Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 13
Evaluating Nephrocheck® as a Predictive Tool for Acute Kidney Injury
Authors Nalesso F , Cattarin L, Gobbi L, Fragasso A , Garzotto F, Calò LA
Received 30 November 2019
Accepted for publication 1 April 2020
Published 24 April 2020 Volume 2020:13 Pages 85—96
DOI https://doi.org/10.2147/IJNRD.S198222
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Federico Nalesso,1 Leda Cattarin,1 Laura Gobbi,1 Antonio Fragasso,1 Francesco Garzotto,2 Lorenzo Arcangelo Calò1
1Department of Medicine, Nephrology, Dialysis and Transplantation Unit, University of Padova, Padova, Italy; 2Healthcare Directorate Unit, Veneto Institute of Oncology IOV-IRCCS, Padova, Italy
Correspondence: Federico Nalesso
Department of Medicine, Nephrology, Dialysis and Transplantation Unit, University of Padova, Via Giustiniani, 2, Padova 35128, Italy
Tel +39 049 8213128
Email [email protected]
Abstract: Acute kidney injury (AKI) is a common complication in critically ill patients in the intensive settings with increased risks of short- and long-term complications and mortality. AKI is also associated with an increased length of stay in intensive care units (ICU) and worse kidney function recovery at hospital discharge. The management of AKI is one of the major challenges for nephrologists and intensivists overall for its early diagnosis. The current KDIGO criteria used to define AKI include the serum creatinine and urinary output that are neither sensitive nor specific markers of kidney function, since they can be altered only after hours from the kidney injury. In order to allow an early AKI detection, in the last years, several studies focused on the identification of new biomarkers. Among all these markers, urinary insulin-like growth factor-binding protein (IGFBP-7) and tissue inhibitor of metalloproteinase (TIMP-2) have been proven as the best-performing and have been proposed as a predictive tool for the AKI detection in the critical settings in order to perform an early diagnosis. Patients undergoing major surgery, cardiac surgery, those with hemodynamic instability or those with sepsis are believed to be the top priority patient populations for the biomarker test. In this view, the urinary [TIMP-2] x [IGFBP-7] becomes an important tool for the early detection of patients at high risk for AKI and its integration with the local ICU experience has to provide a multidisciplinary management of AKI with the institution of a rapid response team in order to assess patients and customize AKI management.
Keywords: TIMP-2, IGFBP-7, NGAL, KIM-1, interleukin-18, L-FABP
Introduction
Acute kidney injury (AKI) is a common complication in critically ill patients in the intensive care settings associated with increased short- and long-term complications and higher mortality risk.
In the current literature, the most frequent cause of AKI in critically ill patients is sepsis, accounting for almost half of the cases, followed by major surgery (34%), cardiogenic shock (27%), hypovolemia (26%) and lastly administration of nephrotoxic drugs (19%).1
According to KDIGO criteria2 more than 50% of critical patients in the Intensive Care Unit (ICU) will develop AKI, requiring renal replacement therapy (RRT) in 13% of cases.3
It is known that AKI is associated with higher mortality risk, increased length of stay in ICU and worse kidney function recovery at hospital discharge with the presence of chronic kidney disease (CKD) criteria in almost 50% of the patients.3 This clinical evolution of renal function is very important since today it is not possible to identify patients at high risk of evolution toward CKD by a specific marker.
For this reason, AKI represents an economic burden for its high costs, longer hospitalization and need of RRT.
According to all these issues, the management of AKI is today one of the major challenges for nephrologists and intensivists overall for its early diagnosis and management. The current KDIGO diagnostic criteria of AKI include the serum creatinine and the urinary output, but it is well known that they are neither sensitive nor specific markers of kidney function since they can be altered only after hours from the kidney injury and they can depend from various patient’s characteristics. Furthermore, the consequent glomerular filtration rate (GFR) reduction is not synchronous with the renal injury, thus these two parameters vary with a certain delay. In effect, these markers seem to be potentially associated with many factors (volume status, sex, age, muscle mass) with poor correlation with the kidney injury.4 To overcome this problem, it would be necessary to identify a marker able to detect a renal damage even in the absence of GFR alterations, discovering episodes of sub-clinical AKI5 compensated by the renal functional reserve that can be lost after a pathological noxa, as heart surgery.6 In this point of view, in the last years, several studies focused on the identification of new biomarkers including neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL18), liver-type fatty acid binding protein (L-FABP), kidney injury molecule-1 (KIM-1), tissue inhibitor of metalloproteinases-2 (TIMP-2), and insulin-like growth factor-binding protein 7 (IGFBP-7).7,8 In this spectrum of new markers, urinary insulin-like growth factor-binding protein (IGFBP-7) and tissue inhibitor of metalloproteinase (TIMP-2) have been proven as the best-performing.9
In September 2014 the test “NephroCheck®” (Astute Medical, San Diego, CA, USA) was approved by the Food and Drug Administration. This immunoassay test measures the urinary concentrations of [TIMP-2] x [IGFBP-7], and combines them into a single numerical result that is the product of their concentrations. The AKIRisk® Score provides a quantitative risk index ([(TIMP-2]x[IGFBP-7])/1000, units = (ng/mL)2/1000). A 0.3 cut-off has been established to achieve high sensitivity while preserving acceptable specificity.10 Since its approval, several studies focused on the evaluation of its clinical utility.
The aim of this review is to analyse the research status of cell-cycle arrest biomarkers (TIMP-2 and IGFBP7) and their efficacy in predicting AKI in order to be used as a predictive tool for the early diagnosis of AKI in critical patients.
In order to verify the utility of TIMP-2 and IGFBP7 as early predictors of AKI, we reviewed the most significant clinical researches reported in PubMed. Since AKI can have different etiologies and can occur in several clinical contests, we arranged the clinical studies on the basis of the specific clinical conditions in which the biomarkers [TIMP-2] x [IGFBP-7] were validated.
Tissue Inhibitor of Metalloproteinase 2 (TIMP-2) and Insulin-Like Growth Factor-Binding Protein 7 (IGFBP-7)
Tissue inhibitor of metalloproteinase 2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP-7) are expressed and secreted in kidney and other tissues. Some authors postulated that these proteins cause cell-cycle arrest during the very early phase of cellular damage leading the cell-cycle arrest in G1 phase in response to various insults (eg oxidative stress, toxins, ischemia, sepsis, inflammation).11,12 Cells react to injury by repairing while entering and exiting different phases of proliferation assisted by kinases. G1 cell-cycle arrest prevents division of cells with damaged DNA, allowing an adequate repair (Figure 1). Several proteins regulate the cell cycle, particularly kinases. During renal injury in the early phases of AKI, several tumor suppressor proteins like p53, p27 and p21 can be activated, and they upregulate different proteins.11 IGFBP-7 directly increases the expression of p53 and p21, while TIMP-2 enhances p27 expression. These effects are carried in an autocrine and paracrine pattern through IGFBP-7 and TIMP-2 receptors. The p-proteins block the effect of the cyclin-dependent protein kinase complexes (CyclD-CDK4 and CyclE-CDK2) on the cell cycle promotion, resulting in G1 cell-cycle arrest. This protective mechanism is designed to avoid the cells to divide when DNA is damaged.13 If tubular cells become arrested at the G1 phase for prolonged periods, will develop senescence and fibrosis.14
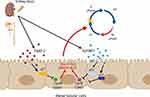 |
Figure 1 Tissue inhibitor of metalloproteinase 2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP-7) (Created with BioRender). |
Markers of cell-cycle arrest such as TIMP-2 and IGFBP7 may act as a signal warning that the tubular cells have been stressed and have to shut down their functions in the aim of preserve energy. TIMP-2 and IGFBP7 appear to be able to signal in autocrine and paracrine fashions, spreading the alarm from the site of injury11 to other sites. In Kashani et al15 prospective, multicentre investigation conducted in 2013, IGFBP-7 and TIMP-2 were validated using a clinical assay and compared to existing markers of AKI in a cohort of heterogeneous critically ill patients without evidence of AKI at the enrolment. The primary end point was moderate-severe AKI (KDIGO stage 2–3) within 12 hours which occurred in 14% of the study subjects. [TIMP-2] x [IGFBP-7] were validated with an area under the curve (AUC) of 0.80 together (0.76 and 0.79 alone). According to this trial [TIMP-2] x [IGFBP-7] was remarkably superior to other biomarkers of AKI (P < 0.002), including neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1), as neither of them reached an AUC > 0.72 in this investigation.
Cardiac Surgery
AKI has a high incidence in postsurgical cardiac patients, and it is estimated to be around 22%.16
The pathogenesis of cardiac surgery-associated acute kidney injury (CSA-AKI) is complex and multi-factorial; there are several mechanisms of damage involved such as hemodynamic and inflammation factors, ischemia-reperfusion injury and oxidative stress. Especially patients undergoing cardiopulmonary bypass (CPB) surgery are at high risk of developing postoperative AKI not only for hemodynamic alterations but also for the inflammation and oxidative stress related to the not biocompatible surface of CPB devices. In fact, during CPB the renal perfusion may be inadequate17 and the inflammatory and oxidative stress is increased.
Due to the clinical relevance of CSA-AKI, several studies focused on assessing the performance of [TIMP-2] x [IGFBP-7] as early biomarkers of AKI. In 2014 Meersch et al enrolled 50 patients undergoing cardiac surgery including cardiopulmonary bypass. In serial urine samples [TIMP-2] x [IGFBP-7] concentrations were measured. The primary outcome was the development of CSA-AKI. While creatinine and urine output alterations did not occur until 1–3 days after CPB, urinary [TIMP-2] x [IGFBP-7] started to rise 4 hours after CPB in patients who developed AKI. For urine [TIMP-2] x [IGFBP-7], a cut-off of 0.3 showed a good sensitivity and specificity at 4 hours after CPB, with the area under the ROC curve of 0.81 (CI: 0.68–0.93). Additionally, according to this study, the decline of urinary [TIMP-2] and [IGFBP-7] at the discharge was a strong predictor for renal recovery.18
In a recent analysis urine samples were collected from the patients enrolled in a randomized controlled trial, Cummings et al19 compared [TIMP-2] x [IGFBP-7] levels between patients undergoing cardiac surgery who developed AKI stage 2 or 3 according to KDIGO criteria and who did not. In patients who developed KDIGO AKI stage 2 and 3, [TIMP-2] x [IGFBP-7] levels showed a biphasic elevation with the first peak occurring during cardiac surgery and the second elevation at the 6th hour in the postoperative period. The authors suggest that both intraoperative and postoperative elevations of [TIMP-2] x [IGFBP-7] can predict moderate or severe AKI. In this study, there was no elevation of [TIMP-2] x [IGFBP-7] in patients who did not develop AKI confirming the validity of the test in this setting.
The intraoperative rise of [TIMP-2] x [IGFBP-7] was also confirmed in Mayer et al study20 in which the biomarkers levels were significantly higher after 1 hour of CPB in patients who developed AKI. According to the authors [TIMP-2] x [IGFBP-7] levels > 0.4 were the best cut-off (with a sensitivity of 0.778 and a specificity of 0.641) for predict AKI at an early time.
In Oezkur study,21 [TIMP-2] x [IGFBP-7] were measured in urinary samples at baseline, at ICU-admission, and 24 hours post-surgery using a cut-off of 0.3. The primary endpoint was the rate of AKI within the first 48 hours postoperatively. [TIMP-2] x [GFBP-7] values >0.3 at admission in ICU were strongly associated with the incidence of AKI (OR 11.8, p <0.001) with a sensitivity of 0.60 and a specificity of 0.88 while measurements before surgery (at baseline) were not related with the risk of AKI.
In the purpose of increase [TIMP-2] x [IGFBP-7] accuracy in predicting AKI, some authors tried to combine the test with other predictive tools. Zaouter et al22 validated the combination of urinary [TIMP-2] x [IGFBP-7] and the RRI (Renal Resistive Index) in patients scheduled for elective on-pump cardiac surgery at high risk for CSA-AKI. The incidence of AKI according to the KDIGO criteria was the primary outcome. Serial RRI measurements and the urinary [TIMP-2] x [IGFBP-7] were obtained simultaneously. [TIMP-2] x [IGFBP7] at 12 hours were significantly higher in patients that developed AKI (0.62, [IQR] [0.20–1.18] with an area under the curve of 0.69 [0.53–0.84]). The best sensitivity (65%) and specificity (62%) were achieved for a cut-off value of 0.3. Thus, the authors showed that the combination of [TIMP-2] x [IGFBP-7] and RRI at 12 hours had increased accuracy with an area under the receiver-operating characteristic curve of 0.78 [0.62–0.93].
Grieshaber et al23 tested an algorithm to predict CSA-AKI. Patients at high risk for CSA-AKI undergoing surgical procedures with CPB were identified using a combination of clinical scores, the Cleveland Clinic Score and the Leicester score.24 In these patients [TIMP-2] x [IGFBP-7] test was performed 4 hours postoperatively. The primary end point was the rate of postoperative AKI stages 2 to 3 within the sixth day after surgery. The secondary end points were all-stage AKI within the sixth day after surgery, AKI stage 3 within the sixth day after surgery, early AKI within the first day after surgery, and in-hospital mortality. None of the prediction scores used by the authors reached a good accuracy for AKI prediction. [TIMP-2] x [IGFBP-7] was fairly predictive for early all-stage AKI (AUC 0.63). All-stage AKI until the sixth day after surgery was noticed more frequently in patients with [TIMP-2] x [IGFBP-7] > 0.3 (odds ratio [OR] 2.9; 95% confidence interval 1.1–7.6). However, considering the ROC, [TIMP-2] x [IGFBP-7] were neither significantly predictive for the primary end point nor the secondary end points. In brief [TIMP-2] x [IGFBP7] ability to identify high-risk patients was weak and did not contribute to early diagnosis of CSA-AKI.
Wetz et al in 201525 analysed the adequacy of [TIMP-2] x [IGFBP-7] as a diagnostic test to identify early AKI after cardiac surgery. The authors included patients undergoing coronary artery bypass graft (CABG) with the use of CPB. The [TIMP-2] x [IGFBP-7] levels were measured at the baseline pre-surgery, at the end of surgery, 4 hours after cardiopulmonary bypass and on the first postoperative day. On the day of surgery, the concentration of [TIMP2] × [IGFBP-7] did not significantly differ between patients with or without AKI. At the first postoperative day, the median [TIMP-2] x [IGFBP-7] concentration of patients without AKI was 0.28, whereas patients with AKI had a significantly higher [TIMP-2] x [IGFBP-7] of 0.79. Applying previously published cut-off of 0.3 in the first postoperative day has been obtained a sensitivity of 53% and a specificity of 54%. The author concluded that the [TIMP-2] × [IGFBP-7] test may allow the identification of patients at increased AKI risk after cardiac surgery starting at the first postoperative day, but not during the early postoperative phase. They also believe that [TIMP-2] × [IGFBP-7] test may be more accurate in patients who are at high risk for AKI and may be less precise in patients at low risk.
In 2017 Meersch et al26 conducted a randomized clinical trial in order to prove that an implementation of supportive care in high-risk patients for cardiac surgery-related AKI could reduce their mortality and improve renal recovery. Patients with high risk for developing AKI undergoing cardiac surgery with cardiopulmonary bypass were enrolled. High-risk patients for AKI were defined by urinary [TIMP-2] x [IGFBP7] ≥ 0.3 after 4 hours of CPB. In the control group patients received standard care while patients in the intervention group received a rigorously controlled implementation of KDIGO clinical practice guidelines (discontinuation of nephrotoxic agents and ACEi and ARBs for the first 48 hours after surgery, close monitoring of serum creatinine and urine output, tight glycemic control, avoidance when possible radiocontrast agents, close hemodynamic monitoring, optimization of the fluid).2 The rate of AKI defined by KDIGO criteria within the first 72 hours after surgery was the primary outcome. Secondary end points were AKI severity, need for RRT, length of stay, and major adverse kidney events. In the intervention group, moderate and severe AKI showed lower rate while there were no significant differences for any of the secondary outcomes. The authors disclosed that early predictive biomarkers such as [TIMP-2] x [IGFBP7] can identify AKI high-risk patients and take preventing measures to reduce the occurrence of AKI.
In the point of view that a marker can identify patients to manage by preventive measures, Levante et al27 assessed the ability of the urinary [TIMP-2] x [IGFBP7] to predict the probability of developing CSA-AKI and evaluated its accuracy as a diagnostic test, by building a multivariate logistic regression model for CSA-AKI prediction. Based on their findings, when the urinary [TIMP-2] x [IGFBP7] is included in a multivariate model its performance is improved, moreover when the test result is >0.3, an automated electronic alert prompts the physician to intervene by following a checklist of preventive measures introducing an innovative way to early identify and manage patients at high risk of AKI.
Cardiac Arrest
The prevalence of AKI after cardiac arrest (CA) resuscitation ranges widely from 12% to 81%. In fact, hemodynamic shock28 decreases renal perfusion and ischemia-reperfusion injury induces systemic inflammatory response syndrome mediated by cytokine release and expression of inducible nitric oxide synthase. In this setting, early detection of patients with AKI may improve their management and outcomes. Serum creatinine measurement is inadequate for the early detection of AKI because it requires 48–72 hours for its elevation. Beitland et al used [TIMP‑2] x [IGFBP-7] to assess the risk of AKI in 195 patients resuscitated from out-of-hospital cardiac arrest (CA).29 Increased urine [TIMP-2] x [IGFBP-7] levels at admission were significantly associated with the development of AKI (0.65 vs 0.25, p<0.01) but no at day 3 (0.15 vs 0.24, p=0.079) probably due to the short half-lives of these markers. According to Beitland’s study, Adler et al30 observed, in a group of 48 patients, that increased urine [TIMP-2] x [IGFBP-7] levels, checked 3 hours after CA, can predict AKI. Titeca-Beauport et al,31 analyzing 115 patients after CA, found that urinary [TIMP-2] x [IGFBP-7] levels after 240 minutes of CA predict the development of AKI. These studies showed that [TIMP‑2] x [IGFBP-7] could be early predictive urinary biomarkers of AKI in patients resuscitated after CA with limitations related to their short half-lives.
Decompensated Heart Failure
Cardio-Renal syndrome type 1 refers to acute decompensation of cardiac function leading to AKI.32 AKI complicates from 24% to 45% of hospitalized acute decompensated heart failure (ADHF) patients, with consequent increase in length of stay and a higher likelihood of rehospitalization.33 Schanz et al34 examined the predictive ability of urinary [TIMP-2] x [IGFBP-7] for the development of moderate-severe AKI in 40 ADHF patients. The cell-cycle arrest biomarker discriminated for AKI within 24 hours of sample collection with an area under the curve of 0.84 (95% confidence interval: 0.72–0.93; sensitivity was 86% and specificity was 95%). Atici et al35 found in 111 acute decompensated heart failure (ADHF) patients a significant correlation between high urinary [TIMP-2] x [IGFBP-7] levels at admission and the occurrence of AKI. This may indicate that cell-cycle arrest biomarkers are useful in early detection of Cardio-Renal syndrome type 1. Nevertheless, further studies are needed to recommend these new renal biomarkers in ADHF.
Kidney Transplantation and Delayed Graft Function
Delayed graft function (DGF) is defined as failure of the renal transplant to function immediately, with the need for dialysis in the first post-transplantation week. DGF is associated with increased risk of rejection and long-term graft function loss. Yang et al36 assessed urinary [TIMP-2] x [IGFBP-7] levels at 0 hours post-transplantation in 74 patients who underwent kidney transplant from living or deceased donor. 23 were diagnosed with DGF. There were no differences in age, gender, causes of end-stage renal disease, ischemic time, or degree of HLA mismatch between the patients with early graft function and DGF. In multivariate analysis adjusting other factors, deceased donor and urinary [TIMP-2] x [IGFBP7] at 0 hours post-transplantation could predict the development of DGF with an area under the curve of 0.867 (sensitivity 0.86, specificity 0.71). Pianta et al37 demonstrated in 56 recipients from deceased donors that [TIMP-2] x [IGFBP-7] levels at 4 hours post-transplantation were good predictors of DGF in comparison with serum creatinine (p 0.05) with an area under the curve of 0.76 (sensitivity 0.72, specificity 0.81). The authors observed high urinary concentration of TIMP-2 in DGF group suggesting that DGF is associated with tubular epithelial G1 cell-cycle arrest. They also noticed that urinary concentrations of IGFBP7 in patients with slow graft function were elevated to intermediate levels compared with patients with immediate or delayed graft function. Consequently, IGFBP7 may poorly discriminate patients with DGF and non-DGF but help discriminate immediately from reduced graft function. Schmitt et al.38 observed in a group of 91 transplanted patients that urinary [TIMP-2] x [IGFBP-7] levels at day 1 and day 3 from transplantation significantly increase for patients suffering from a DGF in comparison with those with a normal graft function. Urinary [TIMP-2] x [IGFBP7] was shown to be significantly increased in the decreased donors group in comparison with the living donors group probably due to increased ischemia-reperfusion injury. Partially in contrast, Bank et al39 found in urine samples of 74 transplanted patients from deceased donor, that only urinary TIMP-2 on day 1 and on day 10 adequately identified patients with DGF (area under the curve 0.91) and prolonged DGF (area under curve 0.80), whereas IGFBP-7 did not (AUC 0.63 and 0.60). Using new biomarkers or their combination in predicting DGF could potentially improve long-term graft outcome by identifying these patients earlier and allowing clinicians to individually adjust their therapy.
Sepsis
Sepsis is the most common condition associated with AKI in critically ill patients, accounting for 50% or more of cases of AKI in ICU settings.40 Sepsis-induced AKI is strongly associated with increased mortality and adverse outcomes. An early diagnosis will allow for appropriate and timely interventions that may contribute to improve renal recovery and global outcomes of patients. Cuartero et al41 assessed the urinary [TIMP‑2] x [IGFBP-7] in 98 septic patients. Patients were stratified based on the presence of AKI and their highest level of [TIMP-2] x [IGFBP-7] within the first 12 hours of stay in ICU. [TIMP‑2] x [IGFBP-7] were significantly related to AKI severity according to AKIN criteria (p < 0.0001). The AUROC curve to predict AKI of the worst [TIMP-2] x [IGFBP7] index value was 0.798 (sensitivity 73.5%, specificity 71.4%, p < 0.0001). Index values below 0.8 ruled out any need for renal replacement (NPV 100%), whereas an index >0.8 predicted a rate of AKI of 71% and AKIN ≥ 2 of 62.9%. In this study [TIMP-2] x [IGFBP-7] was an early predictor of AKI in ICU patients regardless of sepsis. Honore et al42 observed significantly higher [TIMP‑2] x [IGFBP-7] levels in patients with AKI than without (p < 0.001). In multivariate analysis, the addition of urinary [TIMP‑2] x [IGFBP-7] significantly improved the performance of a clinical model for predicting AKI (p = 0.015). Maizel et al43 used [TIMP-2] x [IGFBP-7] to assess the risk of AKI in 112 patients admitted for septic shock with mild and moderate AKI. They observed that urinary [TIMP‑2] x [IGFBP-7] concentrations were independent factors to identify the subjects at high risk of progression from mild and moderate to severe AKI over the next 24 but not 72 hours. A urinary [TIMP‑2] x [IGFBP-7] concentration >2.0 quadruples the risk of KDIGO 3 AKI within 24 hours. Urinary [TIMP‑2] x [IGFBP-7] at the early phase of septic shock may be useful to identify the population at high risk of AKI.
Non-Cardiac Surgery
Non-cardiac postoperative AKI is associated with an increase in short and long-term complications and mortality. The use of urinary biomarkers for the early AKI detection can allow the precocious initiation of renal protection measures, including CRRT initiation,44 in the new concept of evaluating renal function in critically ill patients. Gocze et al45 observed in 107 non-cardiac postoperative patients a prevalence of 42% of AKI. The highest median values of [TIMP-2] × [IGFBP-7] were detected in sepsis, transplantation and patients after hepatic surgery with, respectively, the value of 1.24 vs 0.45 vs 0.47. The area under curve was 0.85 for the risk of AKI, 0.83 for early use of renal replacement therapy and 0.77 for 28-day mortality. In a multivariable model with established peri-operative risk factors, the [TIMP-2] × [IGFBP-7] test was a strong predictor of AKI (p<0.001).
Platinum-Induced Nephrotoxicity
Cisplatin is a widely used and highly effective cancer chemotherapeutic agent, but its nephrotoxicity is often the limiting factor. The incidence of cisplatin-induced AKI has been reported before, up to 69% of patients treated.46 However, AKI development during high-dose cisplatin-based chemoradiation is underreported using the KDIGO criteria as the most recent and preferred criteria for AKI diagnosis and staging. Moreover, the early detection of AKI can enable early intervention, which might lessen treatment burden and improves efficacy and cost-effectiveness of care.47 Schanz et al48 measured urinary [TIMP-2] × [IGFBP-7] levels within 6 hours prior to platinum administration and within 12 hours after the end of chemotherapy. They found that [TIMP-2] × [IGFBP-7] levels after platinum administration discriminated for the risk of AKI with an area under curve of 0.92, sensitivity was 50%, specificity was 87%, negative predictive value was 95% and positive predictive value was 25% for the prediction of AKI within 72 hours. In contrast, Toprak et al49 observed 45 patients with lung cancer treated with cisplatin: AKI prevalence was 28% in this group of patients. There was no difference between creatinine and [TIMP-2] × [IGFBP-7] levels before and after treatment. [TIMP-2] × [IGFBP-7] values were not different between patients with or without AKI. The area under the curve of [TIMP-2] × [IGFBP-7] at 24 hours of the treatment was 0.46 (CI 0.26–0.67).
Urine Dilution and Urine Osmolality
Several studies found a considerable overlap in the score of AKI of different RIFLE groups; this aspect makes interpretation of the score uncertain. A possible reason for the overlap between different RIFLE groups could be dependent on the urine dilution. Noto et al50 observed that fluid intake in healthy volunteers is able to modify the urinary concentration of [TIMP-2] x [IGFBP-7] levels, thus, urine osmolality should be considered in the interpretation of the results of the test. They collected urine samples from healthy subjects after 8 h of thirsting (T0) and after a water load of 0.5 L (T1). Osmolality as well as [TIMP-2] x [IGFBP-7] levels were measured. A significant difference was found between the mean AKIRisk® score at T0 (0.82, 95% CI 0.15 to 1.48) vs T1 (0.24, 95% CI 0.02 to 0.50), p = 0.01. The Pearson correlation between osmolality and a score of AKI at T0 and T1 was r = 0.93, p = 0.02, and r = 0.80, p = 0.03. L’Acqua et al51 examined 68 patients electively scheduled for cardiac surgery and described the correlation between urine creatinine and [TIMP-2] x [IGFBP-7] levels. All the samples with low [TIMP-2] x [IGFBP-7] levels (<0.2) also have low urine creatinine (less than 50 mg/dL), detecting a potential diluted sample. [TIMP-2] x [IGFBP-7] values could be related to the urine concentration; thus, urine osmolality should be considered in the interpretation of the results of the test.
Discussion
It is evident that the AKI syndrome affects very heterogeneous patients in Critical Care settings. The need to early identify AKI episodes, including sub-clinical ones,52 is a challenge for nephrologists and intensivists. In fact, the early AKI diagnosis allows the immediate measures necessary for the reduction of kidney damage and its short- and long-term complications.
In this particularly complex scenario, the need is to introduce in the clinical practice an AKI marker that is synchronous with renal damage (Figure 2). As previously discussed, the classic AKI diagnosis criteria, based on serum creatinine and urine output, have been shown not to be timely in identifying patients at AKI risk, as they undergo significant variations only when there is a clinical evident reduction in glomerular filtration53 and after that renal damage was expressed by reducing the GFR beyond the patient’s functional reserve. This clinical evidence occurs when the renal functional reserve54 is affected, and the residual glomerular filtrate is no longer able to maintain the renal compensation. Considering these clinical factors, it is clear that it is necessary to introduce a marker in clinical practice able to identify patients at high risk of kidney damage in order to apply all preventive manoeuvres and the AKI management, even before a clinical alteration of serum creatinine and diuresis.
 |
Figure 2 The AKI assessment (Created with BioRender). |
In the current clinical practice, clinicians evaluate high-risk patients for AKI on the basis of their clinical and biochemical trend, including the urinary output and serum creatinine that result neither sensitive nor specific markers of kidney function since they can be altered with a certain delay from the kidney injury and they can depend from patient’s characteristics. As validated by the recent literature, the use of new biomarkers could help to identify earlier patients with high risk for AKI, addressing properly the resources available55 and allowing clinicians to provide tailored therapies.
In cardiac surgery urinary [TIMP-2] x [IGFBP-7] seems to identify patients at high risk for AKI. Urinary [TIMP-2] x [GFBP7] values > 0.3 at admission in ICU were strongly associated with the incidence of AKI22 good sensitivity and specificity at 4 hours after CPB, and its decline at the discharge was a strong predictor for renal recovery.19 Both intraoperative and postoperative elevations of urinary [TIMP-2] x [IGFBP-7] can predict moderate or severe AKI.20 According to these characteristics, it is possible to increase the ability of the test to identify patients at AKI risk by the combination of urinary [TIMP-2] x [IGFBP-7] and the Renal Resistive Index (RRI) in patients scheduled for elective on-pump cardiac surgery at high risk for CSA-AKI.23 This concept can also open the use of ultrasound56,57 and biomarkers in the assessment of renal function reserve before and after episode of clinical and subclinical AKI. In this setting it is also possible to describe and assess the hemodynamic alterations of renal perfusion in AKI by the RRI58,59 while [TIMP‑2] x [IGFBP-7] levels can describe the renal injury. In order to improve the test efficacy, it is also possible to insert it in an algorithm that considers several clinical scores24 but, in this configuration, the urinary [TIMP-2] x [IGFBP-7] ability to identify high-risk patients is weak and the algorithm does not contribute to early diagnosis of CSA-AKI.
In patients undergoing coronary artery bypass graft (CABG) with the use of CPB, urinary [TIMP-2] × [IGFBP-7] test might allow the identification of patients at increased AKI risk after cardiac surgery starting at the first postoperative day, but not during the early postoperative phase showing a more accuracy in patients at high risk.26
Using [TIMP‑2] x [IGFBP-7] for early AKI diagnosis and management could improve the mortality and improve renal recovery by a rigorous controlled implementation of KDIGO clinical practice guidelines including the discontinuation of all nephrotoxic agents when possible, ensure volume status and perfusion pressure, consider functional hemodynamic monitoring, close monitoring of serum creatinine and urine output, tight glycemic control, avoidance when possible radiocontrast agents, check for changes in drug doses.27 The early diagnosis and management of the AKI are a focal element that allows to improve the patient’s outcome and the reduction of short- and long-term complications.
The ability of urinary [TIMP-2] x [IGFBP-7] to predict CSA-AKI can be increased by building a multidisciplinary team model, for CSA-AKI management, where an automated electronic alert prompts the physicians to intervene by following a checklist of preventive measures, introducing an innovative way to maximize the early AKI diagnosis and the specific treatment.60
In the setting of cardiac arrest, the early detection of patients with AKI may improve their management and outcomes. Serum creatinine measurement is inadequate for the early detection of AKI because it requires 48–72 h for its elevation. Increased urinary [TIMP-2] x [IGFBP-7] levels at admission in ICU is associated with the development of AKI only in the first hours probably due to the short half-lives of these markers,30 with a time window of about 3 hours after CA.31,32 These findings show that [TIMP‑2] x [IGFBP-7] could be an early predictive urinary biomarker of AKI in patients resuscitated after CA with limitations related to the markers short half-lives.
Cardio-Renal syndrome type 1 refers to acute decompensation of cardiac function leading to AKI that complicates from 24% to 45% of hospitalized acute decompensated heart failure (ADHF) patients, with increased length of stay and rehospitalisation.34 In ADHF patients a significant correlation between high urinary [TIMP-2] x [IGFBP-7] levels at admission and the occurrence of AKI was found36 indicating that cell-cycle arrest biomarkers are useful in early detection of Cardio-Renal syndrome type 1.
Another interesting field of application of this new marker is the renal transplant where the Delayed Graft Function (DGF) is associated with increased risk of rejection and long-term graft function loss. The urinary [TIMP-2] x [IGFBP-7] levels at 4 hours post-transplantation could predict the development of DGF more than creatinine and urine output.37,38 This data is also confirmed for the assessment of patient at 1 and 3 days from the transplant.39
The results suggested a plausible correlation between elevated urinary concentrations of cell-cycle arrest biomarkers with tubular epithelial damage due to DGF.
Of particular interest in the Critical Care is the urinary [TIMP-2] x [IGFBP-7] application in Sepsis that is the most common condition associated with AKI in critical patients, accounting for 50% or more of cases of AKI in ICU settings. In this scenario, urinary [TIMP‑2] x [IGFBP-7] resulted significantly related to AKI severity according to AKIN criteria and resulted an independent factor to identify the subjects at high risk of progression from mild and moderate to severe AKI over the next 24 hours from sepsis. [TIMP‑2] x [IGFBP-7] levels are able to anticipate the time window for the assessment of patients and the high risk of AKI in the critical care.
In a recent editorial, Ronco61 underlined the widespread resistance of physicians to adopt this diagnostic tool despite the consistent results obtained in the last years in several studies. Moreover, the author mentioned the McCullogh et al62 retrospective post hoc analysis of an ICU patients’ cohort in which they focused on the utility of [TIMP-2] × [IGFBP-7] measurements to predict stage 2–3 AKI over the course of the first 7 days of ICU stay. According to McCullogh, urinary levels of [TIMP-2] × [IGFBP-7] in critically ill patients at baseline, 12 hours, 24 hours and up to 3 days may predict the progressive risk of stage 2–3 AKI up to 7 days in the ICU, while three consecutive negative values were related with a very low incidence of AKI. Thus, an implementation of the patient’s biochemical profiles with serial measurements of these biomarkers may help to assess the risk of develop AKI and guide its management.
According to the current literature, [TIMP-2] × [IGFBP-7] showed an overall good sensitivity for moderate to severe AKI within 24h after the injury. False-positive results appear to be more common when the test is used in low-risk patients.13 Thus, [TIMP-2] × [IGFBP7] urinary level of >0.3, seems to have a greater predictability in patients at high risk for AKI, but it is certainly less useful in patients at low risk. For that reason, these biomarkers should be used in an appropriate patient population, undergoing cardiac or other major surgery, in the presence of sepsis in the critical settings, with at least one other risk factor for AKI.63
Of particular importance is the emergent possibility to identify patients who could evolve from AKI to CKD. In a Korean prospective trial64 enrolling 124 patients with established AKI according to KDIGO criteria, urinary [TIMP-2] × [IGFBP7] on the day of the diagnosis could predict the need for RRT and renal recovery at the time of discharge (defined as the return of serum creatinine to 25% of the baseline value). Except for this investigation, [TIMP-2] × [IGFBP7] levels have not been validated yet as a predictor tool for the risk of developing CKD after an episode of AKI. Emerging literature focused on discovering other predictors in order to identify patient at high-risk to develop CKD and requiring dialysis or follow up after the discharge. James et al65 validated a multivariable model to predict the progression of AKI to advanced CKD that includes six clinical variables (age, sex, baseline serum creatinine value, albuminuria, AKI stage and the discharge serum creatinine). More recently, post-discharge proteinuria has been suggested as a strong predictor of future kidney function decline after AKI,66 while urinary C–C motif chemokine ligand 14 (CCL14) has proved to be the best predictor of persistence or progression of AKI in a large cohort of critically ill patients with severe AKI.
Finally, a possible limitation in the use of [TIMP-2] x [IGFBP-7] is that several studies found a considerable overlap in their levels of different RIFLE groups; this aspect makes interpretation of the score in some cases uncertain. A possible reason for the overlap in the score of AKI between different RIFLE groups could be dependent on the urine dilution as Noto et al50 observed that fluid intake in healthy volunteers is able to modify the urinary concentration of [TIMP-2] x [IGFBP-7] levels. Similar results were proposed by L’Acqua et al51 describing the correlation between low [TIMP-2] x [IGFBP-7] levels (<0.2) and low urine creatinine (less than 50 mg/dL), in patients under heart surgery, suggesting that the [TIMP-2] x [IGFBP-7] values could be related to the urine concentration.
Conclusions
There is strong consensus for whom to test with urinary [TIMP‑2] x [IGFBP-7], how to interpret a test result, and for the actions to take based on test results.67 Patients undergoing major surgery, cardiac surgery, those with hemodynamic instability, or those with sepsis are believed to be the top priority patient populations for the biomarker test. For what concerns the timing, testing needs to occur early but only after a potential inciting event has occurred, with the top actions for positive tests to involve management of nephrotoxic drugs as well as fluids. In this view, the urinary [TIMP-2] x [IGFBP-7] becomes an important test for the early detection of patients at high risk for AKI and its integration with the local ICU reality has to provide a multidisciplinary management of AKI in Critical Care Medicine with the institution of a rapid response team that can assess patients and customize AKI management. It is, therefore, useful to proceed with this new tool by specific protocols and checklists for the prevention, early diagnosis and treatment of patients with AKI. This action has to be undertaken not only for the immediate management of AKI and its complications, but also for the management of long-term complications including the development of CKD.
Disclosure
Financial support: no grants or funding have been received for this study.
The authors report no conflicts of interest in this work.
References
1. Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients. a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi:10.1001/jama.294.7.813
2. Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving Global Outcomes (KDIGO) acute kidney injury work group. KDIGO Clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:19–36.
3. Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi:10.1007/s00134-015-3934-7
4. Bellomo R, Ronco C, Kellum JA, Mehta RL; Acute Dialysis Quality Initiative workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care. 2004;8:R204–12. doi:10.1186/cc2872
5. Haase M, Kellum JA, Ronco C. Subclinical AKI–an emerging syndrome with important consequences. Nat Rev Nephrol. 2012;8(12):735–739. doi:10.1038/nrneph.2012.197
6. Husain-Syed F, Ferrari F, Sharma A, et al. Persistent decrease of renal functional reserve in patients after cardiac surgery-associated acute kidney injury despite clinical recovery. Nephrol Dial Transplant. 2019;34(2):308–317. doi:10.1093/ndt/gfy227
7. Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238.
8. Murray P, Mehta R, Shaw A, et al. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int. 2020;180(3):513–521.
9. Fan W, Ankawi G, Zhang J, et al. Current understanding and future directions in the application of TIMP-2 and IGFBP7 in AKI clinical practice. Clin Chem Lab Med. 2019;57(5):567–576. doi:10.1515/cclm-2018-0776
10. Di Leo L, Nalesso F, Garzotto F, et al. Predicting acute kidney injury in intensive care unit patients: the role of tissue inhibitor of metalloproteinases-2 and insulin-like growth factor-binding protein-7 biomarkers. Blood Purif. 2018;45(1–3):270–277. doi:10.1159/000485591
11. Ortega LM, Heung M. The use of cell cycle arrest biomarkers in the early detection of acute kidney injury. Is this the new renal troponin? Nefrologia. 2018;38(4):361–367. doi:10.1016/j.nefro.2017.11.013
12. Gomez H, Ince C, De Backer D, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11. doi:10.1097/SHK.0000000000000052
13. Barnum KJ, O’Connell MJ. Cell cycle regulation by checkpoints. Methods Mol Biol. 2014;1170:29–40.
14. Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–543. doi:10.1038/nm.2144
15. Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi:10.1186/cc12503
16. Hu J, Chen R, Liu S, Yu X, Zou J, Ding X. Global incidence and outcomes of adult patients with acute kidney injury after cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2016;30:82–89. doi:10.1053/j.jvca.2015.06.017
17. Nadim MK
18. Meersch M, Schmidt C, Van Aken H, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9(3):e93460. doi:10.1371/journal.pone.0093460
19. Cummings JJ, Shaw AD, J S, Lopez MG, O’Neal JB, Billings FT. Intraoperative prediction of cardiac surgery-associated acute kidney injury using urinary biomarkers of cell cycle arrest. J Thorac Cardiovasc Surg. 2019;157(4):1545–1553. doi:10.1016/j.jtcvs.2018.08.090
20. Mayer T, Bolliger D, Scholz M, et al. Urine biomarkers of tubular cell damage for the prediction of acute kidney injury after cardiac surgery- a pilot study. J Cardiothorac Vasc Anesth. 2017;31:2072–2079. doi:10.1053/j.jvca.2017.04.024
21. Oezkur M, Magyara A, Thomasa P, et al. TIMP-2*IGFBP7 (Nephrocheck®) measurements at intensive care unit admission after cardiac surgery are predictive for acute kidney injury within 48 hours. Kidney Blood Press Res. 2017;42(3):456–467. doi:10.1159/000479298
22. Zaouter C, Potvin J, Bats ML, Beauvieux MC, Remy A, Ouattara A. A combined approach for the early recognition of acute kidney injury after adult cardiac surgery. Anaesth Crit Care Pain Med. 2018;37(4):335–341. doi:10.1016/j.accpm.2018.05.001
23. Grieshaber P, Möller P, Arneth B, et al. Predicting cardiac surgery-associated acute kidney injury using a combination of clinical risk scores and urinary biomarkers. Thorac Cardiovasc Surg. 2019.
24. Birnie K, Verheyden V, Pagano D, et al. UK AKI in cardiac surgery collaborators. predictive models for kidney disease: improving global outcomes (KDIGO) defined acute kidney injury in UK cardiac surgery. Crit Care. 2014;18(6):606. doi:10.1186/s13054-014-0606-x
25. Wetz AJ, Richardt EM, Wand S, et al. Quantification of urinary TIMP-2 and IGFBP-7: an adequate diagnostic test to predict acute kidney injury after cardiac surgery? Crit Care. 2015;19:3. doi:10.1186/s13054-014-0717-4
26. Meersch M, Schmidt C, Hofmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi:10.1007/s00134-016-4670-3
27. Levante C, Ferrari F, Manenti C, et al. Routine adoption of TIMP2 and IGFBP7 biomarkers in cardiac surgery for early identification of acute kidney injury. Int J Artif Organs. 2017;40(12):714–718. doi:10.5301/ijao.5000661
28. Rundgren M, Ullén S, Morgan MPG, et al. Renal function after out-of-hospital cardiac arrest; The influence of temperature management and coronary angiography, a post hoc study of the target temperature management trial. Crit Care. 2019;23(1):1–10. doi:10.1186/s13054-019-2390-0
29. Beitland S, Waldum-Grevbo BE, Nakstad ER, et al. Urine biomarkers give early prediction of acute kidney injury and outcome after out-of- hospital cardiac arrest. Crit Care. 2016;20(1):1–11.
30. Adler C, Heller T, Schregel F, et al. TIMP-2/IGFBP7 predicts acute kidney injury in out-of-hospital cardiac arrest survivors. Crit Care. 2018;22(1):1–9. doi:10.1186/s13054-018-2042-9
31. Titeca-Beauport D, Daubin D, Chelly J, et al. The urine biomarkers TIMP2 and IGFBP7 can identify patients who will experience severe acute kidney injury following a cardiac arrest: a prospective multicentre study. Resuscitation. 2019;141:104–110. doi:10.1016/j.resuscitation.2019.06.008
32. House AA, Anand I, Bellomo R, et al.; Acute Dialysis Quality Initiative Consensus Group. Definition and classification of cardio-renal syndromes: workgroup statements from the 7th ADQI consensus conference. Nephrol Dial Transplant. 2010;25(5):1416–1420.
33. Ismail Y, Kasmikha Z, Green HL, McCullough PA. Cardio-renal syndrome type 1: epidemiology, pathophysiology, and treatment. Semin Nephrol. 2012;32(1):18–25. doi:10.1016/j.semnephrol.2011.11.003
34. Schanz M, Shi J, Wasser C, Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol. 2017;40(7):485–491. doi:10.1002/clc.22683
35. Atici A, Emet S, Cakmak R, et al. Type I cardiorenal syndrome in patients with acutely decompensated heart failure: the importance of new renal biomarkers. Eur Rev Med Pharmacol Sci. 2018;22(11):3534–3543. doi:10.26355/eurrev_201806_15180
36. Yang J, Lim SY, Kim MG, Jung CW, Cho WY, Jo SK. Urinary tissue inhibitor of metalloproteinase and insulin-like growth factor-7 as early biomarkers of delayed graft function after kidney transplantation. Transplant Proc. 2017;49(9):2050–2054. doi:10.1016/j.transproceed.2017.09.023
37. Pianta TJ, Peake PW, Pickering JW, Kelleher M, Buckley NA, Endre ZH. Evaluation of biomarkers of cell cycle arrest and inflammation in prediction of dialysis or recovery after kidney transplantation. Transpl Int. 2015;28(12):1392–1404. doi:10.1111/tri.12636
38. Schmitt FCF, Salgado E, Friebe J, et al. Cell cycle arrest and cell death correlate with the extent of ischaemia and reperfusion injury in patients following kidney transplantation – results of an observational pilot study. Transpl Int. 2018;31(7):751–760. doi:10.1111/tri.13148
39. Bank J, Ruhaak R, Soonawala D, et al. Urinary TIMP-2 predicts the presence and duration of delayed graft function in donation after circulatory death kidney transplant recipients. Transplantation. 2019;103(5):1014–1023. doi:10.1097/TP.0000000000002472
40. Wang K, Xie S, Xiao K, Yan P, He W, Xie L. Biomarkers of sepsis-induced acute kidney injury. Biomed Res Int. 2018;24(2018):6937947.
41. Cuartero M, Ballús J, Sabater J, Pérez X, Nin N, Ordonez-Llanos J. Betbesé AJ Cell-cycle arrest biomarkers in urine to predict acute kidney injury in septic and non-septic critically ill patients. Ann Intensive Care. 2017;7(1):92. doi:10.1186/s13613-017-0317-y
42. Honore PM, Nguyen HB, Gong M, et al., Sapphire and Topaz Investigators. Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for risk stratification of acute kidney injury in patients with sepsis. Crit Care Med. 2016;44(10):1851–1860. doi:10.1097/CCM.0000000000001827
43. Maizel J, Daubin D, Vong LV, et al. Urinary TIMP2 and IGFBP7 identifies high risk patients of short-term progression from mild and moderate to severe acute kidney injury during septic shock: a prospective cohort study. Dis Markers. 2019;2019:3471215. doi:10.1155/2019/3471215
44. Clark WR, Neri M, Garzotto F, et al. The future of critical care: renal support in 2027. Crit Care. 2017;21(1):92. doi:10.1186/s13054-017-1665-6
45. Gocze I, Koch M, Renner P, et al. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS One. 2015;10(3):e0120863. doi:10.1371/journal.pone.0120863
46. van der Vorst MJDL, Neefjes ECW, Toffoli EC, et al. Incidence and risk factors for acute kidney injury in head and neck cancer patients treated with concurrent chemoradiation with high-dose cisplatin. BMC Cancer. 2019;19(1):1066. doi:10.1186/s12885-019-6233-9
47. Lewington AJ, Cerdá J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84:457–467. doi:10.1038/ki.2013.153
48. Schanz M, Hoferer A, Shi J, Alshcer MD, Kimmel M. Urinary TIMP2·IGFBP7 for the prediction of platinum induced acute renal injury. Int J Nephrol Renovasc Dis. 2017;10:17581. doi:10.2147/IJNRD.S135271
49. Toprak Z, Cebeci E, Helvaci SA, et al. Cisplatin nephrotoxicity is not detected by urinary cell-cycle arrest biomarkers in lung cancer patients. Int Urol Nephrol. 2017;49(6):1041–1047. doi:10.1007/s11255-017-1556-4
50. Noto A, Cortegiani A, David A. Nephrocheck: should we consider urine osmolality? Crit Care. 2019;23(1):1–2. doi:10.1186/s13054-019-2341-9
51. L’Acqua C, Sisillo E, Salvi L, Introcaso G, Biondi ML. Nephrocheck after cardiac surgery: does it play a role in daily practice? A sequel of “Nephrocheck results should be corrected for dilution.”. Int J Artif Organs. 2019;42(11):665–667. doi:10.1177/0391398819852958
52. Ronco C, Kellum JA, Haase M. Subclinical AKI is still AKI. Crit Care. 2012;16(3):313. doi:10.1186/cc11240
53. Ronco C. Acute kidney injury: from clinical to molecular diagnosis. Crit Care. 2016;20(1):201. doi:10.1186/s13054-016-1373-7
54. Husain-Syed F, Ferrari F, Sharma A, et al. Preoperative renal functional reserve predicts risk of acute kidney injury after cardiac operation. Ann Thorac Surg. 2018;105(4):1094–1101. doi:10.1016/j.athoracsur.2017.12.034
55. Kashani K, Cheungpasitporn W, Ronco C. Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption. Clin Chem Lab Med. 2017;55(8):1074–1089. doi:10.1515/cclm-2016-0973
56. Samoni S, Nalesso F, Meola M, et al. Intra-Parenchymal Renal Resistive Index Variation (IRRIV) describes Renal Functional Reserve (RFR): pilot study in healthy volunteers. Front Physiol. 2016;7:286. doi:10.3389/fphys.2016.00286
57. Meola M, Nalesso F, Petrucci I, Samoni S, Ronco C. Ultrasound in acute kidney disease. Contrib Nephrol. 2016;188:11–20.
58. Meola M, Nalesso F, Petrucci I, Samoni S, Ronco C. Pathophysiology and clinical work-up of acute kidney injury. Contrib Nephrol. 2016;188:1–10.
59. Meola M, Nalesso F, Petrucci I, Samoni S, Ronco C. Clinical scenarios in acute kidney injury: pre-renal acute kidney injury. Contrib Nephrol. 2016;188:21–32.
60. Rizo-Topete LM, Rosner MH, Ronco C. Acute kidney injury risk assessment and the nephrology rapid response team. Blood Purif. 2017;43(1–3):82–88. doi:10.1159/000452402
61. Ronco C. Acute kidney injury biomarkers: are we ready for the biomarker curve? Claudio ronco. Cardiorenal Med. 2019;9:354–357. doi:10.1159/000503443
62. McCullough PA, Ostermann M, Forni LG. Serial urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 and the prognosis for acute kidney injury over the course of critical illness. Cardiorenal Med. 2019;9:358–369. doi:10.1159/000502837
63. Vijayan A, Faubel S, Askenazi DJ, et al. Clinical use of the urine biomarker [TIMP-2] × [IGFBP7] for acute kidney injury risk assessment. AJKD. 2016;68:19–28. doi:10.1053/j.ajkd.2015.12.033
64. Cho WY, Lim SY, Yang JH, Oh SW, Kim MG, Jo SK. Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 as biomarkers of patients with established acute kidney injury. Korean J Intern Med. 2019;23.
65. James MT, Pannu N, Hemmelgarn BR, et al. Derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury. JAMA. 2017;318(18):1787–1797. doi:10.1001/jama.2017.16326
66. Hsu C, Chinchilli VM, Coca S, et al. Post–acute kidney injury proteinuria and subsequent kidney disease progression.The Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) study for the ASSESS-AKI Investigators. JAMA Intern Med. 2020;180(3):402–410. doi:10.1001/jamainternmed.2019.6390
67. Guzzi LM, Bergler T, Binnall B, et al. Clinical use of [TIMP-2]•[IGFBP7] biomarker testing to assess risk of acute kidney injury in critical care: guidance from an expert panel. Crit Care. 2019;23(1):225. doi:10.1186/s13054-019-2504-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
