, discrete event model was used in this analysis to quantify the cost and patient burden associated with CD. We compare common monitoring practices (CMP, namely ileocolonoscopy plus MRE/CTE as required) to SBC in the monitoring of patients previously diagnosed with CD and estimate outcomes up to 20 years.
Methods
A discrete events simulation model was created to assess a change in monitoring modality from CMP to SBC for patients diagnosed with CD. The use of ileocolonoscopy plus the use of imaging in the form of MRE or CTE as required to evaluate the small bowel is considered to define CMP. In the simulation, 4000 individual patient entities are followed for between five and 20 years. Every 3 months, each patient has the potential to visit a physician for assessment of their current care. Depending on patient characteristics, this visit may include disease monitoring. The monitoring modality each patient receives (CMP or SBC) is fixed for the duration of the simulation. The efficacy of the monitoring modalities was modeled using their sensitivity and specificity to detect active CD. As such, each monitoring modality is associated with a rate of true positive, true negative, false positive, and false negative diagnoses. These data along with safety event incidence and procedure completion rates are provided in Table 1, with further details on monitoring and a detailed model description in the supplement.
 |
Table 1 Efficacy and safety of monitoring |
Source data
A structured literature review identified peer-reviewed publications that could inform model development. Searches were initially performed in PubMed on November 7, 2016, and were repeated periodically, most recently in June 2019. The aim was to identify high-quality data on efficacy and safety, the costs of care, and patient quality of life as measured by the EQ-5D index. The structured searches provided in the supplement (Tables S1–S4) returned 1599 abstracts for screening. Title and abstract screening using the Sourcerer webtool identified 168 articles for full-text review.
Decision analytic model structure
This model of the American Gastroenterological Association patient care pathway for CD22 was developed to conform to good practice guidelines.23,24 Each patient (N=4000) in the model had diagnosed CD and was in one of nine disease states: Remission (non-active CD, non-symptomatic), Non-active Symptomatic (non-active CD, but with symptomatology), Active Symptomatic (active CD with symptomatology), Active Non-symptomatic (active CD without symptomatology), On Treatment (receiving medication for management of active CD), Treatment Failure, Surgery, Post-surgery, or Death.
The status of each patient was assessed and updated quarterly. Within this process, the patient’s CD was assessed via CMP or SBC or assessment of patient-reported information and marker assessment. The patient’s actual CD state is known, allowing for tracking of correct (true positive or true negative) or incorrect (false positive or false negative) diagnoses; the diagnosed disease state was also tracked. Given the diagnosis and known patient characteristics, the patient is stratified into low, medium, or high-risk.22 Subsequently, a treatment decision was made, which can be no treatment if a negative (true or false) diagnosis was made (full treatment options in the supplement). The disease then progresses, regresses, or stabilizes. Adverse events associated with monitoring, treatment, surgery, and disease progression are evaluated and mortality assessed. The patient was lastly assigned to the health state that reflects their current CD profile, treatment, and life status. Further information on the model is available in the supplement section 1.4 and Figure S1, details on treatment efficacy in Table S6.
Patient profile
The model population was taken from summary statistics of patients in the practice of Illinois Gastroenterology Group Project Sonar Database (supplementary material).25 Where parameters were unavailable, these were sourced from peer-reviewed literature. A detailed overview is available in Table S5.
Model parameters
The model takes a payer perspective in assigning costs. Costs include, but are not limited to, physician fees, treatment purchase and administration costs, and cost of monitoring, surgery, (re)admission, adverse events, and symptomatic treatments (see Table S7). Costs are in 2016 USD and discounted at 3.5% per annum.
Model outcomes
A budget impact analysis determined cost outcomes over a 5-year time horizon. In this analysis, costs are compared between a scenario where all patients receive monitoring with CMP and a second scenario where 50% of them are switched to SBC monitoring. The outcome is total cost of care for a payer with 12,000 CD patients.
The cost-effectiveness analysis balances cost outcomes with patient outcomes measured in quality-adjusted life years (QALYs). Each CD state is associated with a baseline quality of life measured on a scale from 0 (no quality of life) to 1 (perfect quality of life). Each event in the model can apply a decrement to the patient’s baseline quality of life (see Table S7). As with costs, quality of life is discounted by 3.5% per annum. The cost-effectiveness analysis runs for 20 years. In this analysis, outcomes are compared between all patients receiving CMP or SBC. The outcome is the cost per QALY gained.
Model rigor
Although the budget-impact and cost-effectiveness models used similar approaches, they were programmed separately (one by RTT and one by RS). This independence allowed for evaluation of structural uncertainty in the model and its influence on outcomes. The budget-impact model assesses all 4000 patients simultaneously with sensitivity analyses performed separately. In contrast, the cost-effectiveness model iterates over 80 patients until all 4000 patients have been assessed. The sensitivity analyses are performed simultaneously with the base-case analysis (see supplement section 1.5 for more information on modality). During sensitivity analyses, the variance in the results of the clinical model is explored by varying the input parameters. In each simulation, every model input (except time horizon, discount rates, and currency) was varied (full details in the supplement). For the budget-impact analysis, bootstrapping was performed 1000 times with populations of 750 patients and in the cost-effectiveness analysis, bootstrapping was performed 2000 times with populations of varying sizes. In sensitivity analysis, the bootstrapped populations are used to determine the percentage of comparisons in which SBC was cost-saving or cost-effective and the 95% credible interval (CrI) for differences between monitoring modalities in terms of costs and QALYs. To determine the influence of the key parameters of sensitivity and specificity, the cost-effectiveness analysis was repeated for lower and higher boundary efficacies identified in a systematic review of comparative trials between SBC and CMP.26–29 The overall range of values was between 92–100% for sensitivity and 67–100% for specificity.27 For SBC, the least favorable data reported were sensitivity 93% and specificity 67%,26 with the most favorable being sensitivity 91% and specificity 100%.28
Results
Model validity
The impact of model findings regarding the cost-effectiveness and budget-impact of SBC relative to CMP can only be considered valuable, if the model outcomes with CMP reflect expectations from current clinical practice. Using CMP, the prevalence of surgical intervention at 6 years (30%) was similar to that reported by Frøslie et al for a Norwegian population (Figure 1).30 Over the longer term, our estimate of 45–60% of patients undergoing bowel resection over 20 years is comparable to the range reported by Bernstein et al of 40–60% of patients post-index event over the same time frame.31
Budget impact
The mean cost of care for a patient with CD was determined to be $22,681 per year using CMP. The cost of care consisted mainly of: CD treatments ($11,993), surgeries and inpatient procedures ($5560), and disease monitoring ($2496). Use of SBC by 50% of patients reduced total costs of care over a 5-ear period to $22,113 per patient-year, with substantial decreases in the cost of monitoring (–$469) and costs of surgery (–$698). Costs for active treatments were higher in SBC patients (+$717) due to more patients receiving active treatments. Cost of care was increased in early years but resulted in fewer surgeries over the 5-year period. Reduction in surgery accounted for almost all cost increases associated with active treatments. Overall, the annual saving per SBC patient was $1135. The total savings for the population of 12,000 patients over the 5-year period were $36,465,960 (−2.5%).
Sensitivity analyses showed a 90.6% chance of SBC being cost saving in a population of 750 patients over 5 years. The median cost saving was $1991 [–$914; $4964 95% CrI]. Analysis of individual patient costs showed a bimodal distribution of cost differentials between CMP and SBC. Many patients (48.3%) had a small cost differential between monitoring modalities of less than ±$5000, indicating little change in their care pathway related to the monitoring modality used. A cost increase of between $5000 and $10,000 with SBC occurred in 3.1% of patients, often due to slightly earlier onset of biologic treatment. Substantial cost increases with the use of SBC were rare, but where they occurred, were linked to capsule retention that required surgical removal. The majority of patients had a reduction in costs with the use of SBC; a cost saving of over $50,000 was found in 9.2% of patients.
Cost-effectiveness
Over the 20-year time horizon, use of CMP resulted in accumulated care costs of $320,015 and patients had an average life expectancy of 17.9 years, yielding total cost per patient life-year of $18,616. Patients achieved 8.0 QALYs. In the same time frame, total care costs with the use of SBC were $313,367 and patients had an average life expectancy of 18.15 years, yielding a cost of $17,265 per patient life-year. These patients accrued 8.7 QALYs. SBC thus provided a savings of $6648 over CMP over the 20-year time horizon. As SBC decreased care costs and resulted in increased QALYs, it dominated CMP and may be considered the best option for monitoring some patients with CD.
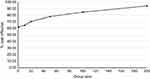
Over the 4000 patients, SBC was a cost-effective alternative to CMP in 71% of individuals. Sensitivity analysis of this result was assessed by bootstrap replication of individual patients from this population to simulate group sizes. This type of analysis permits estimation of potential results for a patient group, of a size of interest to health care payers and providers who will consider aggregate population or group outcomes. As the group size evaluated increased, it became increasingly likely that SBC would be considered a cost-effective option for monitoring of CD (Figure 2). At a group size of 50 patients, SBC would be considered cost-effective in 78% of those groups. The percentage considered cost-effective reached 84.4% and 94% with group sizes of 100 and 200 patients, respectively. Examination of the simulation data stratified by the patient’s starting health state revealed that every patient group had on average an increase in quality of life with the use of SBC. Quality of life benefit was lowest in patients starting in Remission and highest in those with Active Symptomatic Disease.
During the 20-year time horizon of the cost-effectiveness analysis, the time to event was assessed depending on monitoring modality between the two scenarios (Figure 3). As with the budget-impact analysis, use of SBC was associated with earlier initiation of active treatment for CD (Figure 3A). The time to 50% population exposure to biologics was 3 years with SBC and 4 years with CMP. For those patients in whom SBC was cost saving, this saving was under $10,000 per patient-year in 62% of cases. Where costs increased with SBC, this was generally (in 69% of cases) less than $10,000 per patient-year; often these patients had earlier initiation of active treatment but no change in overall health outcomes. Over the 20-year window, there was a 16%-point reduction in patients receiving colonic resection (Figure 3B). Examining individual patient results, most patients had only minor changes in quality of life, indicating that for most patients there is no significant difference in the care pathway between using SBC or CMP. The same was true for health care costs. As such, most patients benefit from SBC with the considerable benefits in quality of life and costs seen at the population level driven by a minority of patients who have large quality of life gains and/or cost reductions.
The results presented above used sensitivity and specificity data for SBC of 100% and 91% for the small bowel and 93% and 84% for the colon, respectively.29,32
In this scenario, SBC was a cost-effective alternative to CMP in 71% of individuals. For the least favorable data reported for SBC, SBC would be considered cost-effective in 56.7% of patients and in 74.9% of patients using the most favorable data identified. In all cases, a majority of the CD population are expected to benefit from SBC in a cost-effective way.
Discussion
The optimized management of chronic diseases is an ongoing challenge for health care providers. Depending on the perspective taken, optimization can be defined as cost minimization, patient outcome maximization, but ideally the balance between costs and outcomes. Payers wish to ensure that a health care intervention is affordable within their budget constraints, whereas patients are in general more concerned with health outcomes, irrespective of cost. Physicians, as the common point of this interaction, often aim to provide cost-effective solutions that balance patient outcomes against the cost of care. A recent conceptual shift in CD management is the targeting of mucosal healing and prevention of long-term complications as opposed to simply improving symptomatology.33 A large part of this is optimization of biologic therapies, and studies have supported their use earlier in the disease course to maximize beneficial outcomes.33 The costs associated with biologic therapies means, however, that patient selection is critical.
Use of endoscopy and imaging to identify patients with active disease is a key step in patient selection. Identifying these patients earlier, and before symptoms present, is a further challenge. Clinical data show that monitoring with VCE can help identify patients with active CD.13,17,18,34 Furthermore, Nishikawa et al showed in 2019 that there is a significant relationship between a Lewis score of over 264 and subsequent clinical relapse.35 Early work on the use of VCE for diagnosis of CD indicated that it was likely to be less costly than use of combined small-bowel follow-through and ileocolonoscopy.36 In 2010, Levesque et al evaluated alternative imaging strategies for the diagnosis of CD in the small-bowel and determined that use of VCE is beneficial in patients with moderate to high suspicion of small-bowel CD, largely because ileocolonoscopy cannot always examine the small bowel.37,38 In their study, VCE in addition to CMP was found not to be cost-effective,37 a finding supported by analyses performed with our model (data not shown). Nevertheless, the Canadian Association of Gastroenterlogy recommends this addition in case of inconclusive CMP results.34 The analysis presented here is the first to evaluate the cost-effectiveness and budget-impact of replacing CMP with SBC in patients already diagnosed with CD.
At a population or health care-plan level, our analysis suggested that SBC is expected to be cost saving over a period of 5 years. This payer benefit is complemented by the finding that most patients would be expected to see gains in quality of life. Although results for individual patients vary, overall use of SBC is likely to be considered cost-effective in the US. Considering populations as small as 15 patients, there was a 67% chance that SBC would cost less than $50,000 per QALY gained. Our analyses indicated that patients in remission had a lower likelihood of QALY benefits. As such, patients with symptoms may be the best candidates for initiation and continued use of SBC.
Use of SBC increased the costs associated with the use of biologics. The proportion of patients using biologics during the 20-year simulation was, however, equivalent: 95.4% (SBC) and 91.3% (CMP, Figure 3A). The increase in costs was mainly due to earlier initiation and longer duration of use of biologics with SBC. This effect was likely due to earlier identification of active disease (which is in line with optimized care provision) and a higher rate of false positive diagnosis. The sensitivity of SBC is generally higher than CMP (Table 1), which means that more true positive cases of CD are identified and placed on treatment, but its lower specificity means that more patients in remission are given a positive (active) diagnosis. The latter factor that may underlie the lower benefit for patients in remission, as being on treatment leads to reduced quality of life through the need for injectables and the potential for treatment side effects. This may change with the higher uptake of fecal calprotectin tests which were associated with a high specificity for absent disease and a British study has shown that the number of CMP referrals were decreased after pathway adoption.39 These hypotheses require testing in sufficiently powered clinical studies before conclusions can be drawn.
Increased costs of treatment were offset by reduced need for surgery and inpatient procedures with SBC. Of surgeries considered in this analysis, timeliness of intervention is critical to patient well-being where bowel resection and abscess drainage are required. Both had lower incidence with use of SBC. This result may in part be due to earlier treatment initiation. Additionally, some patients in remission and incorrectly assigned to treatment may have benefitted from “prophylactic” use of biologics. This is supported by the work of Holko et al who were able to show a positive effect of increased biologics treatment on surgical and hospitalization rates for CD.34
As with all studies, the present analysis has both strengths and weaknesses. The outcomes of this study provide clinically relevant insight into the potential impact of including SBC in CD monitoring. Results are only applicable to patients without a contraindication to VCE and who pass the patency capsule. The model assumes a fixed monitoring plan, that might not fully reflect real-life practice where patient can switch between VCE and ileocolonoscopy or may receive both. This implies certain limitations on supplemental diagnoses like biopsy, which in real practice can be used as confirmatory measures. Biopsy was not considered in our model, and one potential advantage of ileocolonoscopy is the ability to perform an adjunct biopsy if deemed necessary. The interpretation or application of results must, thus, be cautious as they are derived from a computational model representing the CD care pathway, which cannot precisely capture the intricacies of biology and real-life decision making. A model is dependent on the data used to inform it.
For certain parameters, insufficient data were available, and assumptions were required. Where made, these were intended to be realistic and conservative. For example, the quality of life associated with SBC was unknown. Research has shown a decrement of 0.0025 with ileocolonoscopy.40 As data support increased patient acceptance of VCE,41 a lower decrement was applied but it was not considered that SBC would be without any decrement as bowel preparation is still required. Most importantly, sensitivity and specificity data for SBC from a large cohort of patients with CD are required. Current data are from small cohorts or focused on small bowel disease, where VCE is expected to be superior to CMP.19,42 Furthermore, as the image processing software included with some SBC systems can help physicians track CD changes over time, longitudinal performance of SBC requires investigation and quantification.
Although computational in nature, the analysis provides results that reflect published clinical practice and support the utility of the results reported. Use of active treatments contributed 53% to the total cost of care, a result closely aligned to the recent work reporting that the cost share of injectable drugs was between 45.3% and 48.6%.43 It must also be noted that not all active treatments in use in the model were injectables. Kappelman et al reported that inpatient costs accounted for 31% of total costs and this is in line with surgery and complication costs in our model.44 The total cost of care reported here is also similar to published values. Previous studies estimate the annual cost of CD to be between $18,637 and $24,000.8,43 A recent study by Rao et al in published 2017 determined the interquartile range of cost at even between $9128 to $48,079.4 That our results, determined independently of any claims database data, are in line with cost data from claims databases lends further support to our findings. Finally, the consistency between model-derived rates of surgical intervention and published literature not used to inform model development is encouraging.30,31
Conclusion
SBC is likely to be cost saving compared with CMP over a 5-year time horizon. Increased costs of biologic treatments with SBC are offset by reduced surgery costs and a lower cost of monitoring. Over 20 years, SBC would likely be considered a cost-effective monitoring option for CD.
Ethics statement
Data from the Illinois Gastroenterology Group Project Sonar Database were made freely available and no ethics approval was required to access anonymized, aggregate, summary statistic data.
Disclosure
This work was funded by Medtronic. Rhodri Saunders is the owner and Rafael Torrejon Torres an employee of Coreva Scientific GmbH & Co KG, which received consultancy fees as part of this research collaboration. Lawrence Kosinski is a clinician who has ownership in the Sonar MD database and has provided expert input for Allergan Pharmaceuticals and Medtronic. Rhodri Saunders reports consultancy fees from Medtronic, ownership in Coreva Scientific, during the conduct of the study; consultancy fees from Cardinal Health, outside the submitted work; Rafael Torrejon Torres reports personal fees from Medtronic, personal fees from Coreva Scientific, during the conduct of the study; personal fees from Cardinal Health, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Iacucci M, Ghosh S. Looking beyond symptom relief: evolution of mucosal healing in inflammatory bowel disease. Therap Adv Gastroenterol. 2011;4(2):129–143. doi:10.1177/1756283X11398930
2. Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol. 2017;15(6):857–863. doi:10.1016/j.cgh.2016.10.039
3. Ganz ML, Sugarman R, Wang R, Hansen BB, Håkan-Bloch J. The economic and health-related impact of Crohnʼs disease in the United States. Inflamm Bowel Dis. 2016;22(5):1032–1041. doi:10.1097/MIB.0000000000000742
4. Rao BB, Click BH, Koutroubakis IE, et al. The cost of Crohnʼs disease. Inflamm Bowel Dis. 2017;23(1):107–115. doi:10.1097/MIB.0000000000000977
5. Lichtenstein GR, Yan S, Bala M, Hanauer S. Remission in patients with Crohn’s disease is associated with improvement in employment and quality of life and a decrease in hospitalizations and surgeries. Am J Gastroenterol. 2004;99(1):91–96.
6. Orlando A, Guglielmi FW, Cottone M, Orlando E, Romano C, Sinagra E. Clinical implications of mucosal healing in the management of patients with inflammatory bowel disease. Dig Liver Dis. 2013;45(12):986–991. doi:10.1016/j.dld.2013.07.005
7. Bassi A, Dodd S, Williamson P, Bodger K. Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut. 2004;53(10):1471–1478. doi:10.1136/gut.2004.041616
8. Loomes DE, Teshima C, Jacobs P, Fedorak RN. Health care resource use and costs in Crohn’s disease before and after infliximab therapy. Can J Gastroenterol. 2011;25(9):497–502. doi:10.1155/2011/157604
9. De Cruz P, Kamm MA, Prideaux L, Allen PB, Moore G. Mucosal healing in Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2013;19(2):429–444. doi:10.1002/ibd.22977
10. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009;15(9):1295–1301. doi:10.1002/ibd.20927
11. Samuel S, Bruining DH, Loftus EV, et al. Endoscopic skipping of the distal terminal ileum in Crohn’s disease can lead to negative results from ileocolonoscopy. Clin Gastroenterol Hepatol. 2012;10(11):1253–1259. doi:10.1016/j.cgh.2012.03.026
12. Lazarev M, Huang C, Bitton A, et al. Relationship between proximal Crohn’s disease location and disease behavior and surgery: a cross-sectional study of the IBD genetics consortium. Am J Gastroenterol. 2013;108(1):106–112. doi:10.1038/ajg.2012.389
13. Leighton JA, Gralnek IM, Cohen SA, et al. Capsule endoscopy is superior to small-bowel follow-through and equivalent to ileocolonoscopy in suspected Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12(4):609–615. doi:10.1016/j.cgh.2013.09.028
14. Boroff ES, Leighton JA. The role of capsule endoscopy in evaluating both suspected and known Crohn’s disease. Tech Gastrointest Endosc. 2015;17(1):5–11. doi:10.1016/j.tgie.2015.02.001
15. Papay P, Ignjatovic A, Karmiris K, et al. Optimising monitoring in the management of Crohn’s disease: a physician’s perspective. J Crohn’s Colitis. 2013;7(8):653–669. doi:10.1016/j.crohns.2013.02.005
16. NICE. Colonoscopic surveillance for preventing colorectal cancer in adults with ulcerativecolitis, Crohn’s disease or adenomas (CG 118). NICE Guidel. 2011;(March).
17. Jensen MD, Kjeldsen J, Nathan T. Fecal calprotectin is equally sensitive in Crohn’s disease affecting the small bowel and colon. Scand J Gastroenterol. 2011;46(6):694–700. doi:10.3109/00365521.2011.560680
18. Fernández-Urien I, Carretero C, González B, et al. Incidence, clinical outcomes, and therapeutic approaches of capsule endoscopy-related adverse events in a large study population. Rev Esp Enfermedades Dig. 2015;107(12):745–752. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26671587. Accessed June 16, 2017.
19. Leighton JA, Helper DJ, Gralnek IM, et al. Comparing diagnostic yield of a novel pan-enteric video capsule endoscope with ileocolonoscopy in patients with active Crohn’s disease: a feasibility study. Gastrointest Endosc. 2017;85(1):196–205.e1. doi:10.1016/j.gie.2016.09.009
20. Rezapour M, Amadi C, Gerson L. Retention associated with video capsule endoscopy: systematic review and meta-analysis. Gastrointest Endosc. 2017;85(6):1157–1168.e2. doi:10.1016/j.gie.2016.12.024
21. Kopylov U, Ben-Horin S, Seidman EG, Eliakim R. Video capsule endoscopy of the small bowel for monitoring of Crohnʼs disease. Inflamm Bowel Dis. 2015;21(11):1. doi:10.1097/MIB.0000000000000497
22. Sandborn WJ. Crohn’s disease evaluation and treatment: clinical decision tool. Gastroenterology. 2014;147(3):702–705. doi:10.1053/j.gastro.2014.07.022
23. Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis – principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Heal. 2014;17(1):5–14. doi:10.1016/j.jval.2013.08.2291
24. Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices – overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Value Heal. 2012;15(6):796–803. doi:10.1016/j.jval.2012.06.012
25. Kosinski L, Brill J, Sorensen M, et al. P-045 Validation of American Gastroenterological Association (AGA) Crohnʼs diseases care pathway risk assessment metrics against Crohnʼs related costs. Inflamm Bowel Dis. 2016;22:S23–S24. doi:10.1097/01.MIB.0000480091.25715.73
26. Biancone L, Calabrese E, Petruzziello C, et al. Wireless capsule endoscopy and small intestine contrast ultrasonography in recurrence of Crohnʼs disease. Inflamm Bowel Dis. 2007;13(10):1256–1265. doi:10.1002/ibd.20199
27. Choi M, Lim S, Choi MG, Shim KN, Lee SH. Effectiveness of capsule endoscopy compared with other diagnostic modalities in patients with small bowel crohn’s disease: a meta-analysis. Gut Liver. 2017;11(1):62–72. doi:10.5009/gnl16015
28. Albert JG, Martiny F, Krummenerl A, et al. Diagnosis of small bowel Crohn’s disease: a prospective comparison of capsule endoscopy with magnetic resonance imaging and fluoroscopic enteroclysis. Gut. 2005;54(12):1721–1727. doi:10.1136/gut.2005.069427
29. Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Diagnostic accuracy of capsule endoscopy for Small Bowel Crohn’s disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol. 2011;9(2):124–129.e1. doi:10.1016/j.cgh.2010.10.019
30. Frøslie KF, Jahnsen J, Moum BA, Vatn MH. Mucosal healing in inflammatory Bowel disease: results from a norwegian population-based cohort. Gastroenterology. 2007;133(2):412–422. doi:10.1053/j.gastro.2007.05.051
31. Bernstein CN, Loftus EV, Ng SC, Lakatos PL, Moum B. Hospitalisations and surgery in Crohn’s disease. Gut. 2012;61(4):622–629. doi:10.1136/gutjnl-2011-301397
32. Ladas SD, Triantafyllou K, Spada C, et al. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy. 2010;42(3):220–227. doi:10.1055/s-0029-1243968
33. Moss AC. Optimizing the use of biological therapy in patients with inflammatory bowel disease. Gastroenterol Rep. 2015;3(1):63–68. doi:10.1093/gastro/gou087
34. Enns RA, Hookey L, Armstrong D, et al. Clinical practice guidelines for the use of video capsule endoscopy. Gastroenterology. 2017;152(3):497–514. doi:10.1053/j.gastro.2016.12.032
35. Nishikawa T, Nakamura M, Yamamura T, et al. Lewis score on capsule endoscopy as a predictor of the risk for Crohn’s disease-related emergency hospitalization and clinical relapse in patients with Small Bowel Crohn’s disease. Gastroenterol Res Pract. 2019;2019:1–8. doi:10.1155/2019/4274257
36. Flemming J. Diagnosing Crohn’s disease: an economic analysis comparing wireless capsule endoscopy with traditional diagnostic procedures. Dis Manag. 2004;7(4):292–304. doi:10.1089/dis.2004.7.292
37. Levesque BG, Cipriano LE, Chang SL, Lee KK, Owens DK, Garber AM. Cost effectiveness of alternative imaging strategies for the diagnosis of small-bowel Crohn’s disease. Clin Gastroenterol Hepatol. 2010;8(3):
38. García-bosch O, Ordás I, Aceituno M, Rodríguez S, Ramírez AM. Comparison of diagnostic accuracy and impact of magnetic resonance imaging and colonoscopy for the management of Crohn’ s disease. J Crohn’s Colitis. 2016:663–669. DOI:10.1093/ecco-jcc/jjw015
39. Turvill J, Turnock D, Holmes H, Jones A, Hilton V, Marriott S. Evaluation of the clinical and cost- effectiveness of the York faecal calprotectin care pathway. Frontline Gastroenterol. 2018:1–10. DOI:10.1136/flgastro-2018-100962
40. Saini SD, Schoenfeld P, Vijan S. Surveillance colonoscopy is cost-effective for patients with adenomas who are at high risk of colorectal cancer. Gastroenterology. 2010;138(7):2292–2299.e1. doi:10.1053/j.gastro.2010.03.004
41. Rex DK, Lieberman DA. A survey of potential adherence to capsule colonoscopy in patients who have accepted or declined conventional colonoscopy. J Clin Gastroenterol. 2012;46(8):691–695. doi:10.1097/MCG.0b013e31824432df
42. Hudesman D, Mazurek J, Swaminath A. Capsule endoscopy in Crohn’s disease: are we seeing any better? World J Gastroenterol. 2014;20(36):13044–13051. doi:10.3748/wjg.v20.i36.13044
43. Park KT, Bass D. Inflammatory bowel disease-attributable costs and cost-effective strategies in the United States: a review. Inflamm Bowel Dis. 2011;17(7):1603–1609. doi:10.1002/ibd.21488
44. Kappelman MD, Rifas-shiman SL, Porter C, et al. Direct health care costs of Crohn’s disease and ulcerative colitis in United States children and adults. Gastroenterology. 2009;135(6):1907–1913. doi:10.1053/j.gastro.2008.09.012.Direct
45. Bourreille A. Wireless capsule endoscopy versus ileocolonoscopy for the diagnosis of postoperative recurrence of Crohn’s disease: a prospective study. Gut. 2006;55(7):978–983. doi:10.1136/gut.2005.081851
46. Ranasinghe I, Parzynski CS, Searfoss R, et al. Differences in colonoscopy quality among facilities: development of a post-colonoscopy risk-standardized rate of unplanned hospital visits. Gastroenterology. 2016;150(1):103–113. doi:10.1053/j.gastro.2015.09.009
47. Arora G, Mannalithara A, Singh G, Gerson LB, Triadafilopoulos G. Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc. 2009;69(3SUPPL.):654–664. doi:10.1016/j.gie.2008.09.008
48. Schwartz LM, Woloshin S, Welch HG. Using a drug facts box to communicate drug benefits and harms: two randomized trials. Ann Intern Med. 2009;150(8):516–527. doi:10.7326/0003-4819-150-8-200904210-00106
49. Nelson DB. Infectious disease complications of GI endoscopy: part I, endogenous infections. Gastrointest Endosc. 2003;57(4):546–556. doi:10.1067/mge.2003.139
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.