Back to Journals » Clinical Ophthalmology » Volume 12
Endophthalmitis rates among patients receiving intravitreal anti-VEGF injections: a USA claims analysis
Authors Kiss S, Dugel PU, Khanani AM , Broder MS , Chang E , Sun GH, Turpcu A
Received 24 March 2018
Accepted for publication 23 May 2018
Published 30 August 2018 Volume 2018:12 Pages 1625—1635
DOI https://doi.org/10.2147/OPTH.S169143
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Szilárd Kiss,1 Pravin U Dugel,2,3 Arshad M Khanani,4 Michael S Broder,5 Eunice Chang,5 Gordon H Sun,5 Adam Turpcu6
1Department of Ophthalmology, Weill Cornell Medical College, New York, NY, USA; 2Retinal Consultants of Arizona, Phoenix, AZ, USA; 3USC Roski Eye Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA; 4Sierra Eye Associates, Reno, NV, USA; 5Partnership for Health Analytic Research, LLC, Beverly Hills, CA, USA; 6Genentech, Inc., South San Francisco, CA, USA
Purpose: Intravitreal (IVT) injections of the anti-vascular endothelial growth factor (VEGF) agents aflibercept, bevacizumab, and ranibizumab are commonly prescribed to treat neovascular age-related macular degeneration (nAMD). Studies comparing inflammation rates in large populations of patients receiving these agents and the treatment of ocular inflammation post-IVT anti-VEGF injections are scarce. In this study, we compared rates of endophthalmitis claims (sterile and infectious) following IVT anti-VEGF injections to determine the risk factors associated with developing endophthalmitis, and examined the claims for subsequent treatment.
Patients and methods: This retrospective cohort study of USA claims data examined the risk of developing endophthalmitis following IVT injection of aflibercept, bevacizumab, or ranibizumab in patients with nAMD between 11/18/2011 and 5/31/2013. The primary study outcome was occurrence of endophthalmitis within 30 days of a claim for an IVT anti-VEGF injection. Endophthalmitis rates were calculated separately for aflibercept, bevacizumab, and ranibizumab, followed by pairwise comparisons of endophthalmitis frequencies among the 3 treatments.
Results: This analysis included 818,558 injections from 156,594 patients with nAMD. The rates (% [n/N]) of endophthalmitis following aflibercept, bevacizumab, and ranibizumab IVT injections were 0.100% (136/135,973), 0.056% (268/481,572), and 0.047% (94/201,013), respectively. In a multivariate analysis, aflibercept was associated with a significantly higher risk of endophthalmitis vs ranibizumab (adjusted odds ratio, 2.19; 95% CI: 1.68–2.85; P<0.0001). The risk of endophthalmitis was similar for bevacizumab and ranibizumab. Within 14 days after endophthalmitis, 38.6% of cases received injectable antibiotics, 15.3% received injectable steroids, and 30.3% underwent vitrectomy.
Conclusion: The rate of endophthalmitis was very low, but higher following IVT injection with aflibercept compared with both bevacizumab and ranibizumab in patients with nAMD.
Keywords: ranibizumab, aflibercept, bevacizumab, regression analysis
Introduction
The anti-vascular endothelial growth factor (VEGF) agents aflibercept and ranibizumab have been widely adopted for the treatment of neovascular age-related macular degeneration (nAMD),1,2 both inside and outside of the USA. The off-label use of bevacizumab is also widespread for this indication, based on efficacy in case series and clinical trials.3
One of the most serious complications following intravitreal (IVT) anti-VEGF injections is endophthalmitis – severe intraocular inflammation. Endophthalmitis can be infectious or sterile (noninfectious), with limited available evidence suggesting that the 2 forms differ in their pathogenesis, although this is currently not well understood.4 Another consideration is the inability to isolate an organism in some cases of infection.
The rate of ocular inflammation subsequent to IVT anti-VEGF injections is generally thought to be low, with the largest meta-analysis of published data to date estimating the frequency to be 0.056% (197/350,535 injections) across all anti-VEGF agents.5 In comparison, a retrospective claims study suggested that the rate of endophthalmitis after IVT steroid injections (N=18,666) was 0.13%, compared with 0.019% for anti-VEGF injections (N=387,714).6 The reported frequency of ocular inflammation following IVT anti-VEGF injections varies widely in retrospective clinical case series. Data from the largest studies generally support the low incidence of ocular inflammation after IVT anti-VEGF injections (<0.1%; summarized and corroborated by Dossarps et al7), although some smaller-sized studies have reported much higher rates.8–11 A high rate of acute intraocular inflammation was observed following IVT bevacizumab in the retrospective case series carried out by Wickremasinghe et al (14/1,278 injections; 1.10%)11 and Johnson et al (9/693 injections; 1.30%).10 Furthermore, there are a number of case reports of ocular inflammation following IVT bevacizumab associated with contamination during preparation of the drug for injection.12 A retrospective medical record review by Goldberg et al identified 20 instances of sterile inflammation after a total of 5,356 aflibercept injections (0.37%; 17/20 cases were from 1 retinal specialist).9 Similarly, Fine et al reported inflammation in 28 instances from a total of 5,905 aflibercept injections from their retrospective series (0.47%).8
The present study builds upon the analysis by Goldberg et al9 and explores rates of endophthalmitis following ~1 million IVT anti-VEGF injections for the treatment of nAMD using data from a large database capturing adjudicated claims from across the USA. The term “endophthalmitis” was used in this study because it captures both infectious and sterile ocular inflammation via easily identifiable International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Because endophthalmitis is perhaps the most feared and visually devastating complication following IVT injections, underdiagnosis is likely not an issue. As such, the data from this source are likely to accurately represent how often clinicians are making this diagnosis in the “real-world” setting. The 3 objectives of this study were as follows: 1) to compare rates of endophthalmitis (sterile and infectious) following IVT injections of aflibercept, bevacizumab, and ranibizumab, 2) to determine if there are risk factors associated with developing cases of endophthalmitis, and 3) to investigate how physicians are currently treating cases of post-IVT-injection endophthalmitis.
Patients and methods
Study design
This claims-based retrospective cohort study examined the risk of developing endophthalmitis following IVT injection of aflibercept, bevacizumab, or ranibizumab in a national cohort of insured patients. Analyses were conducted using the Wolters Kluwer Health’s Source® Lx database, which contains Health Insurance Portability and Accountability Act-compliant administrative patient data. Prescription claims from commercial plans, Medicare Part D plans, cash, and Medicaid claims were included in this data source. According to the US Department of Human Services, if research does not involve intervention or interaction with individuals, and if information on subjects is not individually identifiable, the research is considered not to involve human subjects and therefore does not even require a waiver from an institutional review board [45 CFR 46.102(f)(1),2].
The study only included deidentified data, therefore neither institutional review board approval, nor a waiver of such approval, was required.
All nAMD patient encounters were captured from the USA Food and Drug Administration approval date for aflibercept for treatment of nAMD (11/18/2011) until the last date of available data at the time of analysis (5/31/2013). The study was designed to assess the rate of endophthalmitis in patients with nAMD because this indication had the largest sample size and aflibercept has been approved for the longest period of time for this indication. Data for retinal vein occlusion (RVO) and diabetic macular edema (DME) are not reported here because the sample size for the RVO analysis for aflibercept was small compared with those for ranibizumab and bevacizumab, and aflibercept was not approved for treatment of DME at the time of the study. A patient encounter was an instance in which a patient diagnosed with nAMD had a claim for an IVT anti-VEGF injection on the same day as the diagnosis. Each patient may have had multiple encounters; each encounter for a given patient was considered separately. The date of the encounter (diagnosis/anti-VEGF claim) served as the index date, and each encounter was followed for 30 days. Encounters were excluded if the patient had a prior diagnosis of endophthalmitis (before the first anti-VEGF treatment in the identification period), had a claim for cataract surgery within 30 days of the encounter, or if the patient ever had a claim for glaucoma surgery. Furthermore, once a patient received a diagnosis of endophthalmitis, regardless of whether he or she switched treatment, all subsequent encounters for that patient were excluded. Therefore, only 1 endophthalmitis event per patient was included in these analyses.
Diagnoses of nAMD and endophthalmitis were based on ICD-9-CM13 codes detailed in Table S1. Claims for IVT anti-VEGF injections (aflibercept, bevacizumab, or ranibizumab) were identified based on the Healthcare Common Procedure Coding System codes detailed in Table S2.
The primary study outcome was occurrence of endophthalmitis during the 30-day follow-up period, which was selected based on previous endophthalmitis research.14
Data analysis
Patient encounters were stratified based on the type of anti-VEGF agent used on the index date. Endophthalmitis rates were calculated separately for aflibercept, bevacizumab, and ranibizumab. Pairwise comparisons of frequencies between therapies were made. In addition, the 18-month study period was divided into three 6-month periods and endophthalmitis rates were calculated for each anti-VEGF agent in each of the 3 periods; this was to confirm whether patterns seen overall were consistent over time or being driven by a certain time period, which could have indicated a short-lived manufacturing issue vs an underlying issue with the product.
To investigate whether there were risk factors associated with developing endophthalmitis, a repeated-measure analysis with generalized estimating equations was performed to adjust for baseline differences between treatment groups and the correlation among patients with multiple encounters. The models adjusted for the following confounding variables: baseline patient characteristics (age, sex, and region), comorbidities, and type of retinal disease (cataract, glaucoma).
Resource utilization, including rates of specific injectable antibiotic use (amikacin, vancomycin, garamycin, ceftriaxone, cefuroxime, and ceftazidime), vitrectomy, and injectable steroids within 14 days of endophthalmitis, was estimated. In addition, the rate of patients who continued on the same anti-VEGF agent, switched to a different anti-VEGF therapy, or discontinued anti-VEGF therapy during the 6-month period post-endophthalmitis was calculated.
All data transformations and statistical analyses were performed using SAS® version 9.4 (SAS Institute, Cary, NC, USA).
Results
Incidence of endophthalmitis
A total of 818,558 nAMD patient encounters were identified for this analysis (Figure S1). Baseline demographic characteristics for the first observed encounter per patient are shown in Table 1. The mean age (± SD) at the first encounter was 75.0±6.1 years. Most of the administered injections were IVT bevacizumab, followed by ranibizumab and aflibercept (58.8%, 24.6%, and 16.6%, respectively). Comorbid conditions (diabetes mellitus, cataract, and glaucoma) were similarly distributed among the treatment groups.
The overall rate of endophthalmitis was 0.061% (498/818,558) for nAMD patient encounters. The rate of endophthalmitis was higher following aflibercept (0.100%, 95% CI: 0.083–0.117) than bevacizumab (0.056%, 95% CI: 0.049–0.062) or ranibizumab (0.047%, 95% CI: 0.037–0.056) injections (P<0.001 for both vs aflibercept) (Figure 1).
After adjusting for factors, including baseline patient characteristics (age, sex, and region), comorbidities, and type of retinal disease (cataract, glaucoma), aflibercept was found to be associated with a significantly higher risk of endophthalmitis vs ranibizumab (adjusted odds ratio: 2.19; 95% CI: 1.68–2.85; P<0.0001) (Table 2). The risk of endophthalmitis was similar for bevacizumab and ranibizumab (Table 2). None of the other factors examined, including patient age, sex, geographical region, comorbid conditions, or whether the injection was the first received or otherwise, were associated with increased risk of endophthalmitis (Table 2).
The rate of endophthalmitis during each 6-month period of the 18-month study period is shown in Figure 2. For aflibercept, the rate of endophthalmitis appeared to increase as the study progressed. The rate of endophthalmitis remained relatively stable throughout the study period for ranibizumab and bevacizumab (Figure 2).
  | Figure 2 Rate of endophthalmitis during each 6-month period of the 18-month study in nAMD patients. |
The rate of endophthalmitis was higher at every injection of the sequence for patient encounters at which aflibercept was administered (range, 0.02%–0.18%) compared with ranibizumab (range, 0.01%–0.10%) and bevacizumab (range, 0.02%–0.07%), with the exception of injection 5 (Figure S2).
Following nAMD encounters, endophthalmitis occurred within a median of 3 days of aflibercept injections (mean: 7.6 days), compared with a median of 5 days for ranibizumab (mean: 8.5 days) and bevacizumab (mean: 7.6 days) (Figure 3).
Treatment received within 14 days after endophthalmitis
A total of 38.6% (192/498) of endophthalmitis cases in patients with nAMD received treatment with injectable antibiotics within 14 days of endophthalmitis; vancomycin and ceftazidime were the most frequently administered antibiotics (Figure S3). Injectable steroids were administered within 14 days of endophthalmitis for 15.3% (76/498) of patient encounters. The number of patients undergoing vitrectomy within 14 days of endophthalmitis was 30.3% (151/498) (Figure S4). There were no major differences between the treatment groups with regard to number of patients who received injectable antibiotics or steroids, or underwent vitrectomy (Figures S3 and S4). It should be noted that patients may have received more than 1 antibiotic or course of treatment.
Anti-VEGF treatment after incidences of endophthalmitis
Within the 6-month period after endophthalmitis was identified, 38.0% (189/498) of nAMD patient encounters were receiving the same anti-VEGF agent, 20.7% (103/498) had switched to another anti-VEGF agent, and 41.4% (206/498) were not receiving any anti-VEGF treatment. A greater proportion of nAMD encounters receiving ranibizumab (48.9%, 46/94) were continuing the same drug at 6 months (ie, had not switched or discontinued) compared with aflibercept (34.6%, 47/136) or bevacizumab (35.8%, 96/268). This was accompanied by a lower rate of discontinuation of anti-VEGF therapy for patient encounters receiving ranibizumab (27.7%, 26/94) compared with aflibercept (39.7%, 54/136) or bevacizumab (47.0%, 126/268) (Figure S5).
Discussion
This retrospective cohort study of USA claims data for patients with nAMD demonstrated that the overall rate of endophthalmitis was low (0.061%) following IVT anti-VEGF injections. This is broadly consistent with much of the previously published literature from large, retrospective studies (summarized by Dossarps et al7).
After adjusting for baseline patient differences, aflibercept was associated with a significantly higher risk of endophthalmitis vs ranibizumab in patients with nAMD. The risk of endophthalmitis was not significantly different between ranibizumab and bevacizumab. During the clinical trials of these agents, no statistically significant differences in the rates of ocular inflammation were observed (although these studies were neither designed nor powered to detect differences in safety outcomes).12 Because ocular inflammation following IVT anti-VEGF injections is encountered with relatively low frequency and most studies only encounter a few cases, there is little evidence in the published literature to corroborate our findings regarding potential differences in rates of endophthalmitis among these agents. A similar medical claims data-based retrospective cohort study by VanderBeek et al15 demonstrated no significant difference between bevacizumab and ranibizumab in the rate of post-injection endophthalmitis (0.017% [49/296,565 injections] and 0.025% [22/87,245 injections], respectively). However, a smaller retrospective case series demonstrated that aflibercept was associated with higher rates of ocular inflammation than bevacizumab and ranibizumab when considering sterile vitritis (0.16% [13 cases], 0.10% [67], and 0.02% [6], respectively), culture-positive endophthalmitis (0.02% [2], 0.003% [2], and 0.01% [3], respectively), and indeterminate cases administered antibiotics without a positive culture result (0.16% [13], 0.06% [40], and 0.03% [9], respectively).16
There are a number of factors that have the potential to impact rates of ocular inflammation following IVT anti-VEGF injections. Contamination with bacteria or endotoxin during preparation of the drug or the injection procedure is implicated in many reported cases, with clusters of post-IVT injection ocular inflammation associated with individual physicians and anti-VEGF drug lots or batches.9,17–21 Variations in molecular structure may mean that there are differences in immunogenicity among the agents, which may be increased by degradation, meaning that drug stability may have a role.21,22
Data regarding the treatment patients received within 14 days after occurrences of endophthalmitis revealed that injectable antibiotics were administered in 38.6% of cases, injectable steroids in 15.3%, and vitrectomy was carried out in 30.3%. There were no major differences among the aflibercept, ranibizumab, or bevacizumab groups with regard to the treatment received following cases of endophthalmitis for nAMD patient encounters. The presumed use of vitrectomy for treatment of endophthalmitis was more common than expected, given that the only clinical trial evaluating its effectiveness is limited to a 20-year-old study of postoperative endophthalmitis, the Endophthalmitis Vitrectomy Study.23 This study only reported a benefit of vitrectomy in eyes with light perception only at presentation. The use of vitrectomy was also more common than expected in a recent retrospective cohort study of post-cataract surgery endophthalmitis using Medicare billing claims (45%, 279/615).24 This study demonstrated no association between higher rates of vitrectomy and better visual outcomes for patients with vision better than light perception.
Data from patients with nAMD indicated that patients who received ranibizumab were more likely to still be receiving the same drug and not to have discontinued anti-VEGF therapy after 6 months compared with patients who received aflibercept or bevacizumab. The reasons behind this cannot be determined from these study data, but potential differences in long-term tolerability among the drugs warrant further investigation.
The present study, evaluating nearly 1 million injections, is the largest conducted to date regarding the incidence of endophthalmitis and the treatment following IVT anti-VEGF injections. Furthermore, the contemporary data mean that it is the first to comparatively assess endophthalmitis rates following bevacizumab, ranibizumab, and the more recently approved aflibercept. This study is based on a patient claims database; therefore, the risk of selection bias (overestimating the number of cases of endophthalmitis) is reduced compared with some practice-based studies, particularly studies at practices to which patients with endophthalmitis may be referred. A general limitation of studies based on patient claims databases is that the data are collected for billing, rather than research purposes. Billing codes lack clinical information (including visual acuity) and the data necessary to distinguish between sterile and infectious endophthalmitis, which are thought to differ in their pathogenesis, although this is currently not well understood.4 In addition, these retrospective data cannot be used to identify differences in the injection practices that may have contributed to our findings. Furthermore, claims reflect the care received and not the reasons for that care.
Conclusion
In summary, this large, retrospective claims analysis of patients with nAMD demonstrated that the rate of endophthalmitis following IVT anti-VEGF agents was generally low, but higher following aflibercept compared with following either ranibizumab or bevacizumab. The findings of this study support the expectation of a low rate of endophthalmitis following IVT anti-VEGF treatment. Nevertheless, awareness of the risk remains clinically relevant due to the requirement for regular repeat injections for treatment of nAMD.
Abbreviations
DME, diabetic macular edema; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; IVT, intravitreal; nAMD, neovascular age-related macular degeneration; RVO, retinal vein occlusion; VEGF, vascular endothelial growth factor.
Acknowledgments
Third-party writing assistance for this manuscript was provided by Grace H Lee of Envision Pharma Group, funded by Genentech, Inc., a member of the Roche Group. Dr Turpcu had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Genentech, Inc., South San Francisco, CA, USA provided support for the study and participated in the study design; conducting the study; data collection, management, analysis, and interpretation; and preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. Portions of these data, including odds ratio and injection frequency analyses, were presented at the 38th Annual Meeting of the Macula Society, February 25–28, 2015, in Scottsdale, AZ, USA. The study was funded by Genentech, Inc.
Author contributions
MSB, EC, GHS: all portions of the manuscript. All authors (SK, PUD, AMK, MSB, EC, GHS, AT) contributed to data analysis, drafting and revising the paper, and agree to be accountable for all aspects of the work.
Disclosure
Dr Kiss has served as a consultant to Alcon, Alimera, Allergan, Genentech, Inc., Optos, and Regeneron, and has received research funding from Alimera, Allergan, Genentech, Inc., Optos, and Regeneron. Dr Dugel has served as a consultant for Genentech, Inc., Novartis, and Regeneron. Dr Khanani has served as a consultant for Alimera, Allergan, Genentech, Inc., and Novartis Pharma AG and has received research funding from Genentech, Inc. Dr Broder and Dr Chang are employees of Partnership for Health Analytic Research, LLC, which was paid by Genentech, Inc. to conduct the research, and have served as consultants for Genentech, Inc. Dr Sun was an employee of Partnership for Health Analytic Research, LLC, at the time of preparation of this manuscript, is currently employed by Rancho Los Amigos National Rehabilitation Center, and has served as a consultant for Genentech, Inc. and Medscape from WebMD. Dr Turpcu is an employee of Genentech, Inc. The authors report no other conflicts of interest in this work.
References
Genentech, Inc. LUCENTIS® (ranibizumab injection) [package insert]; 2015. Available from: http://www.gene.com/download/pdf/lucentis_prescribing.pdf. Accessed May 5, 2015. | ||
Regeneron, Inc. EYLEA® (aflibercept injection) [package insert]; 2015. Available from: https://www.regeneron.com/Eylea/eylea-fpi.pdf. Accessed May 5, 2015. | ||
Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014;8:CD005139. | ||
Hahn P, Chung MM, Flynn HW Jr, et al. Postmarketing analysis of aflibercept-related sterile intraocular inflammation. JAMA Ophthalmol. 2015;133(4):421–426. | ||
Fileta JB, Scott IU, Flynn HW Jr. Meta-analysis of infectious endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmic Surg Lasers Imaging Retina. 2014;45(2):143–149. | ||
VanderBeek BL, Bonaffini SG, Ma L. The association between intravitreal steroids and post-injection endophthalmitis rates. Ophthalmology. 2015;122(11):2311.e1–2315.e1. | ||
Dossarps D, Bron AM, Koehrer P, Aho-Glélé LS, Creuzot-Garcher C, FRCR net (FRenCh Retina specialists net). Endophthalmitis after intravitreal injections: incidence, presentation, management, and visual outcome. Am J Ophthalmol. 2015;160(1):17.e1–25.e1. | ||
Fine HF, Roth DB, Shah SP, Haque T, Wheatley HM. Frequency and characteristics of intraocular inflammation after aflibercept injection. Retina. 2015;35(4):681–686. | ||
Goldberg RA, Shah CP, Wiegand TW, Heier JS. Noninfectious inflammation after intravitreal injection of aflibercept: clinical characteristics and visual outcomes. Am J Ophthalmol. 2014;158(4):733.e1–737.e1. | ||
Johnson D, Hollands H, Hollands S, Sharma S. Incidence and characteristics of acute intraocular inflammation after intravitreal injection of bevacizumab: a retrospective cohort study. Can J Ophthalmol. 2010;45(3):239–242. | ||
Wickremasinghe SS, Michalova K, Gilhotra J, et al. Acute intraocular inflammation after intravitreous injections of bevacizumab for treatment of neovascular age-related macular degeneration. Ophthalmology. 2008;115(11):1911–1915. | ||
Fine HF, Despotidis GD, Prenner JL. Ocular inflammation associated with antivascular endothelial growth factor treatment. Curr Opin Ophthalmol. 2015;26(3):184–187. | ||
Centers for Disease Control and Prevention. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Last update: June 18, 2013. Available from: http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed May 27, 2014. | ||
Fintak DR, Shah GK, Blinder KJ, et al. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina. 2008;28(10):1395–1399. | ||
VanderBeek BL, Bonaffini SG, Ma L. Association of compounded bevacizumab with postinjection endophthalmitis. JAMA Ophthalmol. 2015;133(10):1159–1164. | ||
Williams PD, Chong D, Fuller T, Callanan D. Noninfectious vitritis after intravitreal injection of anti-VEGF agents: variations in rates and presentation by medication. Retina. 2016;36(5):909–913. | ||
Wang F, Yu S, Liu K, et al. Acute intraocular inflammation caused by endotoxin after intravitreal injection of counterfeit bevacizumab in Shanghai, China. Ophthalmology. 2013;120(2):355–361. | ||
Yamashiro K, Tsujikawa A, Miyamoto K, et al. Sterile endophthalmitis after intravitreal injection of bevacizumab obtained from a single batch. Retina. 2010;30(3):485–490. | ||
Entezari M, Ramezani A, Ahmadieh H, Ghasemi H. Batch-related sterile endophthalmitis following intravitreal injection of bevacizumab. Indian J Ophthalmol. 2014;62(4):468–471. | ||
Fielden M, Nelson B, Kherani A. Acute intraocular inflammation following intravitreal injection of bevacizumab – a large cluster of cases. Acta Ophthalmol. 2011;89(8):e664–e665. | ||
Agrawal S, Joshi M, Christoforidis JB. Vitreous inflammation associated with intravitreal anti-VEGF pharmacotherapy. Mediators Inflamm. 2013;2013:943409. | ||
Koren E, Zuckerman LA, Mire-Sluis AR. Immune responses to therapeutic proteins in humans – clinical significance, assessment and prediction. Curr Pharm Biotechnol. 2002;3(4):349–360. | ||
Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113(12):1479–1496. | ||
Gower EW, Keay LJ, Stare DE, et al. Characteristics of endophthalmitis after cataract surgery in the United States Medicare population. Ophthalmology. 2015;122(8):1625–1632. |
Supplementary materials
  | Table S2 Healthcare Common Procedure Coding System codes |
  | Figure S2 Rate of endophthalmitis by injection order in patients with nAMD. |
  | Figure S3 Injectable antibiotic treatment within 14 days after endophthalmitis for nAMD patient encounters. |
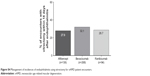  | Figure S4 Management of incidences of endophthalmitis using vitrectomy for nAMD patient encounters. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







