Back to Journals » International Journal of Nanomedicine » Volume 13
Enhanced permeability of blood–brain barrier and targeting function of brain via borneol-modified chemically solid lipid nanoparticle
Authors Song H, Wei M, Zhang N, Li H, Tan XC, Zhang YJ, Zheng WS
Received 1 January 2018
Accepted for publication 17 February 2018
Published 28 March 2018 Volume 2018:13 Pages 1869—1879
DOI https://doi.org/10.2147/IJN.S161237
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Hui Song, Man Wei, Nan Zhang, He Li, Xiaochuan Tan, Yujia Zhang, Wensheng Zheng
Beijing Key Laboratory of Drug Delivery Technology and Novel Formulation, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Introduction: The incidence of central nervous system disease has increased in recent years. However, the transportation of drug is restricted by the blood–brain barrier, contributing to the poor therapeutic effect in the brain. Therefore, the development of a new brain-targeting drug delivery system has become the hotspot of pharmacy.
Materials and methods: Borneol, a simple bicyclic monoterpene extracted from Dryobalanops aromatica, can direct drugs to the upper body parts according to the theory of traditional Chinese medicine. Dioleoyl phosphoethanolamine (DOPE) was chemically modified by borneol as one of the lipid materials of solid lipid nanoparticle (SLN) in the present study.
Results: The borneol-modified chemically solid lipid nanoparticle (BO-SLN/CM), borneol-modified physically solid lipid nanoparticle (BO-SLN/PM), and SLN have similar diameter (of about 87 nm) and morphological characteristics. However, BO-SLN/CM has a lower cytotoxicity, higher cell uptake, and better blood–brain barrier permeability compared with BO-SLN/PM and SLN. BO-SLN/CM has a remarkable targeting function to the brain, while BO-SLN/PM and SLNs are concentrated at the lung.
Conclusion: The present study provides an excellent drug delivery carrier, BO-SLN/CM, having the application potential of targeting to the brain and permeating to the blood–brain barrier.
Keywords: blood–brain barrier, solid lipid nanoparticle, phospholipid modification, BBB model in vitro, body distribution
Introduction
One of the most challenging fields for pharmaceutical and biotechnological products is the drug delivery to the central nervous system.1,2 The blood–brain barrier (BBB) is a tightly packed layer formed by the brain capillary endothelial cells, which strictly restricts the drug transfer from blood to brain. It is vital in maintaining the homeostasis and normal function of central nervous system by controlling the exchange of endogenous and exogenous substances between blood and brain.3 Only a small class of drugs or molecules with lipid solubility and molecular mass <400–500 Da can actually pass through the BBB.1 An efficient design for noninvasive nanocarrier systems, such as liposomes, solid polymer, and lipid nanoparticle, can facilitate the uptake of drugs to specific regions of the brain, which may be advantageous in drug development and delivery for neurological diseases.4–6 These carriers can not only disguise the therapeutic drug’s BBB-limiting characteristic and prevent the drug from chemical or enzymatic degradation but also provide an additional opportunity for the sustained release of the therapeutic drug.7
Borneol (BO) is a simple bicyclic monoterpene extracted from Dryobalanops aromatica.8 It has peripheral and centrally acting analgesic properties as well as anti-inflammatory action.9 In many prescriptions of traditional Chinese medicine (TCM), certain drugs are considered to be messengers introducing the active ingredients to the target part of human body.10 According to the theory of TCM, BO can direct drugs to the upper body parts and be widely used as a messenger for drugs to treat cardiovascular and cerebrovascular diseases. It can also reversibly open the tight junction of the BBB when seen from the grounds of modern medicine.11,12 BO used in TCM is derived from both synthetic and natural sources. Synthetic BO is a mixture of D-BO and iso-BO, and natural BO contains D-BO only. No significant difference is noted in the pharmacological action between synthetic and natural BO.13 Synthetic BO is easier to purchase and hence chosen as the experimental material. It enhances drug permeation through the skin, gastrointestinal mucous membrane, nasal mucosa, and cornea. Recently, BO was reported to accelerate the opening of BBB, improving the permeability through a physiological process, and enhancing penetration and distribution of drugs in the brain.14,15 Ren et al16 prepared BO-modified ganciclovir-loaded solid lipid nanoparticles using a physical mixing (BO-SLN/PM) method and demonstrated that BO-modified SLNs could increase the distribution ratio in the brain compared with SLNs without BO modification.16 Xu et al17 designed a novel targeting drug carrier using the polyamidoamine G5 dendrimer (PAMAM G5) chemically modified with BO and folic acid (FA-BO-PAMAM) at the periphery. The results exhibited that BO-modified conjugates decreased the cytotoxicity of PAMAM against both human brain microvascular endothelial cells (HBMECs) and C6 cells and displayed a higher BBB penetration ability compared with the BO-unmodified conjugates.
Although BO-modified carriers were shown to improve the BBB transportation ability, whether the chemical modification method exhibits different enhancing efficiency has not been explored. Therefore, the main purpose of the present study was to develop BO-modified SLNs using covalently modified materials and investigate differences in the enhancing effect of BBB penetration in vitro and in vivo.
Materials
BO, stearic acid (SA), succinic anhydride, coumarin-6 and cyanine7 (Cy-7), polyethylene glycol monostearate (PEG-SA), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride, N-hydroxysuccinimide, and Brij S10 were purchased from Aladdin Chemical Co. (Rogers, MN, USA). Lecithin and dioleoyl phosphoethanolamine (DOPE) were purchased from Lipoid GmbH (Ludwigshafen, Germany). Dulbecco’s Modified Eagle’s Medium (DMEM), penicillin–streptomycin, D-Hank’s buffer solution, fetal bovine serum, MTT, dimethyl sulfoxide (DMSO), and trypsin solution were purchased from Corning Co. (New York, NY, USA). All the other chemicals and solvents were analytical grade, and purified water was produced using a MILLI-Q® HR 7000 water purification system (Merck KGaA, Darmstadt, Germany). HBMECs were kindly donated by Prof NH Chen (Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical School, Beijing, People’s Republic of China).
Kunming mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, People’s Republic of China). All the animals were cared and used for the experiment in accordance with the protocols established by Chinese Academy of Medical Sciences and Peking Union Medical College and approved by experimental animal management committee, Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College.
Methods
Synthesis and characterization of BO-DOPE
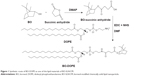
BO-DOPE was synthesized by coupling BO on the hydrophilic side of DOPE using succinic anhydride as a linker (Figure 1). In brief, succinic anhydride, 4-dimethylaminopyridine, and BO (3:2:1, mol/mol/mol) were dissolved in anhydrous methylene chloride (dichloromethane, 50 mL). The reaction mixtures were stirred at room temperature for 48 h and extracted three times with 1 N HCl in a 250 mL separating funnel. Subsequently, the hydrophobic layer was washed three times with distilled water. The product was separated by silica gel column chromatography using cyclohexane and ethyl acetate (9:1, v/v) as the mobile phase. The solvent of eluent containing the targeted compound was removed, and a yellow viscous liquid, BO-succinic anhydride, was obtained.
BO-succinic anhydride was dissolved in 1 mL of dimethylformamide, which was mixed with the phosphate-buffered saline (PBS) solution (pH 5.7) containing twofold molar N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride and N-hydroxysuccinimide. The mixture was stirred for 2 h at room temperature. An equimolar DOPE chloroform solution was dropped into the former solution. The mixture was vigorously stirred overnight at room temperature. Finally, the crude product was washed three times with distilled water, and the hydrophobic layer was separated and dried with anhydrous sodium sulfate. BO-DOPE was obtained by removing the solvent. 1H and 13C nuclear magnetic resonance (NMR) spectra of BO-DOPE were recorded on a Mercury Plus 600 MHz spectrometer (Mercury Computer Systems, Andover, MA, USA) using CDCl3 as solvents. The mass spectrum was measured using liquid chromatography–electrospray ionization–tandem mass spectrometry (Agilent 1200 serious high-performance liquid chromatography, Agilent triple quadrupole mass spectrometer).
Preparation of SLNs and BO-SLN
A modified emulsion and solvent evaporation method was used to prepare SLNs. That is, 5 mg SA, 5 mg PEG-SA, and 3 mg lecithin were dissolved in 0.5 mL anhydrous ethanol. The solution was subsequently mixed with 2 mL 0.2% Brij S10 aqueous solution to form an oil/water emulsion using a probe sonicator, and the solution was further diluted with 10 mL 0.2% Brij S10 aqueous solution under magnetic stirring. Anhydrous ethanol was evaporated at a low pressure at 30°C using a rotary evaporator. Finally, the clear suspension was placed in an ice bath for 2 h (Table 1).
The BO-SLN/PM was prepared following almost the same procedure as for SLNs. The only difference was that 0.4 mg BO was dissolved in anhydrous ethanol together with other lipid materials. The BO-SLN by chemical modification (BO-SLN/CM) was prepared using emulsion and solvent evaporation method. In brief, 5 mg BO-DOPE was dissolved in 0.25 mL DCM and 1 mL anhydrous ethanol. The next steps were the same as for the preparation of SLNs. Three kinds of drug-loaded SLNs were prepared by adding coumarin-6 or Cy-7 into the solvent together with other lipid materials.
Characterization of three kinds of SLNs
The particle size, polydispersity index (PDI), and zeta potential of three kinds of SLNs were measured by a dynamic light scattering method using the zeta potential/particle sizer (NICOMPTM 380ZLS PSS, Nicomp Particle Size Systems, Santa Barbara, CA, USA) at a concentration of 0.5 mg/mL in purified water. The morphological examination was performed using a transmission electron microscope (TEM, HT7700, Hitachi, Tokyo, Japan), and samples for TEM were made by casting a drop of 5 mg/mL aqueous sample on carbon-coated copper grids.
Encapsulation efficiency of coumarin-6-loaded SLN and BO-SLN
Two slightly smaller filter papers, smaller than the diameter of a 2 mL syringe, were placed at the bottom of the syringe. Subsequently, Sephadex G-50 (Sigma-Aldrich, St Louis, MO, USA) was swollen for 12 h using distilled water and placed into the syringe. Following the removal of bubbles, the column was balanced with distilled water at three-fold column volume and excess water was removed by centrifuging at 3,000 rpm for 2 min. Finally, the Sephadex G-50 microcolumn with a height of 1.7 cm was prepared. Subsequently, 0.1 mL coumarin-6-loaded SLN and BO-SLN solutions were slowly added onto the Sephadex G-50 microcolumn, and the column was centrifuged at 500 rpm for 1 min. Following this, 0.2 mL distilled water was added onto the microcolumn, and the column was centrifuged at 2,000 rpm for 2 min. The eluate was diluted and demulsified using methanol to obtain a 10 mL sample. The fluorescence intensity was determined at 502 nm with the excitation wavelength of 465 nm in a 96-well plate using a microplate reader.
Cytotoxicity assay in vitro
HBMECs were cultured in the DMEM cell culture medium containing 20% FBS, endothelial cell growth factor (100 μg/mL), L-glutamine (2 mmol/mL), heparin (20 μg/mL), insulin (40 μU/mL), penicillin (100 U/mL), and streptomycin (100 U/mL). The cells were maintained at 37°C with 5% CO2.
The cytotoxicity of SLN and two kinds of BO-SLN on HBMECs was evaluated. The cells were seeded in a 96-well plate with a density of 5×103 cells/well in 200 μL growth medium. Subsequently, the cells were treated with various carriers at a concentration of 0.001–2.0 mg/mL in a culture medium. Following 48 h of incubation, the media were replaced with fresh culture media containing MTT solution (0.5 mg/mL). The cells were incubated for an additional 4 h, followed by the removal of the medium and addition of DMSO into it. The optical density values were measured using a Stat Fax-2100 microplate reader (Awareness Co., Palm City, FL, USA) at a wavelength of 570 nm. All the experiments were repeated three times.
Cell uptake assay in vitro
HBMECs were cultured in six-well plates at a density of 5×105 cells/well and grown overnight, followed by treatment with a drug-free culture medium, coumarin-6, coumarin-6-loaded SLN, and two kinds of coumarin-6-loaded BO-SLN with 20 μmol/L coumarin-6. At predetermined time intervals, the cells were washed with PBS and harvested. The fluorescence intensity of coumarin-6 was determined using the FACScan flow cytometer (Guava easyCyte, Merck Millipore, Darmstadt, Germany) equipped with an argon laser for 468 nm and emission filter for 484 nm. The cells incubated without the sample were used to account for the background fluorescence. All the experiments were repeated three times.
BBB penetration in vitro
The BBB model was established using the method described in a previous study.18 The HBMECs were seeded into the Transwell inserts (Polycarbonate Membrane Transwell Inserts of 3 μm mean pore size, Corning Co.) at a density of 1×105 cells/well and allowed to grow for 4 days. The medium was changed every 2 days. The integrity of the BBB model was examined according to the transepithelial electrical resistance (TEER) value (threshold value, 250/cm2) measured using a transendothelial electrical resistance instrument (Millicell-ERS-2, Millipore). The TEER can represent the extent of tight junction of the BBB model in vitro.
Free coumarin-6, coumarin-6-loaded SLN, and two kinds of coumarin-6-loaded BO-SLN were added into the donator compartments to evaluate the ability of the carriers across the BBB. Then, 200 μL solution was taken out from the acceptor compartments at 10, 20, 30, 60, 90, 120, and 240 min after treatment, followed by 200 μL of fresh medium added to the compartments immediately. The BBB transport ratios of coumarin-6 were evaluated using high-performance liquid chromatography with the fluorescence detector.
Body distribution in vivo
The SLN and two kinds of BO-SLN injected via tail vein were evaluated using in vivo imaging systems. Further, 15 Kunming mice (20±2 g) were divided into five groups randomly, and free Cy7 solution, Cy7-loaded SLN, and different Cy7-loaded BO-SLN were injected at a volume of 0.1 mL. The mice were anesthetized with isoflurane at a predetermined time and visualized in the imaging system. The mice were placed in the transparent chamber to obtain images. The fluorescence intensity of the brain was quantified to exhibit the permeability of BBB.19 The excitation and emission wavelengths were set at 750 and 800 nm, respectively, to measure the fluorescence intensity of Cy7.20
Results and discussion
Characterization of BO-DOPE
The synthesized product was dissolved in chloroform and analyzed using mass spectrometry. The molecular ion peak with m/z of 980.7 could be observed, which was consistent with the theoretical molecular weight of BO-sa-DOPE. Moreover, the amide bond formed by the terminal carboxyl groups of BO-succinic anhydride and the terminal amino groups of DOPE was confirmed by 13C-NMR and 1H-NMR (Figure 2). The specific analysis was performed as follows.
  | Figure 2 (A) 1H and (B) 13C NMR spectrum of DOPE, (C) 1H and (D) 13C NMR spectrum of BO-DOPE. |
In the 13C-NMR of DOPE, the two peaks between chemical shifts at 168–178 ppm were the signals of two carbonyl carbons in DOPE; their chemical shifts were 173.62 and 173.33. In the 13C-NMR of BO-DOPE, the four peaks between the chemical shifts at 168–178 ppm were the signals of four carbonyl carbons in the synthesized BO-DOPE; their chemical shifts were 175.64, 173.32, 172.99, and 172.68. They were the sum of two carbonyl carbons regarded as the ring-opening product of succinic anhydride and two carbonyl carbons of DOPE. In the 1H-NMR of DOPE, the signal at 8.51 ppm was attributed to amino hydrogen at the end of DOPE. The amino hydrogen of DOPE moved to a high field because of the formation of amide bond; therefore, the chemical shift was reduced to 8.04 ppm. In summary, BO-DOPE was synthesized successfully with a specific structure.
Preparation and characterization of SLN and BO-SLN
The SLN was prepared using four common methods: emulsion and solvent evaporation, microemulsion, water/oil/water double emulsion and solvent evaporation, and thin-film evaporation and ultrasonic dispersion. Four optimal preparation methods used were applied to obtain a better method evaluated by the particle size, PDI, and stability within 7 days (Table 2). The results demonstrated that SLNs prepared by the water/oil/water double emulsion and solvent evaporation method had smaller particle size, good size distribution, and stability. Subsequently, important elements of water/oil/water double emulsion and solvent evaporation method, including lecithin ratio (w/w), BO-DOPE/PEG-SA, emulsification method, ultrasound power, and time, were screened (Figure 3).
  | Table 2 Comparison of the four methods of preparation |
The particle size first decreased and then increased successively with the increasing amount of lecithin (Figure 3A). Generally speaking, the greater the ratio of the emulsifier, the smaller the particle size. The difference originated probably from the presence of BO-DOPE, whose characterization differed from DOPE. When the proportion of BO-DOPE in the formula increased (Figure 3B), the number of free hydroxyl group from PEG-SA and the bound water molecules reduced, leading to smaller particle size. Besides, the power and time of ultrasound was crucial in the formation and stability of colostrum. If the ultrasonic power was too large and time was too long, it was easy to destroy the stability of soybean phospholipids and lipid materials. Instead, the emulsion was not formed completely (Figure 3D and E). The optimal prescription and process parameters were chosen to prepare the SLN and BO-SLN. The diameter, PDI, and zeta potential of three kinds of SLNs were similar, which was beneficial for keeping the single variable of BBB permeation (Table 3). However, the TEM image (Figure 4) of BO-SLN/CM displayed that its diameter was about 60 nm. The particle size and diameter determined using TEM was that of the dried particle.
  | Figure 4 TEM images of (A) a single BO-SLN/CM and (B) a crowd of BO-SLN/CM. |
Entrapment efficiency and drug loading
The drug-loaded nanoparticles and free drug were separated by the microcolumn centrifugation method.21 Moreover, the method was validated with the following parameters: a linear range with fluorescence intensity of coumarin-6 =8,511; C (concentration of coumarin-6) +442.4 (0.01–100 μg/mL, r=1.000); and precision, stability, and percent recovery as 98.66% (0.5 μg/mL), 99.51% (2.5 μg/mL), and 99.13% (5.0 μg/mL), respectively. The entrapment efficiency and drug loading of BO-SLN/CM were 80.1% and 0.75%, respectively.
Coumarin-6 is a lipophilic substance with high fluorescence efficiency; hence, its content can be rapidly measured using a fluorometer. Coumarin-6 was used as a fluorescent probe in qualitative and quantitative studies in vivo and in vitro. Nanoparticles encapsulating coumarin-6 can be observed at the drug-loading rate of 0.05%. The study demonstrated that coumarin-6 is not a pH-dependent fluorescent probe that can accurately trace the intracellular behavior of nanoparticles. Therefore, coumarin-6 was chosen as the drug to characterize SLNs with different modifications.
Cytotoxicity assay in vitro
The MTT method is a technique to detect cell viability based on the mechanism that mitochondria in living cells contain succinate dehydrogenase, which can change exogenous MTT to water-insoluble blue–violet crystals. However, dead cells do not have this function. The absorbance intensity was measured at 590 nm. The toxicity of three kinds of SLNs to HBMECs was concentration dependent (Figure 5A). No significant difference was observed in the cytotoxicity of BO-modified SLN and non-BO-modified SLN at low concentrations (C <0.1 mg/mL). At higher concentrations (C >0.5 mg/mL), the cytotoxicity of the BO-modified carrier was significantly lesser compared with that of the non-BO-modified carrier. BO significantly reduced the toxicity of SLN to HBMECs at high concentrations.
Cell uptake assay in vitro
The results (Figure 5B) exhibited that the uptake of coumarin-6 by HBMECs increased over time. The uptake of BO-SLN/CM was the fastest; it could be completed in 0.5 h, suggesting that BO could improve the ability of cell migration, which may be related to the improvement in the fluidity of phospholipid molecules in the membrane.22
BBB penetration in vitro
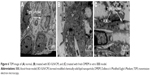
The TEM image (Figure 6A) of the BBB model displayed that HBMECs formed tight junctions, indicating that the BBB model met the morphological requirements. Moreover, the degree of tight junction formation could be assessed by measuring the TEER of the in vitro BBB model. In general, the TEER of the in vitro BBB model reported in previous studies was not the same because of differences in endothelial cells or cell line, model establishment, and other experimental conditions. The TEER of the BBB model formed by HBMEC culture alone can be used for transport studies when the TEER value is more than 250 Ω/cm2. The TEER of the self-made in vitro BBB model was 277.57±2.77 Ω/cm2, meeting the requirements of the in vitro transport study of the model.18,23
As illustrated in Figure 5C, the apparent permeability coefficient (Papp) of BO-SLN/CM reached the maximum Papp value of 98.3×10−6 cm/s within 20 min and was maintained at this level. The variation trend of BO-SLN/PM was the same as that of BO-SLN/CM and reached the maximum value of 95.0×10−6 cm/s. The Papp value of SLN increased slowly, and the maximum value appeared at 4 h. The transmembrane ability of free coumarin-6 was kept at the lowest level at each time. The maximal Papp values of BO-SLN/CM and BO-SLN/PM were 1.91 and 1.85 times that of SLNs, respectively.
The variation curve (Figure 5D) revealed that the TEER value of the BBB model incubated with BO-SLN/CM and BO-SLN/PM decreased within 20 min and remained at a low level. The TEER value of the BBB model incubated with SLN had no obvious change, which did not affect the integrity of the BBB. The change in TEER of SLN/CM and BO-SLN/PM was apparent. The cell uptake assay also exhibited that BO-modified SLN demonstrated excellent enhancement of the BBB permeation. As shown in Figure 6B, the tight junction disappeared, cells shrank, and the gap between cells increased after incubation of the BBB model with BO-SLN/CM or BO-SLN/PM for 1 h. Subsequently, the BBB model was cultured for 1 h with fresh DMEM, and the cells gradually restored as fusiform. The tight junction (Figure 6C) was formed again. Following culture for 4 h, the BBB model had a compact monolayer, indicating that the effect of BO was reversible and did not cause the pathological damage to BBB.24,25
Body distribution in vivo
As shown in Figure 7, the groups A, B, C, and D represented the control group, BO-SLN/CM group, BO-SLN/PM group, and SLN group, respectively. The drug entered the brain rapidly after administration in the BO-SLN/CM and BO-SLN/PM groups, and the fluorescence intensity reached the maximum at 30 min and gradually began to decrease. The drug was completely enriched in the lungs and barely entered into the brain in the SLN group, which might be related to the particle size of the nanoparticles. Moreover, the drug was concentrated in the upper part of the body in the BO-SLN/CM and BO-SLN/PM groups, which may be related to the pharmacological properties of BO.
The fluorescence intensity curve in the brain of each group is illustrated in Figure 7E. The cumulative fluorescence intensity was the highest in the BO-SLN/CM group after 4 h. The BO-modified SLNs after 4 h had a greater ability of BBB permeation compared with common SLN, supporting the results of the in vitro study. The single BO reached the maximum concentration in the brain after 15 min.26 The BO-SLN/CM can prolong the effect of BO. The targeting ability of chemically modified BO was more obvious than that of its physical modification.
Conclusion
BO-DOPE was synthesized by the amide reaction between BO and DOPE with succinic anhydride as the linker. BO-DOPE and PEG-SA were mixed in a certain proportion as a lipid material to prepare BO-SLN/CM by the double emulsion and solvent evaporation method. Moreover, BO and lipid material were mixed physically to prepare BO-SLN/PM. BO-SLN/CM, BO-SLN/PM, and SLN had good morphology, appropriate particle size and distribution, and adequate encapsulation efficiency. Two BO-modified SLNs had less cytotoxicity on HBMECs compared with SLNs. In vitro and in vivo studies demonstrated that the chemical modification of BO had a greater BBB permeation and targeting ability. Therefore, BO-SLN/CM had better BBB permeation compared with BO-SLN/PM and SLN and provided a better drug delivery system for treating brain diseases.
Acknowledgment
This work was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CAMS-2017-I2M-3-012).
Author contributions
Hui Song, Man Wei, Nan Zhang, He Li, Yujia Zhang, Xiaochuan Tan, and Wensheng Zheng participated in conducting the study. Wensheng Zheng and Hui Song designed and performed the majority of the study. All other authors also contributed to data analysis. Hui Song drafted the manuscript. Man Wei provided language help and with writing assistance. Wensheng Zheng proofread the article. All other authors amended the manuscript and agreed to publication.
Disclosure
The authors report no conflicts of interest in this work.
References
Pardridge WM. Why is the global CNS pharmaceutical market so under-penetrated? Drug Discov Today. 2002;7(1):5–7. | ||
Lawrence RN. Sir Richard Sykes contemplates the future of the pharma industry. Interview by Rebecca N Lawrence. Drug Discov Today. 2002;7(12):645–648. | ||
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. | ||
Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv Drug Deliv Rev. 2012;64(7):640–665. | ||
Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 2008;127(2):97–109. | ||
Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood-brain barrier by nanoparticles. J Control Release. 2012;161(2):264–273. | ||
Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007;59(6):454–477. | ||
Lim YM, Loh PHM, Ho ASH, Kua RZ, inventors; Universiti Tunku Abdul RahmanEu Yan Sang International Ltd, assignee. Method to extract borneol from the exudates of dryobalanops aromatica. CN103480169A. June 11, 2012. | ||
Almeida JR, Souza GR, Silva JC, et al. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Scientific World J. 2013;2013:808460. | ||
Cai Z, Hou S, Li Y, et al. Effect of borneol on the distribution of gastrodin to the brain in mice via oral administration. J Drug Target. 2008;16(2):178–184. | ||
Yu B, Ruan M, Dong X, Yu Y, Cheng H. The mechanism of the opening of the blood-brain barrier by borneol: a pharmacodynamics and pharmacokinetics combination study. J Ethnopharmacol. 2013;150(3):1096–1108. | ||
Zhang Q, Wu D, Wu J, et al. Improved blood-brain barrier distribution: effect of borneol on the brain pharmacokinetics of kaempferol in rats by in vivo microdialysis sampling. J Ethnopharmacol. 2015;162:270–277. | ||
Wang Y, Gao X, Zhang B. Pharmacodynamic comparison of natural bingpian (borneol) with compound bingpian in compound dripping pill of danshen. J Tianjin University Trad Chinese Med. 2003;(2):18–20. | ||
Liang M, Liu Q, Huang T, et al. The pharmacokinetic characteristics of borneol in serum and brain tissue of rats. Trad Chinese Drug Res Clin Pharmacol. 1993;(4):38–40. | ||
Wang LP, Feng JF, Hu KL. Progress in regulation effect of aromatic refreshing traditional Chinese medicine on BBB permeability and its mechanism. China J Chinese Materia Medica. 2014;39(6):949–954. | ||
Ren J, Zou M, Gao P, Wang Y, Cheng G. Tissue distribution of borneol-modified ganciclovir-loaded solid lipid nanoparticles in mice after intravenous administration. Eur J Pharm Biopharm. 2013;83(2):141–148. | ||
Xu X, Li J, Han S, et al. A novel doxorubicin loaded folic acid conjugated PAMAM modified with borneol, a nature dual-functional product of reducing PAMAM toxicity and boosting BBB penetration. Eur J Pharm Sci. 2016;88:178–190. | ||
Cecchelli R, Dehouck B, Descamps L, et al. In vitro model for evaluating drug transport across the blood-brain barrier. Adv Drug Deliv Rev. 1999;36(2–3):165–178. | ||
Bhaskar S, Tian F, Stoeger T, et al. Multifunctional nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging. Part Fibre Toxicol. 2010;7:3. | ||
Wu H, Wang S, Shi L, Deng Y. Effects of borneol on the distribution of azidothymidine palmitate liposomes in mice. Chin Pharm J. 2009;(8):590–593. | ||
Liu Y, Tao S, Guo W. Determination of entrapment efficiency of oxaliplatin liposomes by sephadex microcolumn combined with HPLC. Chin J Mod Appl Pharm. 2014;31(1):74–77. | ||
Davda J, Labhasetwar V. Characterization of nanoparticle uptake by endothelial cells. Int J Pharm. 2002;233(1–2):51–59. | ||
Panyam J, Sahoo SK, Prabha S, Bargar T, Labhasetwar V. Fluorescence and electron microscopy probes for cellular and tissue uptake of poly(D,L-lactide-co-glycolide) nanoparticles. Int J Pharm. 2003;262(1–2):1–11. | ||
Ge C, Han M, Bai R, et al. Effect of borneol on the ultrastructure of promoting blood brain barrier open. Chinese J Integrative Med Cardio-/Cerebrovascular Dis. 2008;(10):1183–1185. | ||
Lv X, Sun M, Sun F. Research of bornrol promote drugs through blood-brain barrier. China J Chinese Materia Medica. 2012;37(7):878–881. | ||
Yu B, Ruan M, Dong XP, Jin L, Qu HX. The influence of borneol treatment interval on the concentration of geniposide in rat brains. Chin Pharmacol Bull. 2012;28(6):862–866. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.