Back to Journals » Open Access Journal of Clinical Trials » Volume 11
Effects of pain on cognitive function and mobility
Authors Moreira SA, Novak P
Received 3 August 2018
Accepted for publication 8 February 2019
Published 25 March 2019 Volume 2019:11 Pages 1—10
DOI https://doi.org/10.2147/OAJCT.S182502
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Arthur E. Frankel
Sebastian A Moreira,1 Petr Novak2
1Clinical Research, GlaxoSmithKline Consumer Healthcare, Warren, NJ, USA; 2Medical Affairs, GlaxoSmithKline Consumer Healthcare, Prague, Czech Republic
Background: Chronic pain has been associated with impaired cognitive function as well as limitations in physical mobility in older adults. This study was conducted to determine whether acute pain that is typically self-diagnosed and self-treated with nonprescription analgesics also affects cognitive function or mobility.
Methods: In this placebo-controlled, randomized study, generally healthy adults with recurrent acute joint, back, head, or menstrual pain underwent assessments of cognitive function (Axon Sports Priming Application, Cambridge Neuropsychological Test Automated Battery) and mobility (gait, time to stand, and grip force) during pain (≥5 out of 10 on Brief Pain Inventory – Short Form Q6) vs after pain resolution. Assessments during pain were made before and after treatment with paracetamol 500 mg, paracetamol 500 mg/caffeine 65 mg, or placebo. Primary and secondary outcomes, respectively, were the effects of pain on cognition and mobility vs the pain-free state. The effects of nonprescription analgesics on these outcomes were intended as an exploratory outcome but were not analyzed due to early termination of the study for breaches of Good Clinical Practice guidelines.
Results: At termination, 54 individuals had been screened and 21 randomized (safety population). The primary analysis population (modified intent-to-treat) consisted of 20 participants with no inclusion/exclusion violations. Due to early termination, the study did not meet prespecified recruitment levels; therefore, no conclusions could be drawn regarding the effects of pain on cognitive performance or mobility. There were three treatment-emergent adverse events (placebo: reflux disease and hypercholesterolemia; paracetamol: pharyngeal erythema).
Conclusion: Because the study was terminated before reaching prespecified recruitment levels, conclusions cannot be drawn regarding the effects of pain or relief from pain by analgesics on cognition or mobility. However, the methodology can serve as a model for addressing these important questions in future investigations. Safety results were consistent with the safety profile for nonprescription paracetamol and paracetamol/caffeine.
Keywords: pain, cognition, mobility limitation, paracetamol, caffeine
Introduction
Chronic pain conditions have been associated with impairments in cognitive function, including speed of information processing, reasoning ability, attention, working memory, and long-term memory.1–7 In addition, studies of community-dwelling older adults found that pain, particularly widespread or severe pain, was associated with mobility limitations.8–11 Limitations in physical performance (eg, walking speed, stair climbing, and activities of daily living) have been observed in individuals with knee or hip osteoarthritis and correspond to the pain level.12,13 Finally, both pain and cognitive impairment affect mobility status in older adults, and mobility is affected to a greater extent when both are present.14
Little is known about whether common types of pain that are often self-treated with nonprescription therapies (eg, joint, back, head, and menstrual pain) have similar effects on cognitive function or mobility in the general adult population. If such cognitive and mobility impairments do occur, it is unknown whether over-the-counter analgesics have the ability to reduce these deficits either via direct effects on the central nervous system (CNS) or indirectly by reducing pain. Paracetamol is known to penetrate the blood–brain barrier into the CNS.15 Therefore, it is believed to have a central effect on pain that may involve neurotransmitters (eg, serotonin) that are also involved in decision making and other cognitive tasks.16–18 Data on the effects of paracetamol on cognition and mobility in individuals with pain, however, are lacking. Although one double-blind, randomized, placebo-controlled, repeated-dose clinical study in 72 healthy men with exercise-induced leg muscle pain found no improvement in cognitive or motor skills with ibuprofen,19 another randomized, placebo-controlled study conducted in 40 healthy volunteers found that a single oral 2 g dose of paracetamol improved cognitive function, particularly decision making and spatial memory;18 whether lower nonprescription doses have similar effects has not been established. It is also unknown whether the addition of caffeine to paracetamol would enhance any potential benefits regarding cognitive function or mobility, but such effects are plausible because caffeine increases the analgesic effects of paracetamol, may shorten the time to paracetamol onset,20–22 and has been shown to enhance alertness/vigilance and reaction time among healthy individuals.23–25
To address these knowledge gaps, this study was designed to assess the effects of common joint, back, head, or menstrual pain on cognition (primary objective) and mobility (secondary objective) compared with a pain-free state. Exploratory end points were intended to compare the effects of paracetamol and paracetamol/caffeine vs placebo on cognition and mobility in participants with pain. Unfortunately, it was not possible to fully evaluate the primary and secondary end points or calculate the exploratory end points because the study was terminated due to breaches of Good Clinical Practice guidelines observed during the sponsor’s internal audit. These breaches pertained to technical aspects of data storage, handling, and transmission, such that data were not all transmitted via the secured systems required by the study protocol. These breaches did not affect participant safety or compromise personally identifiable information, as such information was not recorded on the case report forms. The methodology of this study may be useful in designing future studies to address these important questions.
Materials and methods
Study design and procedures
This parallel, assessor-blind, placebo-controlled, stratified, randomized study was conducted from October 31, 2016 to February 6, 2017 (ClinicalTrials.gov Identifier: NCT02974114; EudraCT: 2015-002330-42). Participants made three visits to the study center (GSK Human Performance Lab, Brentford, Middlesex, UK). Visit 1 was for participant screening and collection of baseline and demographic data. At Visit 2 (7–28 days after Visit 1), assessments of cognition (Axon Sports Priming Application and Cambridge Neuropsychological Test Automated Battery [CANTAB]) and mobility were conducted while the participant was experiencing pain. Pain was confirmed by a score of 5 or greater on the Brief Pain Inventory (BPI) – Short Form (question 6; numerical rating of pain severity from 0= no pain to 10= pain as bad as you can imagine).26 Cognition and mobility assessments were made both before and after pain treatment at Visit 2. These assessments were repeated at Visit 3 (2–30 days after Visit 2) with the participant in a pain-free state. The absence of pain at Visit 3 was confirmed by score of 0 on the BPI – Short Form.
Cognitive testing consisted of the Axon Sports Priming Application and the CANTAB,27–29 which were administered via electronic tablet device at Visits 2 and 3. The Axon Sports Priming Application is a mobile version of the simple reaction time task from the CogState computerized cognitive assessment battery;30 it consists of a 5-minute computerized test that measures psychomotor speed (primarily simple reaction time) in milliseconds. The CANTAB assessments used in this study included tests of reaction time, executive function (via the One Touch Stockings of Cambridge task and the Attention Switching Task), spatial working memory, and rapid visual information processing.29
Mobility assessments included evaluations of gait, time to stand, and grip force.31 Gait assessments included duration of contact with the ground (ie, time from initial heel strike to lifting of toe, in m/s), stride length (cm), and walking speed over 5–10 m for each foot (m/s). At each assessment period, participants completed three walk tests over a 15 m track with only a 5- to 10-m section measured and analyzed; three sit-to-stand tests; and three grip force tests; the results for each set were averaged. The Optojump athletic movement analysis system (Microgate) was used in the analyses of gait. Time to stand from a seated position was measured with a stopwatch, and ground reaction force was measured with a force plate. Grip force in the dominant hand was assessed using a dynamometer, with the participant exerting the maximum effort possible during arm swinging from above the head to the side of the body. If pain was present in the dominant hand, grip force was measured in the nondominant hand instead.
Study population and restrictions
Participants were required to be generally healthy men and women, 18–65 years of age, with a body mass index (BMI) of 18.5–30 kg/m2, who were experiencing a transient episode of only one of the following: joint, back, head, or menstrual pain. They had to have a minimum of two recurrent, acute pain episodes in the past 3 months or a current flare-up of recurrent, acute pain at Visit 1, and a score of at least 5 (on a 0–10 numerical rating scale) on question 6 of the BPI – Short Form questionnaire in the pretreatment stage of Visit 2. Women of childbearing potential had to be using a reliable method of contraception, and all participants had to be able and willing to comply with study procedures, assessments, and restrictions. Participants were recruited from GlaxoSmithKline employees, the general local population via advertisement, and from a panel of pain sufferers held by the study center.
Exclusion criteria included the presence of any medical condition that might be aggravated by the test procedures or that might impact interpretation of the results (in the opinion of the investigator), color blindness, pregnancy or lactation, use of antidepressants within 2 years prior to the study, hypersensitivity to study interventions or related compounds, and positive breath test results for alcohol or urine test results for drugs of abuse at Visit 2. Individuals with current (within 14 days of study start) or regular use of any prescription, over-the-counter, or herbal medicine were excluded unless the medication was approved by the study physician. Current smokers, heavy drinkers (>21 units/week for men or >14 units/week for women), and those with frequent caffeine intake equivalent to six cups of brewed coffee or 12 cups of tea per day were also excluded. Potential participants were excluded if they had participated in another clinical trial or received an investigational drug within 1 week of the screening visit, or if they were employees or immediate family members of employees at the study center.
Analgesics, anti-inflammatory agents, and vitamin supplements were prohibited within 48 hours of Visits 2 and 3. Medications that might counteract paracetamol and/or caffeine were prohibited on assessment days, and antidepressants were prohibited for the duration of the study. Consumption of any substance known to induce or inhibit hepatic drug metabolism was prohibited in the 30 days prior to dosing. During the study, participants were required to refrain from excessive consumption of alcohol for 24 hours prior to Visits 2 and 3 and from consuming caffeine for 4 hours prior to these visits. While on site, participants were not permitted to smoke or to consume food, fluids, or medications other than the study medication and water provided.
All participants provided written informed consent at Visit 1, prior to any assessments or interventions. The study was reviewed and approved on August 9, 2016, by the East Midlands – Leicester South Research Ethics Committee, NHS and conducted in accordance with local laws and regulations as well as the Declaration of Helsinki.
Interventions
At Visit 2, participants were randomly assigned to one of the following three treatment groups: paracetamol 500 mg+ caffeine 65 mg (Panadol® Extra Advance; GlaxoSmithKline Consumer Healthcare [GSKCH], Warren, NJ, USA), paracetamol 500 mg (Panadol® Advance, GSKCH), or placebo matched to Panadol® Extend (GSKCH). All groups received a single oral dose consisting of two tablets taken with 200 mL of water. The three treatments had different appearances; however, participants wore an eye mask during dosing, and study site personnel involved in dispensing and administration were not involved in any of the cognition and mobility assessments. The investigators and statisticians were not present during dosing and were blinded to treatment assignment.
Randomization was stratified by baseline pain type (knee or hip joint pain, upper or lower back pain, headache, or menstrual pain). Assessments of pain, cognition, and mobility were conducted prior to treatment and at 75 minutes posttreatment.
Study outcomes
The primary outcome was a comparison of cognitive performance based on Axon Sports and CANTAB assessments during pain (Visit 2, pretreatment) vs a pain-free state (Visit 3). The secondary outcome was a comparison of mobility based on gait, time to stand, and ground reaction force during pain (pretreatment) vs the pain-free state. Safety data (adverse events [AEs], serious AEs) were collected throughout the study beginning with Visit 1, and all AEs were evaluated with regard to severity and causality.
Statistics
According to the study protocol, ~65 participants were to be randomized to ensure that ~60 evaluable participants would complete the study (~20 participants per treatment arm). However, enrollment was not completed because of the early study termination.
The safety population was defined as all participants receiving treatment, and the intent-to-treat (ITT) population was defined as all participants who received treatment and had at least one postbaseline efficacy measurement. The modified ITT (mITT) population, which was the primary analysis population, was defined as participants from the ITT population who had no violations of protocol-defined inclusion/exclusion criteria.
Descriptive statistics were calculated for all efficacy outcomes. A nonparametric Wilcoxon signed-rank test was performed to analyze time-related efficacy end points (Axon Sports assessment, reaction time, attention-switching task, contact phases, walking speed, and time to stand). A two-sided paired t-test was performed for end points that were not time-related (Rapid Visual Information Processing, Spatial Working Memory, One Touch Stockings of Cambridge, stride length, ground reaction force, and grip force), and 95% CIs were calculated. For the parametric analyses, assumptions of normality were investigated and, if unconfirmed, an appropriate data transformation or nonparametric method was used. No adjustments were made for multiplicity, and no imputations were made for missing data. All statistical analyses were performed using SAS software version 9.2.
Planned exploratory analyses comparing the effects of the treatment interventions (posttreatment vs pretreatment) on cognition and mobility during the pain state were not conducted due to early study termination. Therefore, only the pretreatment data from Visit 2 are reported and compared with the assessments in the pain-free state at Visit 3.
Results
Study population
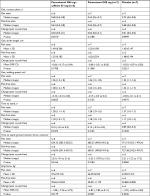
At the time of the early study termination, 54 individuals had been screened and 21 randomized (Figure 1). The 21 randomized participants comprised both the ITT and safety populations. This group was 66.7% female, 57.1% White, and had a mean age of 33.2 years (Table 1). One of the randomized participants had a protocol violation consisting of having two pain sites (hip and lower back) and was therefore excluded from the mITT population. Eighteen participants completed the study.
  | Table 1 Demographic characteristics Abbreviation: BMI, body mass index. |
Effects of pain on cognition and mobility
There were insufficient data to allow for observation of any trends regarding the effects of pain (pretreatment) on cognitive performance compared with the pain-free state (Table 2). Results in this small population were highly variable with few significant differences. Similarly, no clear trend could be discerned regarding how pain affected mobility (Table 3).
Safety
There were seven total AEs (Table 4), of which three were treatment-emergent (acid reflux, worsening hypercholesterolemia, and pharyngeal erythema). All were mild to moderate. None were considered treatment-related or serious, and all resolved. None of the participants withdrew from the study as a result of an AE.
  | Table 4 Adverse events (AEs) |
Discussion
Definitive conclusions about the effects of pain on cognition and mobility cannot be drawn from this study due to the high variability of the results and the insufficient sample size resulting from early study termination. Safety results were consistent with the known safety profile for an over-the-counter dose of paracetamol32 or paracetamol/caffeine.22,33 One case of mild pharyngeal erythema on the day of paracetamol administration was the only treatment-emergent AE associated with either active treatment, and it was not considered treatment-related.
The adverse effect of pain on cognitive function has been demonstrated in numerous studies of patients with various chronic pain conditions such as rheumatoid arthritis, fibromyalgia, neuropathic pain, and persistent low back or neck pain.1–7 These effects may be mediated by depression1 or may occur because cognitive tasks are competing with pain for attentional resources.3,34 Another hypothesis is that closely linked neurologic signaling pathways involved in cognition and pain transmissions may modulate each other.34 In addition, chronic pain may result in altered neuroplasticity or dysregulated neurochemistry that in turn affects cognitive function.34
Data are lacking in terms of whether similar mechanisms would also lead to impairments in cognition in pain that would ordinarily be treated with nonprescription analgesics. Similarly, it is unclear whether the mobility limitations observed in older adults with severe or widespread pain8–10 or osteoarthritis13 would also occur in younger populations or in individuals with more mild, localized, or transient pain.
Although a wide range of tests have been used to assess cognitive function in studies of chronic pain populations,1–6 there does not appear to be a gold standard for such assessments. A systematic review of cognitive function instruments used to assess individuals with chronic pain35 found that none of the 53 instruments identified had been validated specifically for use in patients with pain, and there was limited information in individuals with certain pain types, including musculoskeletal pain. In the current study, we chose the Axon Sports Priming Application and several CANTAB assessments. The CogState simple reaction time test was previously validated against multiple neuropsychological tests in healthy adults aged 35–50 years.30 To our knowledge, it has not previously been used to assess cognition in studies of patients with pain. However, it was previously validated in and found to be sensitive to mild cognitive impairment among individuals with various neuropsychiatric or neurodegenerative disorders. On that basis, we believe it to be an appropriate tool for use in hypothesis-generating group studies to evaluate the nature and severity of cognitive impairment associated with other diseases/disorders.30
The CANTAB combined various tests that had proven useful for neuropsychological testing in animals and humans and converted them into a form that could be administered using digital touch-screen technologies.36 Thus, it offers the advantages of simple and standardized administration and automated recording of responses with millisecond precision.7 Although the CANTAB battery of assessments has not been extensively studied in pain conditions, it has been studied in various other neurological conditions, has the advantage of an easy-to-use subject interface, and has been validated in healthy volunteers aged 55–80 years.28 It was originally designed for use in elderly patients with neurodegenerative diseases (eg, Alzheimer’s disease and Parkinson’s disease) but has been widely used in other patient populations, including those with various neurologic or psychiatric disorders, brain injury, or developmental disorders.36 The CANTAB tests we selected for the current analysis focused on the attention and executive function/decision-making domains; these domains were of primary interest to us because we inferred that they might be impacted by pain.
Three previous studies used CANTAB assessments to evaluate cognition in patients with pain conditions, including postherpetic neuralgia,37 chronic low back pain,7 and chronic nonmalignant pain treated with opioids.38 The study in postherpetic neuralgia utilized CANTAB assessments for choice reaction time, semantic memory (Graded Naming Test), decision making (Information Sampling Task), and spatial memory (Stockings of Cambridge test), with impairments seen in all three, but only in patients taking systemic treatments and not in patients taking topical lidocaine therapy compared with age- and sex-matched controls.37 The study of low back pain used the choice reaction time test, pattern recognition memory test, and the spatial span test of working memory, none of which were found to be affected by pain, although patients using opioid treatment exhibited worsening on the spatial span test compared with healthy controls.7 The study of patients with opioid-tolerant chronic nonmalignant pain used the rapid visual information processing test of general cognitive performance, as well as the tests for spatial recognition memory and spatial working memory, but focused on whether taking oxymorphone extended release with a high-fat meal vs a fasted state affected these cognitive outcomes; it was not designed to assess the impact of pain or opioid therapy on cognition.38
Conclusion
The substantial limitations of this study relating to the small sample size and the breach in data transmission protocol prevent accurate interpretation of the current results and leave the central study questions regarding the effects of pain on cognitive function and mobility unanswered. However, the design of the current study remains pertinent as a model for development of future investigations into these important questions. For future studies, there is strong rationale for pursuing the effects of pain on attention and executive function/decision-making capability, as there seems to be evidence for overlap in the neural regions responsible for pain sensation and cognition, resulting in competition for resources.
Abbreviations
AE, adverse event; BPI, Brief Pain Inventory; CANTAB, Cambridge Neuropsychological Test Automated Battery; GSKCH, GlaxoSmithKline Consumer Healthcare; ITT, intent-to-treat; mITT, modified intent-to-treat.
Data sharing statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Acknowledgments
The authors wish to thank all members involved in the study and the study participants. This study was wholly funded by GSKCH, Warren, NJ, USA. GSKCH reviewed the data report and publication. Medical writing assistance was provided by Peloton Advantage, Parsippany, NJ and was funded by GSKCH.
Author contributions
Both authors were actively involved in preparing this paper for publication and made substantial contributions to: 1) the conception and design of the study, or acquisition of the data, or analysis and interpretation of the data; and 2) drafting the article or revising it critically for important intellectual content. Moreover, they have each 3) granted final approval of the current version for submission to be published and 4) agree to be accountable for all aspects of the work.
Disclosure
Sebastian A Moreira and Petr Novak are employees of GlaxoSmithKline Consumer Healthcare. The authors report no other conflicts of interest in this work.
References
Brown SC, Glass JM, Park DC. The relationship of pain and depression to cognitive function in rheumatoid arthritis patients. Pain. 2002;96(3):279–284. | ||
Berryman C, Stanton TR, Jane Bowering K, Tabor A, Mcfarlane A, Lorimer Moseley G. Evidence for working memory deficits in chronic pain: a systematic review and meta-analysis. Pain. 2013;154(8):1181–1196. | ||
Eccleston C. Chronic pain and attention: a cognitive approach. Br J Clin Psychol. 1994;33(Pt 4):535–547. | ||
Landrø NI, Fors EA, Våpenstad LL, Holthe Ø, Stiles TC, Borchgrevink PC. The extent of neurocognitive dysfunction in a multidisciplinary pain centre population. Is there a relation between reported and tested neuropsychological functioning? Pain. 2013;154(7):972–977. | ||
Veldhuijzen DS, Kenemans JL, van Wijck AJM, Olivier B, Kalkman CJ, Volkerts ER. Processing capacity in chronic pain patients: a visual event-related potentials study. Pain. 2006;121(1–2):60–68. | ||
Park DC, Glass JM, Minear M, Crofford LJ. Cognitive function in fibromyalgia patients. Arthritis Rheum. 2001;44(9):2125–2133. | ||
Schiltenwolf M, Akbar M, Hug A, et al. Evidence of specific cognitive deficits in patients with chronic low back pain under long-term substitution treatment of opioids. Pain Physician. 2014;17(1):9–20. | ||
Eggermont LHP, Shmerling RH, Leveille SG. Tender point count, pain, and mobility in the older population: the MOBILIZE Boston Study. J Pain. 2010;11(1):62–70. | ||
Karttunen N, Lihavainen K, Sipilä S, Rantanen T, Sulkava R, Hartikainen S. Musculoskeletal pain and use of analgesics in relation to mobility limitation among community-dwelling persons aged 75 years and older. Eur J Pain. 2012;16(1):140–149. | ||
Leveille SG, Bean J, Ngo L, Mcmullen W, Guralnik JM. The pathway from musculoskeletal pain to mobility difficulty in older disabled women. Pain. 2007;128(1-2):69–77. | ||
Shah RC, Buchman AS, Boyle PA, et al. Musculoskeletal pain is associated with incident mobility disability in community-dwelling elders. J Gerontol A Biol Sci Med Sci. 2011;66(1):82–88. | ||
Morone NE, Abebe KZ, Morrow LA, Weiner DK. Pain and decreased cognitive function negatively impact physical functioning in older adults with knee osteoarthritis. Pain Med. 2014;15(9):1481–1487. | ||
van Dijk GM, Veenhof C, Lankhorst GJ, Dekker J. Limitations in activities in patients with osteoarthritis of the hip or knee: the relationship with body functions, comorbidity and cognitive functioning. Disabil Rehabil. 2009;31(20):1685–1691. | ||
Schepker CA, Leveille SG, Pedersen MM, et al. Effect of pain and mild cognitive impairment on mobility. J Am Geriatr Soc. 2016;64(1):138–143. | ||
Singla NK, Parulan C, Samson R, et al. Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract. 2012;12(7):523–532. | ||
Pickering G, Loriot M-A, Libert F, Eschalier A, Beaune P, Dubray C. Analgesic effect of acetaminophen in humans: first evidence of a central serotonergic mechanism. Clin Pharmacol Ther. 2006;79(4):371–378. | ||
Rogers RD. The roles of dopamine and serotonin in decision making: evidence from pharmacological experiments in humans. Neuropsychopharmacology. 2011;36(1):114–132. | ||
Pickering G, Macian N, Dubray C, Pereira B. Paracetamol sharpens reflection and spatial memory: a double-blind randomized controlled study in healthy volunteers. Drug Des Devel Ther. 2016;10:3969–3976. | ||
Allen GJ, Hartl TL, Duffany S, et al. Cognitive and motor function after administration of hydrocodone bitartrate plus ibuprofen, ibuprofen alone, or placebo in healthy subjects with exercise-induced muscle damage: a randomized, repeated-dose, placebo-controlled study. Psychopharmacology. 2003;166(3):228–233. | ||
Renner B, Clarke G, Grattan T, et al. Caffeine accelerates absorption and enhances the analgesic effect of acetaminophen. J Clin Pharmacol. 2007;47(6):715–726. | ||
Ali Z, Burnett I, Eccles R, et al. Efficacy of a paracetamol and caffeine combination in the treatment of the key symptoms of primary dysmenorrhoea. Curr Med Res Opin. 2007;23(4):841–851. | ||
Laska EM, Sunshine A, Zighelboim I, et al. Effect of caffeine on acetaminophen analgesia. Clin Pharmacol Ther. 1983;33(4):498–509. | ||
Lieberman HR, Wurtman RJ, Emde GG, Roberts C, Coviella IL. The effects of low doses of caffeine on human performance and mood. Psychopharmacology. 1987;92(3):308–312. | ||
Michael N, Johns M, Owen C, Patterson J. Effects of caffeine on alertness as measured by infrared reflectance oculography. Psychopharmacology. 2008;200(2):255–260. | ||
Wilhelmus MM, Hay JL, Zuiker RG, et al. Effects of a single, oral 60 mg caffeine dose on attention in healthy adult subjects. J Psychopharmacol. 2017;31(2):222–232. | ||
Cleeland CS. The Brief Pain Inventory User Guide. Houston, TX: The University of Texas M. D. Anderson Cancer Center; 2009. | ||
Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med. 1992;85(7):399–402. | ||
Robbins TW, James M, Owen AM, Sahakian BJ, Mcinnes L, Rabbitt P. Cambridge neuropsychological test automated battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–281. | ||
Cambridge Cognition. Cognitive tests; 2018. Available from: http://www.cambridge.exp37.co.uk/cantab/cognitive-tests/. Accessed: June 5, 2018. | ||
Maruff P, Thomas E, Cysique L, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24(2):165–178. | ||
Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. | ||
Rainsford KD, Roberts SC, Brown S. Ibuprofen and paracetamol: relative safety in non-prescription dosages. J Pharm Pharmacol. 1997;49(4):345–376. | ||
Pini LA, Guerzoni S, Cainazzo M, Ciccarese M, Prudenzano MP, Livrea P. Comparison of tolerability and efficacy of a combination of paracetamol + caffeine and sumatriptan in the treatment of migraine attack: a randomized, double-blind, double-dummy, cross-over study. J Headache Pain. 2012;13(8):669–675. | ||
Moriarty O, Mcguire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93(3):385–404. | ||
Ojeda B, Failde I, Dueñas M, Salazar A, Eccleston C. Methods and instruments to evaluate cognitive function in chronic pain patients: a systematic review. Pain Med. 2016;17(8):1465–1489. | ||
Barnett JH, Blackwell AD, Sahakian BJ, Robbins TW. The Paired Associates Learning (PAL) Test: 30 years of CANTAB translational neuroscience from laboratory to bedside in dementia research. Curr Top Behav Neurosci. 2016;28:449–474. | ||
Pickering G, Pereira B, Clère F, et al. Cognitive function in older patients with postherpetic neuralgia. Pain Pract. 2014;14(1):E1–E7. | ||
Spierings ELH, Volkerts ER, Heitland I, Thomson H. A randomized, rater-blinded, crossover study of the effects of oxymorphone extended release, fed versus fasting, on cognitive performance as tested with CANTAB in opioid-tolerant subjects. Pain Med. 2014;15(2):264–271. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.