Back to Journals » Clinical Ophthalmology » Volume 10
Epiretinal membrane: optical coherence tomography-based diagnosis and classification
Authors Stevenson W, Prospero Ponce C, Agarwal D, Gelman R, Christoforidis J
Received 6 October 2015
Accepted for publication 7 January 2016
Published 29 March 2016 Volume 2016:10 Pages 527—534
DOI https://doi.org/10.2147/OPTH.S97722
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
William Stevenson, Claudia M Prospero Ponce, Daniel R Agarwal, Rachel Gelman, John B Christoforidis
Department of Ophthalmology, University of Arizona Medical Center, Tucson, AZ, USA
Abstract: Epiretinal membrane (ERM) is a disorder of the vitreomacular interface characterized by symptoms of decreased visual acuity and metamorphopsia. The diagnosis and classification of ERM has traditionally been based on clinical examination findings. However, modern optical coherence tomography (OCT) has proven to be more sensitive than clinical examination for the diagnosis of ERM. Furthermore, OCT-derived findings, such as central foveal thickness and inner segment ellipsoid band integrity, have shown clinical relevance in the setting of ERM. To date, no OCT-based ERM classification scheme has been widely accepted for use in clinical practice and investigation. Herein, we review the pathogenesis, diagnosis, and classification of ERMs and propose an OCT-based ERM classification system.
Keywords: macular pucker, cellophane macular reflex, preretinal macular fibrosis, optical coherence tomography, central foveal thickness, inner segment ellipsoid band
Introduction
Epiretinal membrane (ERM), also known as macular pucker or cellophane maculopathy, is a disorder of the vitreomacular interface that can cause visual impairment. The clinical presentation of an ERM can range from completely asymptomatic, diagnosed on routine examination, to profoundly symptomatic with metamorphopsia, micropsia or macropsia, photopsia, decreased visual acuity (VA), and loss of central vision. The symptoms associated with ERMs, especially metamorphopsia, can impair vision-related quality of life.1 It has been estimated that 30 million people of advanced age in the US have an ERM in at least one eye.2 Numerous potential risk factors for the development of an ERM have been identified, including race, ethnicity, sex, smoking, diabetes, arteriolar narrowing, and hypercholesterolemia; however, the most consistently identified risk factor is age.3 Most ERMs occur in individuals older than 50 years, and the prevalence of ERM increases as age increases. The prevalence of ERM varies from 2.2% to 28.9% depending on the population being studied.4,5
ERM is a pathologic fibrocellular membrane that lies immediately superjacent to the inner surface of the retina. Clinically, an ERM can be classified as either cellophane macular reflex or preretinal macular fibrosis based on severity.2 Furthermore, an ERM can be classified as either idiopathic or secondary based on etiology.6–13 Historically, ERMs were diagnosed and classified based on clinical examination findings alone. However, recent advances in imaging have allowed clinicians to more accurately diagnose and characterize ERMs and their associated complications, such as vitreomacular traction and macular hole.14 Imaging techniques, such as spectral-domain optical coherence tomography (SD-OCT) with three-dimensional reconstruction, have been introduced for this purpose. In order to fully harness these technological advances, a standardized classification system must be devised. Herein, we review the basics of ERMs and propose an OCT-based classification system for ERMs.
Pathogenesis
The vitreous is the transparent gel that occupies the posterior segment of the eye and is composed primarily of water, collagen, hyaluronan, and hyalocytes.15 The vitreous adheres to the inside of the eye at the posterior lens capsule, peripheral retina, retinal vessels, perimacular region, and optic disk. As the vitreous ages, it liquefies and its retinal adhesions weaken. This can precipitate the separation of the vitreous from its posterior attachments, an occurrence known as posterior vitreous detachment (PVD). PVD has been described in up to 95% of cases of idiopathic ERM.16 Numerous theories have been proposed to explain the association between PVD and idiopathic ERM. A classically accepted theory is that PVD causes breaks in the internal limiting membrane (ILM) that allow cells to migrate to the inner surface of the retina where they form an idiopathic ERM.17,18 However, this theory has been challenged by the finding that breaks are exceedingly rare in the ILMs associated with ERMs.19 An alternative theory has been proposed that involves the concept of anomalous PVD.20 Anomalous PVD occurs when vitreous liquefaction outpaces vitreoretinal adhesion weakening, resulting in vitreoschisis and vitreoretinal traction.21 When vitreoschisis occurs, remnants of the cortical vitreous are left in the premacular region.22 Vitreoretinal traction induces the production of cytokines, such as basic fibroblast growth factor and nerve growth factor, that stimulate the residual vitreous cells to proliferate.23 Moreover, residual vitreous cells may promote the migration of cells or projection of cell processes through an otherwise intact ILM.24
The precise identification of the cells and cell lineages involved in the pathogenesis of idiopathic ERMs has been hindered by the ability of these cells to transdifferentiate.25 Glial cells are thought to predominate in early idiopathic ERMs.26 However, the exact etiology of these glial cells remains unclear; there is evidence that these glial cells derive from Müller cells or astrocytes.26–28 Hyalocytes, likely originating from cortical vitreous remnants following anomalous PVD, have been identified in ERMs, and the transdifferentiation of hyalocytes may play a central role in ERM formation.28,29 The role of macrophages in the pathogenesis of idiopathic ERMs has yet to be determined; hyalocytes are of macrophage lineage, and some glial cells are specialized macrophages.29,30 The presence of retinal pigment epithelial cells in idiopathic ERMs is a subject of debate, and there is evidence that retinal pigment epithelial cells may only be found in secondary ERMs following retinal detachment.31,32 Fibroblasts likely contribute to the pathogenesis of idiopathic ERMs by producing collagen.33 Myofibroblasts, possibly of hyalocyte, Müller cell, or retinal pigment epithelial cell origin, are thought to predominate in late idiopathic ERMs.28,33–35 Myofibroblasts can deposit collagen, secrete contractile proteins, and induce intracellular contraction that may account for the contractile properties of late idiopathic ERMs.
Extracellular matrix production and remodeling plays a central role in the pathogenesis of idiopathic ERM. Given the proposed involvement of anomalous PVD, the extracellular matrix of early idiopathic ERM is likely composed primarily of type II collagen. The ultrastructure of idiopathic ERMs is characterized by a dense, irregular network of extracellular fibrils that are oriented at random.36 The diameter of these extracellular fibrils varies based on the stage of idiopathic ERM, suggesting that there are differences in collagen composition. Cellophane macular reflex fibrils are thin, ranging from 6 nm to 15 nm in diameter, while in preretinal macular fibrosis, the fibers are thick, ranging from 18–26 nm to 36–56 nm in diameter.36 The extracellular matrix components that have been described in ERMs include collagen types I, II, III, IV, and VI, fibronectin, and laminin.36–38 Collagen types III and IV, fibronectin, and laminin are present in both early and late idiopathic ERMs.36 Cellophane macular reflex has been shown to contain large amounts of collagen type VI.36 Type VI collagen presumably anchors the ERM to the ILM of the retina. Preretinal macular fibrosis has been shown to contain large amounts of collagen types I and II.36 Collagen types I and II presumably form the bulk of the extracellular matrix in late ERMs. The retinal distortions induced by ERM contraction are thought to be the primary reason for visual impairment in idiopathic ERM.
Like idiopathic ERMs, secondary ERMs are frequently associated with PVD, suggesting that idiopathic and secondary ERMs share some pathogenic mechanisms.39 However, secondary ERMs differ in that they are associated with etiologies, such as posterior uveitis, cytomegalovirus retinitis, diabetic retinopathy, retinal vein occlusion, blunt force trauma, retinal detachment and repair, argon laser photocoagulation, and cataract surgery.7–12 The involvement of cellular proliferation, migration, and adhesion suggests that secondary ERM formation may be an abnormal wound healing response.40 Inflammation is a central component of many of the disorders associated with secondary ERMs, as evidenced by the increased expression of cytokines, such as interleukin (IL)-6, IL-8, and monocyte chemoattractant protein-1.41 These cytokines support the inflammatory cells that have been identified in secondary ERMs, including macrophages, T-cells, and B-cells.42,43 Interestingly, glial cells are thought to predominate in both idiopathic and secondary ERMs, and IL-6 has been implicated in glial cell activation and proliferation.43,44 Some of the disorders associated with secondary ERMs involve angiogenesis, and the resultant secondary ERMs have been shown to contain proangiogenic factors, such as vascular endothelial growth factor, and variable amounts of vascular tissue.45,46 As compared to idiopathic ERMs, secondary ERMs tend to occur in younger patients and be associated with worse VA and greater central foveal thickness (CFT).39
Diagnosis and classification
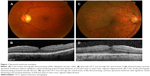
The diagnosis and classification of ERMs has historically been based on clinical examination findings.47 In clinical practice, ERMs are frequently classified as either cellophane macular reflex, the early form, or preretinal macular fibrosis, the late form.2,13 Cellophane macular reflex denotes a thin transparent membrane overlying the macula. Because this membrane does not distort the retina, it typically does not cause visual impairment; therefore, cellophane macular reflex can be an incidental finding on routine clinical examination. Slit lamp biomicroscopy of cellophane macular reflex reveals a glinting, water-silk, shifting light reflex from the inner surface of the retina (Figure 1).48 In select cases, preretinal macular fibrosis develops as the membrane thickens and contracts, with the appearance of superficial retinal folds or traction lines, becoming opaque and gray.48 Preretinal macular fibrosis can distort the retina, resulting in visual impairment in ~80% of cases.49 Slit lamp biomicroscopy of preretinal macular fibrosis reveals a semitranslucent membrane that obscures the underlying retinal features and may be associated with superficial or full-thickness retinal folds or traction lines and vascular tortuosity or dilation (Figure 1). Severe cases can involve retinal hemorrhages, exudates, vascular abnormalities, edema, macular pseudoholes, and macular holes, resulting in further visual impairment.2 In addition to clinical examination, a variety of ancillary tests can assist in the diagnosis and classification of ERM; for example, fluorescein angiography can demonstrate retinal edema. However, OCT is the ancillary test that has had the greatest impact on clinical practice.
OCT is a medical imaging technique used to produce noninvasive high-resolution cross-sectional images of biological tissues. OCT has proven to be more sensitive than clinical examination for the diagnosis of numerous disorders of the vitreomacular interface, including ERM.50 In clinical practice, time-domain OCT has largely been supplanted by SD-OCT, also known as Fourier-domain OCT, because of the improved scanning speed and detection sensitivity of the latter technique.51 The use of broadband light has significantly improved axial resolution, and there are now several commercially available SD-OCT systems with axial resolutions of ~4 μm.52,53 To perform SD-OCT, broadband light is split into several arms: the measurement arm is backscattered by the tissue of interest, and the reference arm is backscattered by a stationary mirror of a known distance.54 The backscattered light from both sources is combined, and the interference spectrum is recorded using a spectrometer and a charge-coupled device camera. Fourier transform of the interference spectrum provides data on the echo time delay of light that is used to form a tissue reconstruction. Transverse scanning is used to form a two-dimensional tissue reconstruction, and the augmented speed of SD-OCT now allows for volumetric three-dimensional tissue reconstruction.14
The International Vitreomacular Traction Study Group recently proposed definitions and classification systems for several disorders of the vitreomacular interface based entirely on OCT findings.55 Vitreomacular adhesion (VMA) was defined as perifoveal vitreous separation with residual vitreomacular attachment and normal foveal morphology. VMA was subclassified as isolated or concurrent based on the absence or presence of associated macular abnormalities, such as diabetic macular edema. Furthermore, VMA was subclassified as either focal (≤1,500 μm) or broad (>1,500 μm) based on the diameter of its vitreous attachment. Vitreomacular traction (VMT) was defined as anomalous PVD in association with an anatomic distortion of the normal foveal morphology, and VMT was subclassified as isolated or concurrent and focal or broad in the same manner as VMA. Full-thickness macular hole (FTMH) was defined as a foveal lesion that interrupts all layers of the macula from the ILM to the retinal pigment epithelium. FTMH was subclassified based on the presence or absence of VMT. FTMH was also subclassified as primary if it was initiated by VMT or secondary if it was associated with a disorder known to cause macular hole in the absence of prior VMT. Furthermore, FTMH was subclassified as small (≤250 μm), medium (>250 μm to ≤400 μm), or large (>400 μm) based on its narrowest diameter. The International Vitreomacular Traction Study Group made note of the relationship between anomalous PVD and idiopathic ERM; however, an ERM classification system was not proposed.
Clinical studies have utilized various disparate systems to classify ERMs based on OCT findings.56–59 For example, an OCT-based idiopathic ERM classification system has been proposed based on foveal morphology.58 The proposed classifications include (1A) fovea-involving ERM with outer retinal thickening and minimal inner retinal change, (1B) fovea-involving ERM with outer retinal inward projection and inner retinal thickening, (1C) fovea-involving ERM with prominent thickening of the inner retinal layer, (2A) fovea-sparing ERM with formation of a macular pseudohole, and (2B) fovea-sparing ERM with schisis-like intraretinal splitting (Table 1). To validate this classification system, multifocal electroretinography was used to demonstrate the functional differences among the various classifications. Another OCT-based ERM classification system has been proposed based on the extensive morphologic classification and subclassification.59 The proposed primary classifications include (A) with PVD and (B) without PVD. Classification (A) was subclassified as (A1) without contraction of the ERM and (A2) with contraction of the ERM; subclassification (A2) was further subclassified as (A2.1) with retinal folding, (A2.2) with edema, (A2.3) with cystoid macular edema, and (A2.4) with lamellar macular hole. Classification (B) was subclassified as (B1) without VMT and (B2) with VMT; subclassification (B2) was further subclassified as (B2.1) with edema, (B2.2) with retinal detachment, and (B2.3) with schisis (Table 2). This classification system provides a framework for thoroughly describing the morphologic characteristics of an ERM; however, it has yet to be validated, and its clinical relevance remains unclear.
  | Table 1 OCT-based morphologic classification of idiopathic ERMs |
  | Table 2 OCT-based morphologic classification of ERMs |
OCT-based classification systems such as the abovementioned ones are poised to supplant the clinical examination-based classification systems currently utilized in clinical practice. In order for an OCT-based ERM classification system to be meaningful, the OCT findings that are included should be evidence-based. CFT is one of the most extensively studied OCT findings, in large part because it was measurable with early iterations of time-domain OCT. CFT is the distance between the inner surface of the retina and the inner surface of the retinal pigment epithelium as measured at the central fovea. At baseline, most ERMs are associated with both increased CFT and worsened VA, and there is overwhelming evidence that successful surgical intervention is associated with both decreased CFT and improved VA.60–78 However, variable and inconsistent findings have been reported regarding the correlation between preoperative CFT and postoperative VA. Overall, these findings suggest that CFT may be useful for evaluating the impact of ERM on baseline VA, but CFT is probably not useful for predicting postoperative VA. Contemporary SD-OCT allows for the characterization of subtle OCT findings, such as the inner segment ellipsoid (ISe) band. The ISe band is the second innermost of the four hyperreflective outer retinal bands on OCT; in the past, this band was erroneously attributed to the boundary between the inner and outer segments of the photoreceptors.79 ISe band integrity is the preoperative OCT finding that has been most consistently associated with postoperative VA.80 A majority of studies have reported that an intact preoperative ISe band is associated with a better postoperative VA than a disrupted preoperative ISe band.60–78 These findings regarding CFT and ISe band integrity are likely applicable to both idiopathic and secondary ERMs.76–78
An ERM classification system should take into account the contemporary understanding of the pathogenesis of ERMs and the clinically relevant SD-OCT findings (Table 3). The diagnosis of ERM is contingent on the recognition of a highly reflective membranous structure at the vitreomacular interface on clinical examination or OCT imaging. An ERM can be classified as idiopathic, primary, or secondary based on its underlying etiology. Idiopathic ERMs are those that occur in the absence of an identifiable etiology. Primary ERMs are those that occur secondary to PVD in the absence of another identifiable etiology. Secondary ERMs are those that occur secondary to other disorders known to cause ERMs regardless of the occurrence of PVD. In the setting of ERM, the SD-OCT findings with the strongest evidence of clinical relevance are CFT and ISe band integrity. CFT is generally reported as either center point thickness or central subfield mean thickness. Although these measures are highly correlated, central subfield mean thickness is preferred because it is the average of more data points and is less dependent on scan centration.81 CFT can be classified as either normal or thickened based on previously reported SD-OCT device-specific values. For the Stratus OCT (Carl Zeiss Meditec AG, Jena, Germany), normal CFT is <250 μm and thickened CFT is ≥250 μm; for the Spectralis OCT (Heidelberg Engineering Inc., Heidelberg, Germany), normal CFT is <320 μm and thickened CFT is ≥320 μm.82,83 Although sex and racial differences in CFT can be disregarded in general clinical practice, these differences should be taken into account when performing rigorous clinical investigation. ISe band integrity on SD-OCT should be evaluated when considering surgical intervention. The ISe band is intact when it is clear and consistent and is disrupted when it is blurred, interrupted, or absent.
  | Table 3 ERM classification scheme that takes into account pathogenesis and clinically relevant SD-OCT findings |
Treatment
The management options for ERM are currently limited and consist of either observation or surgical intervention. Surgical intervention entails pars plana vitrectomy with ERM removal with or without ILM removal.84 Surgical peeling of the ILM may help decrease the risk of ERM recurrence.84 The surgical techniques used in the treatment of ERM generally afford excellent postoperative visual outcomes.85 However, there are definite risks associated with surgical intervention for ERM, including recurrence, endophthalmitis, and retinal detachment.86 Conservative management is supported by the fact that most ERMs are asymptomatic and do not progress, and some ERMs even regress. Aggressive management has been proposed for select cases of ERM based on the fact that patients with better preoperative VA tend to have better postoperative results.87 Surgical ERM removal may be more beneficial for patients with secondary ERM than patients with idiopathic ERM.88 However, secondary ERMs are more likely to recur, potentially because of more extensive damage or ongoing inflammation at the vitreoretinal interface. In clinical practice, surgical intervention is generally deferred until symptoms interfere with daily life. However, this is unlikely to reflect the time at which surgery must be performed to prevent irreversible retinal damage.
Conclusion
OCT has revolutionized the clinical management of numerous disorders of the eye. OCT offers distinct advantages over clinical examination for the diagnosis and classification of disorders of the vitreomacular interface. The OCT-based classification schemes proposed by the International Vitreomacular Traction Study Group will assist in the clinical management and investigation of VMA, VMT, and macular hole. OCT-based classification schemes such as these could potentially allow clinicians to identify cases of VMA or VMT that are at risk of developing anomalous PVD and idiopathic ERM. Managing these cases with prophylactic surgical or pharmacologic intervention could theoretically prevent the formation of primary ERMs. The adoption of a standardized OCT-based classification system for ERMs has the potential to assist in clinical practice and investigation. The inclusion of clinically relevant, objective measures, such as CFT and ISe band integrity, could assist clinicians in identifying the optimal time to perform surgery and predicting postoperative outcomes. Further work will be required to demonstrate the clinical utility of OCT-based ERM classification schemes.
Disclosure
The authors report no conflicts of interest in this work.
References
Ghazi-Nouri SM, Tranos PG, Rubin GS, Adams ZC, Charteris DG. Visual function and quality of life following vitrectomy and epiretinal membrane peel surgery. Br J Ophthalmol. 2006;90(5):559–562. | ||
Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. 1994;92:403–425. | ||
Aung KZ, Makeyeva G, Adams MK, et al. The prevalence and risk factors of epiretinal membranes: the Melbourne Collaborative Cohort Study. Retina. 2013;33(5):1026–1034. | ||
You Q, Xu L, Jonas JB. Prevalence and associations of epiretinal membranes in adult Chinese: the Beijing eye study. Eye (Lond). 2008;22(7):874–879. | ||
Ng CH, Cheung N, Wang JJ, et al. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology. 2011;118(4):694–699. | ||
de Bustros S, Thompson JT, Michels RG, Rice TA, Glaser BM. Vitrectomy for idiopathic epiretinal membranes causing macular pucker. Br J Ophthalmol. 1988;72(9):692–695. | ||
Appiah AP, Hirose T. Secondary causes of premacular fibrosis. Ophthalmology. 1989;96(3):389–392. | ||
Nicholson BP, Zhou M, Rostamizadeh M, et al. Epidemiology of epiretinal membrane in a large cohort of patients with uveitis. Ophthalmology. 2014;121(12):2393–2398. | ||
Kozak I, Vaidya V, Van Natta ML, et al. The prevalence and incidence of epiretinal membranes in eyes with inactive extramacular CMV retinitis. Invest Ophthalmol Vis Sci. 2014;55(7):4304–4312. | ||
Grigorian RA, Castellarin A, Fegan R, et al. Epiretinal membrane removal in diabetic eyes: comparison of viscodissection with conventional methods of membrane peeling. Br J Ophthalmol. 2003;87(6):737–741. | ||
Uemura A, Ideta H, Nagasaki H, Morita H, Ito K. Macular pucker after retinal detachment surgery. Ophthalmic Surg. 1992;23(2):116–119. | ||
Benichou C, Flament J. Epiretinal membrane and photocoagulation with argon laser. Discussion of 3 cases. Bull Soc Ophtalmol Fr. 1989;89:613–619. | ||
Mitchell P, Smith W, Chey T, Wang JJ, Chang A. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104(6):1033–1040. | ||
Koizumi H, Spaide RF, Fisher YL, Freund KB, Klancnik JM Jr, Yannuzzi LA. Three-dimensional evaluation of vitreomacular traction and epiretinal membrane using spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;145(3):509–517. | ||
Lund-Andersen H, Sander B. The vitreous. In: Kaufman PL, Alm A, editors. Adler’s Physiology of the Eye –. 10th ed. St. Louis, MO: Mosby; 2003:293–316. | ||
Wiznia RA. Posterior vitreous detachment and idiopathic preretinal macular gliosis. Am J Ophthalmol. 1986;102(2):196–198. | ||
Foos RY. Vitreoretinal juncture – simple epiretinal membranes. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1974;189(4):231–250. | ||
Foos RY. Vitreoretinal juncture; epiretinal membranes and vitreous. Invest Ophthalmol Vis Sci. 1977;16(5):416–422. | ||
Gandorfer A, Schumann R, Scheler R, Haritoglou C, Kampik A. Pores of the inner limiting membrane in flat-mounted surgical specimens. Retina. 2011;31(5):977–981. | ||
Kampik A. Pathology of epiretinal membrane, idiopathic macular hole, and vitreomacular traction syndrome. Retina. 2012;32(2):S194–S198. | ||
Sebag J. Anomalous posterior vitreous detachment: a unifying concept in vitreo-retinal disease. Graefes Arch Clin Exp Ophthalmol. 2004;242(8):690–698. | ||
Hikichi T, Takahashi M, Trempe CL, Schepens CL. Relationship between premacular cortical vitreous defects and idiopathic premacular fibrosis. Retina. 1995;15(5):413–416. | ||
Joshi M, Agrawal S, Christoforidis JB. Inflammatory mechanisms of idiopathic epiretinal membrane formation. Mediators Inflamm. 2013;2013(4):192582. | ||
Lindqvist N, Liu Q, Zajadacz J, Franze K, Reichenbach A. Retinal glial (Muller) cells: sensing and responding to tissue stretch. Invest Ophthalmol Vis Sci. 2010;51(3):1683–1690. | ||
Vinores SA, Campochiaro PA, McGehee R, Orman W, Hackett SF, Hjelmeland LM. Ultrastructural and immunocytochemical changes in retinal pigment epithelium, retinal glia, and fibroblasts in vitreous culture. Invest Ophthalmol Vis Sci. 1990;31(12):2529–2545. | ||
Bringmann A, Wiedemann P. Involvement of Muller glial cells in epiretinal membrane formation. Graefes Arch Clin Exp Ophthalmol. 2009;247(7):865–883. | ||
Kase S, Saito W, Yokoi M, et al. Expression of glutamine synthetase and cell proliferation in human idiopathic epiretinal membrane. Br J Ophthalmol. 2006;90(1):96–98. | ||
Kohno RI, Hata Y, Kawahara S, et al. Possible contribution of hyalocytes to idiopathic epiretinal membrane formation and its contraction. Br J Ophthalmol. 2009;93(8):1020–1026. | ||
Qiao H, Hisatomi T, Sonoda KH, et al. The characterization of hyalocytes: the origin, phenotype, and turnover. Br J Ophthalmol. 2005;89(4):513–517. | ||
Ohsawa K, Imai Y, Sasaki Y, Kohsaka S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J Neurochem. 2004;88(4):844–856. | ||
Smiddy WE, Maguire AM, Green WR, et al. Idiopathic epiretinal membranes. Ultrastructural characteristics and clinicopathologic correlation. Ophthalmology. 1989;96(6):811–820. | ||
Kampik A, Kenyon WR, Michels RG, Green WR, de la Cruz ZC. Epiretinal and vitreous membranes. Comparative study of 56 cases. Arch Ophthalmol. 1981;99(8):1445–1454. | ||
Michels RG. A clinical and histopathologic study of epiretinal membranes affecting the macula and removed by vitreous surgery. Trans Am Ophthalmol Soc. 1982;80:580–656. | ||
Guidry C, Bradley KM, King JL. Tractional force generation by human Muller cells: growth factor responsiveness and integrin receptor involvement. Invest Ophthalmol Vis Sci. 2003;44(3):1355–1363. | ||
Parapuram SK, Chang B, Li L, et al. Differential effects of TGFbeta and vitreous on the transformation of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2009;50(12):5965–5974. | ||
Kritzenberger M, Junglas B, Framme C, et al. Different collagen types define two types of idiopathic epiretinal membranes. Histopathology. 2011;58(6):953–965. | ||
Okada M, Ogino N, Matsumura M, Honda Y, Nagai Y. Histological and immunohistochemical study of idiopathic epiretinal membrane. Ophthalmic Res. 1995;27(2):118–128. | ||
George B, Chen S, Chaudhary V, Gonder J, Chakrabarti S. Extracellular matrix proteins in epiretinal membranes and in diabetic retinopathy. Curr Eye Res. 2009;34(2):134–144. | ||
Yazici AT, Alagöz N, Celik HU, et al. Idiopathic and secondary epiretinal membranes: do they differ in terms of morphology? An optical coherence tomography-based study. Retina. 2011;31(4):779–784. | ||
Asato R, Yoshida S, Ogura A, et al. Comparison of gene expression profile of epiretinal membranes obtained from eyes with proliferative vitreoretinopathy to that of secondary epiretinal membranes. PLoS One. 2013;8(1):e54191. | ||
Yoshimura T, Sonoda KH, Sugahara M, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4(12):e8158. | ||
Tang S, Scheiffarth OF, Wildner G, Thurau SR, Lund OE. Lymphocytes, macrophages and HLA-DR expression in vitreal and epiretinal membranes of proliferative vitreoretinopathy. An immunohistochemical study. Ger J Ophthalmol. 1992;1(3–4):176–179. | ||
Tamura K, Yokoyama T, Ebihara N, Murakami A. Histopathologic analysis of the internal limiting membrane surgically peeled from eyes with diffuse diabetic macular edema. Jpn J Ophthalmol. 2012;56(3):280–287. | ||
Tilgner J, Volk B, Kaltschmidt C. Continuous interleukin-6 application in vivo via macroencapsulation of interleukin-6-expressing COS-7 cells induces massive gliosis. Glia. 2001;35(3):234–245. | ||
Chen YS, Hackett SF, Schoenfeld CL, Vinores MA, Vinores SA, Campochiaro PA. Localisation of vascular endothelial growth factor and its receptors to cells of vascular and avascular epiretinal membranes. Br J Ophthalmol. 1997;81(10):919–926. | ||
Hsu YR, Yang CM, Yeh PT. Clinical and histological features of epiretinal membrane after diabetic vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2014;252(3):401–410. | ||
McLeod D, Hiscott PS, Grierson I. Age-related cellular proliferation at the vitreoretinal juncture. Eye (Lond). 1987;1:263–281. | ||
Wise GN. Clinical features of idiopathic preretinal macular fibrosis. Schoenberg Lecture. Am J Ophthalmol. 1975;79(3):347–349. | ||
Ryan SJ. Epiretinal membrane. In: Ryan SJ, Schachat AP, Wilkinson CP, Hinton DR, Sadda SR, Wiedemann P, editors. Retina – 5th ed. Philadelphia, PA: Saunders (Imprint) Elselvier; 2013:1955–1957. | ||
Do DV, Cho M, Nguyen QD, et al. The impact of optical coherence tomography on surgical decision making in epiretinal membrane and vitreomacular traction. Trans Am Ophthalmol Soc. 2006;104:161–166. | ||
Choma M, Sarunic M, Yang C, Izatt J. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express. 2003;11(18):2183–2189. | ||
Gabriele ML, Wollstein G, Ishikawa H, et al. Optical coherence tomography: history, current status, and laboratory work. Invest Ophthalmol Vis Sci. 2011;52(5):2425–2436. | ||
Drexler W, Morgner U, Ghanta RK, Kärtner FX, Schuman JS, Fujimoto JG. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat Med. 2001;7(4):502–507. | ||
Wojtkowski M, Leitgeb R, Kowalczyk A, Bajraszewski T, Fercher AF. In vivo human retinal imaging by Fourier domain optical coherence tomography. J Biomed Opt. 2002;7(3):457–463. | ||
Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611–2619. | ||
Wilkins JR, Puliafito CA, Hee MR, et al. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology. 1996;103(12):2142–2151. | ||
Mori K, Gehlbach PL, Sano A, Deguchi T, Yoneya S. Comparison of epiretinal membranes of differing pathogenesis using optical coherence tomography. Retina. 2004;24(1):57–62. | ||
Hwang JU, Sohn J, Moon BG, et al. Assessment of macular function for idiopathic epiretinal membranes classified by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(7):3562–3569. | ||
Konidaris V, Androudi S, Alexandridis A, Dastiridou A, Brazitikos P. Optical coherence tomography-guided classification of epiretinal membranes. Int Ophthalmol. 2015;35(4):495–501. | ||
Suh MH, Seo JM, Park KH, Yu HG. Associations between macular findings by optical coherence tomography and visual outcomes after epiretinal membrane removal. Am J Ophthalmol. 2009;147(3):473.e–480.e. | ||
Mitamura Y, Hirano K, Baba T, Yamamoto S. Correlation of visual recovery with presence of photoreceptor inner/outer segment junction in optical coherence images after epiretinal membrane surgery. Br J Ophthalmol. 2009;93(2):171–175. | ||
Falkner-Radler CI, Glittenberg C, Hagen S, Benesch T, Binder S. Spectral-domain optical coherence tomography for monitoring epiretinal membrane surgery. Ophthalmology. 2010;117(4):798–805. | ||
Inoue M, Morita S, Watanabe Y, et al. Preoperative inner segment/outer segment junction in spectral-domain optical coherence tomography as a prognostic factor in epiretinal membrane surgery. Retina. 2011;31(7):1366–1372. | ||
Shimozono M, Oishi A, Hata M, et al. The significance of cone outer segment tips as a prognostic factor in epiretinal membrane surgery. Am J Ophthalmol. 2012;153(4):698–704;704.e1. | ||
Kinoshita T, Imaizumi H, Okushiba U, Miyamoto H, Ogino T, Mitamura Y. Time course of changes in metamorphopsia, visual acuity, and OCT parameters after successful epiretinal membrane surgery. Invest Ophthalmol Vis Sci. 2012;53(7):3592–3597. | ||
Itoh Y, Inoue M, Rii T, Hirota K, Hirakata A. Correlation between foveal cone outer segment tips line and visual recovery after epiretinal membrane surgery. Invest Ophthalmol Vis Sci. 2013;54(12):7302–7308. | ||
Kim JH, Kang SW, Kong MG, Ha HS. Assessment of retinal layers and visual rehabilitation after epiretinal membrane removal. Graefes Arch Clin Exp Ophthalmol. 2013;251(4):1055–1064. | ||
Brito PN, Gomes NL, Vieira MP, et al. Possible role for fundus autofluorescence as a predictive factor for visual acuity recovery after epiretinal membrane surgery. Retina. 2014;34(2):273–280. | ||
Moisseiev E, Davidovitch Z, Loewenstein A, Barak A. Outcomes of epiretinal membrane removal in eyes with and without concurrent vision-limiting ocular disease. Ophthalmologica. 2011;226(2):71–75. | ||
Kim JH, Kim YM, Chung EJ, Lee SY, Koh HJ. Structural and functional predictors of visual outcome of epiretinal membrane surgery. Am J Ophthalmol. 2012;153(1):103.e–110.e. | ||
Cobos E, Arias L, Ruiz-Moreno J, et al. Preoperative study of the inner segment/outer segment junction of photoreceptors by spectral-domain optical coherence tomography as a prognostic factor in patients with epiretinal membranes. Clin Ophthalmol. 2013;7:1467–1470. | ||
Mayer WJ, Vogel M, Neubauer A, et al. Pars plana vitrectomy and internal limiting membrane peeling in epimacular membranes: correlation of function and morphology across the macula. Ophthalmologica. 2013;230(1):9–17. | ||
Sugiura Y, Okamoto F, Okamoto Y, Hiraoka T, Oshika T. Contrast sensitivity and foveal microstructure following vitrectomy for epiretinal membrane. Invest Ophthalmol Vis Sci. 2014;55(11):7594–7600. | ||
Rii T, Itoh Y, Inoue M, Hirota K, Hirakata A. Outer retinal morphological changes and visual function after removal of epiretinal membrane. Can J Ophthalmol. 2014;49(5):436–442. | ||
Kim HJ, Kang JW, Chung H, Kim HC. Correlation of foveal photoreceptor integrity with visual outcome in idiopathic epiretinal membrane. Curr Eye Res. 2014;39(6):626–633. | ||
Weng CY, Gregori NZ, Moysidis SN, Shi W, Smiddy WE, Flynn HW Jr. Visual and anatomical outcomes of macular epiretinal membrane peeling after previous rhegmatogenous retinal detachment repair. Retina. 2015;35(1):125–135. | ||
Iannetti L, Tortorella P, D’Ambrosio E, Spena R, Zito R, Gharbiya M. Epiretinal membranes in patients with uveitis: morphological and functional analysis with spectral domain optical coherence tomography. Biomed Res Int. 2013;2013:284821. | ||
Kang HM, Koh HJ, Lee SC. Visual outcome and prognostic factors after surgery for a secondary epiretinal membrane associated with branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2015;253(4):543–550. | ||
Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011;31(8):1609–1619. | ||
Scheerlinck LM, van der Valk R, van Leeuwen R. Predictive factors for postoperative visual acuity in idiopathic epiretinal membrane: a systematic review. Acta Ophthalmol. 2015;93(3):203–212. | ||
Browning DJ, Glassman AR, Aiello LP, et al; Diabetic Retinopathy Clinical Research Network. Optical coherence tomography measurements and analysis methods in optical coherence tomography studies of diabetic macular edema. Ophthalmology. 2008;115(8):1366–1371. | ||
Bressler NM, Edwards AR, Antoszyk AN, et al; Diabetic Retinopathy Clinical Research Network. Retinal thickness on Stratus optical coherence tomography in people with diabetes and minimal or no diabetic retinopathy. Am J Ophthalmol. 2008;145(5):894–901. | ||
Chalam KV, Bressler SB, Edwards AR, et al; Diabetic Retinopathy Clinical Research Network. Retinal thickness in people with diabetes and minimal or no diabetic retinopathy: Heidelberg Spectralis optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(13):8154–8161. | ||
Sandali O, El Sanharawi M, Basli E, et al. Epiretinal membrane recurrence: incidence, characteristics, evolution, and preventive and risk factors. Retina. 2013;33(10):2032–2038. | ||
Dawson SR, Shunmugam M, Williamson TH. Visual acuity outcomes following surgery for idiopathic epiretinal membrane: an analysis of data from 2001 to 2011. Eye (Lond). 2014;28(2):219–224. | ||
Guillaubey A, Malvitte L, Lafontaine PO, et al. Incidence of retinal detachment after macular surgery: a retrospective study of 634 cases. Br J Ophthalmol. 2007;91(10):1327–1330. | ||
Rahman R, Stephenson J. Early surgery for epiretinal membrane preserves more vision for patients. Eye (Lond). 2014;28(4):410–414. | ||
Kang KT, Kim KS, Kim YC. Surgical results of idiopathic and secondary epiretinal membrane. Int Ophthalmol. 2014;34(6):1227–1232. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.