Back to Journals » Research and Reports in Urology » Volume 9
Enteric-coated and highly standardized cranberry extract reduces antibiotic and nonsteroidal anti-inflammatory drug use for urinary tract infections during radiotherapy for prostate carcinoma
Authors Bonetta A , Roviello G, Generali D , Zanotti L, Cappelletti MR, Pacifico C, Di Pierro F
Received 31 January 2017
Accepted for publication 27 March 2017
Published 26 April 2017 Volume 2017:9 Pages 65—69
DOI https://doi.org/10.2147/RRU.S133538
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Alberto Bonetta,1 Giandomenico Roviello,2,3 Daniele Generali,3,4 Laura Zanotti,3 Maria Rosa Cappelletti,3 Chiara Pacifico,5 Francesco Di Pierro6
1Oncological Radiotherapy Operative Unit, ASST, Cremona, 2Department of Molecular and Translational Medicine, University of Brescia, Brescia, 3Molecular Therapy and Pharmacogenomics Unit, ASST, Cremona, 4Department of Medical, Surgery and Health Sciences, University of Trieste, Trieste, 5Department of Medical, Surgical and Neurological Sciences, University Hospital of Siena, Siena, 6Velleja Research Scientific Department, Milan, Italy
Introduction: Worldwide, bacterial resistance to antibiotic therapy is a major concern for the medical community. Antibiotic resistance mainly affects Gram-negative bacteria that are an important cause of lower urinary tract infections (LUTIs). Pelvic irradiation for prostate cancer is a risk factor for LUTIs. Cranberry extract is reported to reduce the incidence of LUTIs. The prophylactic role of an enteric-coated, highly standardized cranberry extract (VO370®) in reducing LUTI episodes, urinary discomfort, and nonsteroidal anti-inflammatory drug (NSAID) and antibiotic use during radiotherapy for prostate carcinoma was evaluated.
Methods: A total of 924 patients with prostate carcinoma treated by radiotherapy to the prostatic and pelvic areas were randomized to receive (n=489) or not (n=435) the enteric-coated, highly standardized cranberry extract for 6–7 weeks concurrently with irradiation. Outcomes were analyzed by using Mann–Whitney U test and Pearson’s X2 test. Primary endpoint was the number of patients with LUTI; secondary endpoints were incidence of recurrence, days of treatment with antibiotics and number of subjects treated with NSAIDs, and incidence of dysuria.
Results: The treatment was very well tolerated, and there were no serious side effects. All enrolled patients completed the study. Urinary infections were detected in 53 of the 489 patients (10.8%) treated with enteric-coated, highly standardized cranberry extract, while 107 of the 435 patients (24.6%) in the control group developed LUTIs (p=0.0001). A clear and significant reduction in urinary discomfort of ~50% was seen in treated subjects. The treatment also resulted in ~50% reduction in the use of anti-inflammatory drugs and antibiotics.
Conclusion: The enteric-coated, highly standardized cranberry extract could be used as a prophylactic to reduce the incidence of LUTIs and decrease antibiotic therapy in patients receiving pelvic irradiation for prostate cancer.
Keywords: antibiotic-resistance, PAC-A, Vaccinium macrocarpon, UTI, E. coli, botanicals
Introduction
The medical community is seriously concerned about the increasing resistance of bacteria, especially Gram-negative bacteria, to antibiotics,1 and innovative approaches are needed to deal with the problem. Strategies proposed include the use of phytochemicals to inhibit or eradicate biofilms, to interfere with bacterial quorum sensing signaling pathways, or to act as chelating agents and/or efflux pump inhibitors.2 A-type interflavane bond proanthocyanidins (PAC-A) from Vaccinium macrocarpon (cranberry) fruit, by interacting directly with P-type fimbrial structures found in uropathogenic Escherichia coli strains, seem to prevent their binding to the receptor glycoprotein located on the urinary bladder epithelium. This apparently limits the ability of the bacteria to adhere and, therefore, proliferate, thus preventing effective colonization and disease.3 Therefore, patients with repeated lower urinary tract infections (LUTIs) could possibly avoid recurrence by consuming cranberry preparations.4 LUTI is also a possible adverse event during external beam radiotherapy (EBRT) to the pelvis,5 and E. coli is often the most common pathogen isolated in culture in these LUTIs.6,7 Antibiotic-resistant strains have also been isolated in LUTIs caused by EBRT, and pathogens resistant to trimethoprim/sulfamethoxazole, ciprofloxacin, and nitrofurantoin have been found through antibiotic sensitivity analysis.6 Since it has already been demonstrated that the use of enteric-coated, highly standardized cranberry extract is effective in reducing LUTIs caused by EBRT, with a significant reduction in urinary tract symptoms,7 the aim of the present work was to confirm the previous findings in a larger number of subjects and show that the preventive use of the same cranberry preparation can lead to a considerable decrease in antibiotic use.
Methods
Study patterns
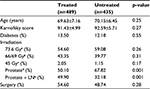
This randomized, open-label, controlled study was performed with the approval of the local Ethics Board of Cremona Hospital (Cremona, Italy), and conducted during routine clinical practice, following international guidelines and in line with the principles outlined in the Declaration of Helsinki. All patients provided written informed consent to participate. Enrolment took place between 2006 and 2016 at the Radiation Oncology Unit of Cremona Hospital (ASST, Cremona, Italy). Patients (n=924) diagnosed with prostatic adenocarcinoma were treated with radiotherapy to the prostatic area and also to the pelvic area if the risk of lymph node (LN) spread was >15% according to Kattan’s nomograms.8 Only patients treated with radical (73.6 Gy delivered as 32 fractions of 2.3 Gy, five fractions per week), postsurgical (66/69 Gy delivered as 32 fractions of 2.23/2.30 Gy, five fractions per week), or personalized (45 Gy delivered as 15 fractions of 3 Gy, five fractions per week) radiotherapy were enrolled in the study. Of the 924 patients, 479 had also undergone surgery. Patients with a history of pelvic EBRT, previous pelvic malignancies, a Karnofsky score9 <80, renal failure, or refusal of preventive, daily treatment with cranberry extract were excluded from the study.
The patients were randomized into two groups by tossing a coin. The treated group consisting of 489 patients was administered one tablet/day containing 200 mg of cranberry extract for 6–7 weeks. The tested cranberry is an enteric-coated, highly standardized extract, titered as 30% PACs according to the European Pharmacopoeia method (version 6.0), and sold in Italy as MonoselectMacrocarpon® by PharmExtracta (Italy) and in the rest of the world as Ressuro® by Helsinn Integrative Care (Helsinn Healthcare SA, Switzerland). An enteric coating was used to prevent possible degradation of PAC-A during processing, and because of their instability in gastric juices and possible interaction with gastric Helicobacter pylori.10,11 No particular suggestion was given to patients in terms of diet, except that the product should not be taken with meals. A second, untreated group of 435 subjects served as controls. No patients exhibited symptoms of cystitis at enrolment, and hence, no urine cultures were carried out at that time.
Radiotherapy was performed using 6 Mv photons with volumetric modulated arc therapy (VMAT) techniques. During treatment planning, the bladder volume and whole bladder average dose were recorded to assess the EBRT impact on urinary symptoms. During the treatment, all the patients underwent a weekly examination to assess urinary symptoms and determine nonsteroidal anti-inflammatory drug (NSAID) and antibiotic use. Two urine cultures were performed at week 3 and at week 6 of treatment, with a further culture performed on request in case of severe dysuria. A UTI was assumed when the findings of bacteriuria exceeded 100,000 units/mL, accompanied by specific symptoms of cystitis. Bacterial species determination has been done by growing bacteria on the selected medium according to conventional methods.12 The primary endpoint of the study was the number of patients who experienced episodes of LUTI in the two different groups. Secondary endpoints included incidence of recurrence, days of treatment with antibiotics (any antibiotic was administered for 5 days but ampicillin administered for 6 days) and number of subjects treated with NSAIDs, and incidence of dysuria in the two groups.
Statistical analysis
For statistical analysis, a preliminary data exploration was performed. Numerical variables were expressed as the median and range and were compared using nonparametric tests (Mann–Whitney U test). Qualitative data were expressed as frequencies and organized into contingency tables; the association between categorical variables was investigated by means of Pearson’s χ2 test. Univariate logistic regression was performed to evaluate the influence of VO370® on the development of LUTIs. Significance was set at p< 0.05. STATA 13.1 software was used for analysis.
Results
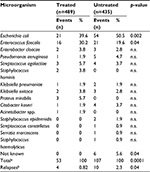
As shown in Table 1, the main characteristics of the two groups were comparable. The only significant difference was the higher percentage of subjects in the treated group (~50% vs 32%) who received both prostate and pelvic LN irradiation. The compliance of the treated group was excellent, and no patient discontinued treatment during the study. In the treated group, four patients with chronic gastritis complained of gastric pain. This required the use of a protecting agent, but no other unpleasant, or allergic, effects were observed. All patients completed the planned EBRT. As shown in Table 2, 107 of the 435 patients (24.6%) in the control group experienced LUTIs compared to 53 of the 489 patients in the treated group (10.8%; p=0.0001). In univariate analysis, the use of cranberry was correlated with a low risk of developing a LUTI (odds ratio 0.39; 95% confidence interval [CI] 0.27–0.55). Recurrent infection was seen in 10 patients (2.3%) in the control group and in four patients (0.8%) in the treated group (p=0.004).
E. coli was the main microorganism involved in LUTIs. Of the 75 cases in the two groups, 54 (72%) were in the control group and 21 (28%) in the treated group. Enterococcus faecalis was involved in 37 cases of LUTI, with 21 such cases (~57%) in the untreated group and 16 (43%) in the treated group. With a few exceptions (ie, Staphylococcus hominis, Proteus mirabilis, and Acinetobacter spp.), all other microorganisms were found less frequently in the treated group than in the control group. Their relatively small number does not allow statistically significant differences to be identified.
As shown in Table 3, the drop in LUTI incidence in the treated group resulted in a reduction in antibiotic use. Cranberry-treated subjects (n=489) were administered an antibiotic for a total of 285 days compared to 585 days in the control group (n=435), showing that cranberry administration significantly reduced (–48.7%) the use of antibiotics (p=0.001).
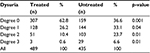
A considerable reduction in dysuria (Table 4) was also seen in the cranberry-treated subjects. Assessment with the Boyarsky scoring system for dysuria discomfort13 revealed an absence of symptoms in ~63% of treated subjects compared to ~37% of the control group. Occasional burning, frequent burning, and constant pain, respectively, were observed in ~26%, 10%, and 0.6% of the treated group and in ~33%, 24%, and 7% of the control group.
NSAIDs are routinely prescribed to treat urinary symptoms. As shown in Table 3, NSAIDs were used by 201 of the 435 subjects in the control group (46.2%) and by 113 of the 489 subjects in the treated group (23.1%). This demonstrates that cranberry administration significantly reduced (–56.2%) the use of NSAIDs.
Finally, urinary symptoms due to radiotherapy were milder in the treated subjects compared to controls. Nocturia affected 29% of treated subjects and 52% of control subjects, urgency affected 30% of treated subjects and 55% of control subjects, and average daily urination frequency increased from 5.65 to 7.45 in the treated group and from 5.22 to 8.57 in the control group (data not shown).
Conclusion
Cystitis is a common infection, affecting 0.5%–1% of the population. About 90% of bladder infections are caused by Gram-negative bacteria, especially of fecal origin, and >50% of these Gram-negative infections are caused by E. coli, with Enterococcus, Pseudomonas, Enterobacter, Klebsiella, and Proteus spp. less frequent.14–16 Also, microorganisms causing LUTIs can demonstrate resistance due to the excessive use of antibiotics. Indeed, LUTI shows a high frequency (20%) of relapse in some populations, such as menopausal women, despite the use of antibiotics.14 Cancer patients treated with pelvic radiotherapy are at increased risk of urinary infections, with an incidence ranging from 14% to 33%, depending on the type of cancer and radiation techniques used.17–20
In a previous work, it was demonstrated that the use of an enteric-coated, highly standardized cranberry extract decreased the incidence and symptoms of LUTI caused by irradiation in prostate cancer patients.7 The aim of the current work was to confirm the previous findings in a larger sample of subjects while also evaluating the reduction in antibiotic use following cranberry treatment. Indeed, the results of this study, independently from Gy intensity used (data not shown), demonstrate once again the beneficial role played by enteric-coated cranberry extract in preventing LUTI episodes (objective endpoint) and in reducing urinary discomfort, such as dysuria, nocturia, and urinary frequency (subjective endpoints). There were 53 patients with LUTIs in the treated group compared to 107 in the control group, while 113 subjects in the treated group were treated with NSAIDs compared to 210 in the control group. These differences clearly indicate that the treatment with enteric-coated cranberry could lessen actinic damage to the bladder mucosa, thus reducing the inflammatory process and, consequently, symptoms in those subjects apparently not having a LUTI. Moreover, for the first time, this study demonstrates that the prophylactic use of cranberry can reduce antibiotic treatment by ~50% with advantages in terms of possible reduced generation of new antibiotic resistance.
This work has some limitations. In particular, it is not a double-blind, placebo-controlled trial. Moreover, despite the randomization process, there is a significant potential that the patients with prostate + LN field radiation (Table 1) had higher risk of LUTI and dysuria. However, it was a study with a considerable number of subjects and a control group. Statistical analysis shows that preventive treatment with an enteric-coated and highly standardized cranberry extract reduces the incidence of LUTI, its symptoms, and antibiotic and NSAID use. Further studies are currently ongoing to validate these findings in a blinded study with a placebo.
Disclosure
F Di Pierro belongs to the Scientific Committee of one of the companies trading the tested product. He has the full control of all primary data and agrees to allow the journal to review the data if requested. The other authors report no conflicts of interest in this work.
References
Falagas ME, Mavroudis AD, Vardakas KZ. The antibiotic pipeline for multi-drug resistant (MDR) Gram negative bacteria: what can we expect. Expert Rev Anti Infect Ther. 2016;14(8):747–763. | ||
Borges A, Abreu AC, Dias C, Saavedra MJ, Borges F, Simões M. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules. 2016;21(7):pii:E877. | ||
Pinzón-Arango PA, Liu Y, Camesano TA. Role of cranberry on bacterial adhesion forces and implications for Escherichia coli-uroepithelial cell attachment. J Med Food. 2009;12(2):259–270. | ||
Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321. | ||
Çetinel B. Chemotherapy and pelvic radiotherapy-induced bladder injury. Urologia. 2015;82(Suppl 3):S2–S5. | ||
Shuford RA, Dulaney CR, Burnett OL 3rd, Byram KW, McDonald AM. Evaluating the role of urinalysis for suspected cystitis in women undergoing pelvic radiotherapy. Int J Gynecol Cancer. Epub 2016 Apr 21. | ||
Bonetta A, Di Pierro F. Enteric-coated, highly standardized cranberry extract reduces risk of UTIs and urinary symptoms during radiotherapy for prostate carcinoma. Cancer Manag Res. 2012;4:281–286. | ||
Flern P, Kattan MW. Prediction tools – prostate cancer nomograms. Memorial Sloane Kattering Cancer Center, NY. Available from: www.mskcc.org/nomograms/prostate. Accessed February 26, 2017. | ||
Marina O, Suh JH, Reddy CA, et al. Treatment outcomes for patients with glioblastoma multiforme and a low Karnofsky Performance Scale score on presentation to a tertiary care institution. Clinical article. J Neurosurg. 2011;115(2):220–229. | ||
Pappas E, Schaich KM. Phytochemicals of cranberries and cranberry products: characterization, potential health effects, and processing stability. Crit Rev Food Sci Nutr. 2009;49(9):741–781. | ||
Matsushima M, Suzuki T, Masui A, et al. Growth inhibitory action of cranberry on Helicobacter pylori. J Gastroenterol Hepatol. 2008;23(Suppl 2):S175–S180. | ||
Mahon CR, Lehman DC, Manuselis G. Textbook of Diagnostic Microbiology. 5th ed. Amsterdam, NL: Elsevier; 2014. | ||
Cetinel B, Obek C, Solok V, Yaycioglu O, Yazici H. Urologic screening for men with Behçet’s syndrome. Urology. 1998;52(5):863–865. | ||
Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997;11(3):551–581. | ||
Anderson RU. Management of lower urinary tract infections and cystitis. Urol Clin North Am. 1999;26(4):729–735, viii. | ||
Alukal J, Mir J, Bergin C. Genitourinary infection. In: Siroky MB, Oates RD, Babayan RK, editors. Handbook of Urology: Diagnosis and Therapy. 3rd ed. New York: Lippincott Williams and Wilkins; 2004:206–229. | ||
Prasad KN, Pradhan S, Datta NR. Urinary tract infection in patients of gynecological malignancies undergoing external pelvic radiotherapy. Gynecol Oncol. 1995;57(3):380–382. | ||
Roberts FJ, Murphy J, Ludgate C. The value and significance of routine urine cultures in patients referred for radiation therapy of prostatic malignancy. Clin Oncol. 1990;2(1):18–21. | ||
Bialas I, Bessell EM, Sokal M, Slack R. A prospective study of urinary tract infection during pelvic radiotherapy. Radiother Oncol. 1989;16(4):305–309. | ||
Bessell EM, Granville-White M. The effect of prophylactic trimethoprim on aerobic urinary tract infection during pelvic radiotherapy and the incidence of infections due to fastidious or anaerobic organisms. Clin Oncol. 1994;6(2):116–120. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.