Back to Journals » Clinical Ophthalmology » Volume 8
Effectiveness and tolerability of dietary supplementation with a combination of omega-3 polyunsaturated fatty acids and antioxidants in the treatment of dry eye symptoms: results of a prospective study
Authors Oleñik A
Received 17 September 2013
Accepted for publication 12 November 2013
Published 6 January 2014 Volume 2014:8 Pages 169—176
DOI https://doi.org/10.2147/OPTH.S54658
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Andrea Oleñik
On behalf of the Dry Eye Clinical Study Group (DECSG)
Ophthalmology Department, Fundación Jiménez Díaz, Madrid, Spain
Background: We assessed the effectiveness and tolerability of a dietary supplement based on the combination of omega-3 essential fatty acids and antioxidants on dry eye-related symptoms.
Methods: A total of 905 patients (72% women, median age 60 years) with dry eye syndrome and using artificial tears to relieve symptoms participated in an open-label prospective intervention study. They were recruited during a routine ophthalmological appointment. Patients were instructed to take three capsules/day of the nutraceutical formulation (Brudysec® 1.5 g) for 12 weeks. Dry eye symptoms (categorized as 0, none; 1, mild; 2, moderate; and 3, severe) included scratchy and stinging sensation in the eyes, eye redness, grittiness, painful eyes, tired eyes, grating sensation, and blurry vision.
Results: The mean intensity of dry eye symptoms varied from 1.1 (± standard deviation [SD] 0.9) for painful eyes to 2.0 (0.9) for grittiness, with a mean value of 11.9 (4.8) for all symptoms together. At week 12, all individual symptoms improved significantly (P<0.001). The mean value for all symptoms together decreased from a mean value of 11.9 (± SD 4.8) at baseline to 6.8 (± SD 4.5) after 12 weeks of treatment (P<0.001). There was a decrease in the percentage of patients in which dry eye symptoms predominated nearly all the time (53.5% versus 34.1%). A total of 68.1% of patients reported better tolerance to contact lenses after treatment. The mean number of daily instillations of artificial tears also decreased significantly (3.8 [± SD 1.6] versus 3.3 [± SD 1.6], P<0.001). A total of 634 patients (70.1%) did not report any adverse events. In the remaining patients with adverse events, the most frequent was fish-tasting regurgitation in 13.5% of cases, followed by nausea in 4.9%, diarrhea in 1.3%, and vomiting in 0.3%.
Conclusion: Dietary supplementation with a combination of omega-3 essential fatty acids and antioxidants was an effective treatment for dry eye.
Keywords: antioxidants, Brudysec 1.5 g, dry eye symptoms, nutraceutics, omega-3, polyunsaturated fatty acids
Introduction
Ocular surface dysfunction (OSD) is a multifactorial condition that results in signs and symptoms of dry eye, which induces inflammation and discomfort.1 The lacrimal gland is the major contributor to the aqueous layer of the tear film which consists of water, electrolytes, and proteins. The amount and composition of this layer is critical for the health, maintenance, and protection of the ocular surface.2 Dry eye disease is also called keratoconjunctivitis sicca or dysfunctional tear syndrome.3 Epidemiological studies have shown that the prevalence of dry eye varies from 5% to 34% at different ages, with a higher incidence in older subjects.4 In 2007, the International Dry Eye WorkShop defined it as:
[…] a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.5
The newer definition emphasizes symptoms and global mechanisms, while recognizing the multifactorial nature of dry eye disease.
The tear film, comprised of lipids (cholesteryl esters, triglycerides, and unsaturated fatty acids),6 water, and mucous, acts not only as a lubricant and nutritional source, but also as the source of the regulatory factors for the maintenance and repair of the corneal epithelium.7 Essential polyunsaturated fatty acids omega-3 (ω-3) and omega-6 (ω-6) are the precursors of eicosanoids, which are locally acting hormones that, among other actions, mediate the inflammatory processes.8,9 Omega-3 essential fatty acids include alpha linoleic acid, eicosapentaenoic acid, and docosahexaenoic acid (DHA).10,11 They are found in cold water fish, including salmon, sardines, tuna, mackerel, and herring, and flaxseed oil.8,12 The omega-6 group includes linoleic acid and its derivatives. Recently, fatty acid supplementations have shown promise in dry eye therapy because of their anti-inflammatory effects.13 However, evidence of the efficacy of omega-3 supplements in treating dry eye is scarce8,14–19 and further studies are needed to substantiate existing evidence for the use of these supplements in the improvement of dry eye syndrome.
The purpose of this open-label intervention study was to assess the effectiveness and tolerability of oral supplementation with a combined formulation of omega-3 essential fatty acids and antioxidants on dry eye-related symptoms, and to determine whether treatment with this nutraceutical formulation can improve treatment with artificial tears.
Materials and methods
Patients of both sexes, aged 16 years or older, on current treatment with artificial tears due to dry eye disease were invited by their ophthalmologist to participate in an open-label, intervention, noncomparative study during a routine ophthalmological appointment in conditions of daily practice. The primary objective of the study was to assess the effectiveness of an oral nutraceutical formulation based on omega-3 and antioxidants in the relief of dry eye-related symptoms. Secondary objectives were as follows: 1) to assess whether this oral formulation could improve the results of treatment with artificial tears, and 2) to evaluate the tolerability of the product. The duration of treatment was 12 weeks. Patients with fish allergy, history of bariatric surgery for morbid obesity, ocular disorders requiring the use of eye drops other than artificial tears, and those deemed unable to participate in the study according to the clinician’s criteria were excluded. The study was conducted in accordance with the principles of the Declaration of Helsinki for the protection of human subjects, and written informed consent was obtained from all participants.
Ophthalmologists all over the country were invited to participate voluntarily in the study by the sales division of the pharmaceutical company who manufacture the supplement (Brudysec® 1.5 g; Brudy Laboratories, Barcelona, Spain). The composition of the supplement formulation is shown in Table 1. Between September 1, 2012 and December 31, 2012, eligible patients who agreed to take part in the study were consecutively recruited by the participating ophthalmologists, with a total of 20 patients per clinician.
  | Table 1 Composition of Brudysec® 1.5 g |
Patients were visited at baseline and at the end of the study (12 weeks). At the baseline visit (visit 0), the patient’s eligibility was assessed, the informed consent form was signed, and the nutraceutical formulation was prescribed. Patients were instructed to take three capsules of Brudysec 1.5 g, one time daily with a main meal (excluding breakfast). Ophthalmologists paid special care to insist on the importance of adherence to the dietary supplement. Data recorded included demographics (age, sex), use of artificial tears and mean daily eye drops, use of medications to relieve dry eye symptoms and frequency of daily use (number of times the patient took the medication), use of contact lenses (categorized as yes/no) and mean daily wear (hours), as well as dry eye symptoms (categorized as 0, none; 1, mild; 2, moderate; and 3, severe) including scratchy and stinging sensation in the eyes, eye redness, grittiness, painful eyes, tired eyes (eye fatigue), grating sensation, blurry vision, and other. Patients were also asked if symptoms predominated in the morning, afternoon, evening, or nearly all the time. At baseline, patients were also instructed to discontinue any medication for dry eye and to use only the study nutraceutical formulation.
At the end of the study (week 12), data recorded included compliance with treatment (“Did you take the three capsules every day?”; categorized as always, some forgetfulness, much forgetfulness); “Have you noticed any change in symptoms” (categorized as yes or no); assessment of dry eye symptoms as at baseline visit; if contact lens wearer, mean daily wear (hours); better tolerance to contact lenses (categorized as yes or no); mean daily eye drops of artificial tears; tolerability to nutraceutical formulation (categorized as fish-tasting regurgitation, nausea, vomiting, diarrhea, none of the above); level of the patient’s satisfaction (categorized as not at all satisfied, satisfied, very satisfied); and clinical assessment of the ophthalmologist (categorized as no improvement, mild improvement, great improvement). Patients could be withdrawn from the study of their own free will or according to the ophthalmologist’s criteria due to adverse events or concomitant diseases that may require a specific nutritional treatment.
Statistical analysis
Differences of continuous variables between the visit 0 (baseline) and the visit at the end of treatment (week 12) were analyzed with the Wilcoxon signed-rank test for paired samples. Changes in each individual dry eye symptom between compliant patients (those who always took the three capsules a day) and noncompliant patients (those who reported some or much forgetfulness), as well as between contact lens and non-contact lens users were compared with the Mann–Whitney U test. Statistical significance was set at P<0.05. Statistical analyses were performed with the R Project for Statistical Computing (R 3.0) program (http://www.r-project.org/).
Results
A total of 905 patients with dry eye symptoms participated in the study. Seventy-two percent of patients were women, with a mean (standard deviation [SD]) age of 58.6 (15.4) years (range 16–101 years). Contact lens users accounted for 16.2% of the study population (n=147), with a mean (SD) daily wear of 9.2 (3.1) hours. All patients used artificial tears to relieve dry eye symptoms, with a daily mean (SD) of 3.3 (1.6) instillations of eye drops. As shown in Table 2, the mean intensity of dry eye symptoms varied from 1.1 (0.9) for painful eyes to 2.0 (0.9) for grittiness, with a mean value of 11.9 (4.8) for all symptoms together. More than 50% of patients reported to suffer from dry eye symptoms nearly all time and 20.3% reported predominance of symptoms in the afternoon.
  | Table 2 Description of variables at baseline (visit 0) in 905 patients with dry eye symptoms |
At the end of the study period (week 12), all individual symptoms improved and in all cases, differences as compared with the baseline visit were statistically significant (P=0.001) (Figure 1). The mean value for all symptoms together decreased from a mean value of 11.9 (4.8) at baseline to 6.8 (4.5) after 12 weeks of treatment (P<0.001).
In relation to changes of symptoms at the end of the study, 83.8% of patients reported improvement of symptoms. Also, there was a decrease in the percentage of patients in which OSD dry eye symptoms predominated nearly all the time (53.5% at baseline versus 34.1% after 3 months) and an increase in those reporting predominance of symptoms in the morning (13.1% versus 19.0%) (Figure 2). On the other hand, 68.1% of patients reported better tolerance to contact lenses after treatment, although the mean (SD) time of daily contact lens wear did not vary between baseline and at the final visit (9.2 [3.1] versus 9.7 [3.0] hours, P=0.164). The mean (SD) number of daily instillations of artificial tears also decreased significantly (3.8 [1.6] versus 3.3 [1.6], P<0.001).
In relation to compliance with treatment, 40.1% of patients reported having taken the three capsules always, 51.2% reported some forgetfulness, and only 8.7% reported much forgetfulness. A total of 634 patients (70.1%) did not report any adverse events. In the remaining patients, in whom adverse events occurred, the most frequent was fish-tasting regurgitation in 13.5% of cases, followed by nausea in 4.9%, diarrhea in 1.3%, and vomiting in 0.3%. None of the patients were withdrawn from the study because of adverse events.
The level of patient satisfaction and ophthalmologist’s opinion regarding clinical improvement of dry eye symptoms coincided, with only 17.9% of patients being “not at all satisfied with treatment” and 12.2% of ophthalmologists considering “no improvement of symptoms” (Table 3). The percentage of patients who were satisfied or very satisfied with treatment and ophthalmologists reporting improvement or large improvement of symptoms was similar (82.1% and 87.8%, respectively).
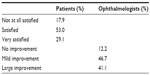  | Table 3 Patients’ level of satisfaction and clinician’s opinion of clinical improvement after 12 weeks of treatment with Brudysec® 1.5 g |
When patients were divided into the groups of compliant (always took the three capsules daily) and noncompliant patients (some and much forgetfulness), differences between mean (SD) values at baseline and at the end of treatment were only statistically significant for symptoms of grittiness (1.02 [0.92] versus 0.80 [0.83], P=0.001) and grating sensation (0.76 [0.83] versus 0.61 [0.82], P=0.007). The comparison of contact lens uses and nonusers showed significant differences for eye redness (0.79 [0.77] versus 0.67 [0.88], P=0.042) and blurry vision (0.64 [0.73] versus 0.53 [0.76], P=0.037).
Discussion
In an open-label prospective design, carried out in routine daily practice, this study provides evidence of the beneficial effect of oral supplementation with a nutraceutical formulation based on the combination of omega-3 fatty acids, vitamins, and essential trace elements to relieve symptoms of dry eye syndrome. In a large study population of 905 patients, who were users of artificial tears to treat dry eye, a 3-month treatment of the supplement prescribed as three capsules a day was associated with a clinical improvement of all characteristic symptoms of dry eye (scratchy and stinging sensation, pain, redness, blurry vision, etc), with statistically significant differences for all comparisons between end of treatment and baseline. It has been shown that omega-3 essential fatty acids have an anti-inflammatory action in the lacrimal gland, preventing apoptosis of the secretory epithelial cells, and that supplementation clears meibomitis-increasing tear secretion from the lacrimal gland, and allows a thinner, more elastic lipid layer to protect the tear film and cornea.20 Also, omega-3 and omega-6 cannot be synthesized in the body and must be obtained from diet; as a result, many dieticians are encouraging the population to incorporate omega-3 into their diet.21
This study has been preceded by three other clinical trials.15,17,22 These clinical trials included limited samples of patients suffering OSD due to dry eye. Two of them15,17 were prospective, comparative, open trials including a control group, and the third one22 was a double blind placebo-controlled trial. In the three previous studies, dry eye diagnosis was made both with a validated symptomatology questionnaire (Ocular Surface Disease Index [OSDI] questionnaire) and by evaluating the clinical objective signs. The patients of previous trials evidenced a significant improvement in the dry eye symptoms. These positive signs should be attributable to the beneficial influence of omega-3 supplementation.
Thus, the present study aimed to assess the patient’s level of satisfaction, based on three previous clinical trials (with controls and placebo), but in this trial we focused on a large sample of patients having a diagnostic of OSD. In the present protocol, we considered patients having been already diagnosed with dry eye and who had an artificial tear prescribed, but without reaching an adequate relief of their symptomatology.
As a result of treatment with omega-3 supplementation, better tolerance to contact lenses was reported, but the mean time of contact lens wear was similar at baseline and at week 12. Although all symptoms of dry eye improved significantly as compared with the baseline, in the subset of contact lens users, improvements in eye redness and blurry vision were significantly higher than in nonusers. It may be argued that these symptoms are common among contact lens wearers so that the range of improvement for these symptoms may be greater than in non-wearers.
We did not assess clinical signs during the trial, because a positive result was already expected. Their improvement were noticed and accepted as a positive influence of omega-3 fatty acids in OSD based on our previous studies.15,17,22 This observational trial has evaluated the degree of patient compliance by means of a questionnaire with three possible answers about patient’s daily supplementation: never forgotten (40.1%), occasionally forgotten (51.2%), and quite often forgotten (8.7%). The quantification of compliance by the patient report is a limitation of the study, in particular because the assessment of some and much forgetfulness is imprecise. However, comparison of compliant and noncompliant subgroups showed improvement in dry eye symptoms especially in the grittiness and grating sensation, before (baseline) and after (3 months later) treatment with the nutraceutical formulation of omega-3, despite some doses being missed. Besides, we also evaluated the level of severity (no symptoms, mild, moderate, or severe) and the temporal predominance (morning, noon, night, or during all day).
Dietary supplementation with omega-3 essential fatty acids has proved to be effective in the treatment of dry eye syndrome. In a randomized, double-blind trial of 45 patients with dry eye syndrome assigned to two capsules/day of omega-3 (each containing 180 mg eicosapentaenoic acid and 120 mg DHA) (n=33) or placebo (n=31) for 30 days, consumption of omega-3 fatty acids was associated with a decrease in the rate of tear evaporation, an improvement in dry eye symptoms, and an increase in tear secretion.14 In a study of patients on long-term treatment with antihypertensive eye drops with glaucoma and dry eye disorders, in which the oral supplement was Brudysec 1.5 g (the same as used in our patients), the main signs and symptoms of dry eye were significantly improved after 3 months of treatment as compared to unsupplemented patients.17 In a study by Oleñik et al22 in which the effect of Brudysec 1.5 g for 3 months was assessed in the treatment of dysfunction of the meibomian gland, the mean OSDI, tear breakup time, lid margin inflammation, and meibomian gland expression showed significant improvement from baseline values. Also, Pinazo-Durán et al15 demonstrated that mediators of expression levels of inflammation and immune response (cytokines/chemokines) in reflex tear samples showed different expression patterns in subjects with dry eye disorders, and that these patterns changed in response to oral intake of Brudysec 1.5 g over 3 months.
This study also confirms the tolerability and safety of the omega-3 dietary supplement. We studied the possible gastrointestinal adverse effects derived from a relative high DHA daily dose. No serious adverse events arose during the study. Digestive upset, particularly fish-tasting regurgitation was the most frequent side effect. In a study by Oleñik et al,22 digestive symptoms developed during the first month of treatment, and patients recovered immediately after stopping the medication. In our study, none of the participants discontinued the nutraceutical supplement because of adverse events.
The present results should be interpreted taking into account some limitations of the study, especially the open-label design; although the large number of participants is a strength of the study, as well as the fact that the data obtained is applicable to daily clinical practice. Next steps and future assessments should be aimed at the evaluation of the evolution of the objective clinical signs. The lack of control over dietary intake of the subjects was among the limitations of the study. Based on the three previous clinical trials (with controls15,17 and placebo22) the present study is aimed to assess the patients’ level of satisfaction after the addition of the supplementation to the use of an artificial tear, but focussing our conclusions on a large sample of patients with OSD diagnostic.
Conclusion
This study, carried out in a large group of patients using artificial tears to relieve dry eye symptoms, has shown that dietary supplementation with a combination of omega-3 essential fatty acids and antioxidants was an effective adjunctive treatment. A statistically significant improvement in dry eye symptoms after 3 months of treatment was demonstrated. Further studies with a longer treatment period are suggested to observe the long-term effectiveness and tolerability of this supplement in the improvement of dry eye syndrome.
Acknowledgments
The author would like to thank Dr Jaume Borras for his coordination and monitoring of the trial, to Sergi Mojal for performing the statistical analysis, and to Marta Pulido, MD, for editing the manuscript and for her editorial assistance.
Members of the DECSG: Mahmoud Zabad Wehbi, Caravaca, Murcia; Constanza García Torres, Lorca, Murcia; Emilia Sánchez Blanque, Mª Dolores López Bernal, María Dolores Miranda Rollón, José Grech Ríos, Encarnación Díaz Guía, Murcia; Juan José Mondéjar García and Javier Clavel Laria, Elche, Alicante; Anouska Rombouts Matamala, Marta Nadal Vall, Isabel Romagosa Robles, Baschir El Hayek Abu-Foul, Luís Fernández Vega Cueto, Susana García Garzón, Abdulrahman Kabbani Bota, Verónica Ribas González, Aníbal Gallardo Pisón, María Iglesias álvarez, ángela Ding Wu, Fuad Hajjar Sayegh, Paloma Martínez de Carneros Llorente, Kattia Milagros Llanos Rotta, Mónica Vásquez de los Ríos, and Hassane Usmud Ismail Hamed, Barcelona; Mariona Darné Freixenet, Girona; Eduardo Conesa Hernández, Eloisa Mejías González, José E Zamora Barrios, Mariano Royo Sans, Luciano A Donzis, Antonio Pérez Esteban, Javier Benítez Herreros, Alejandro Portero Benito, and Mahmoud Said Farah Diab, Madrid; Carlos Izquierdo Rodríguez, Alcalá de Henares, Madrid; Núria Soler Lluis, Reus, Tarragona; José María Gutiérrez Iglesias, Sevilla; Luís Miguel Sádaba Echarri, Pamplona, Navarra; Alesander Bilbao Urtiaga, Amagoia Arteagabeitia González, and Edurne Etxeandía Guisasola, Bilbao, Bizkaia; David Rodriguez Feijoo, Vitoria, Alava; J Antonio Quevedo Aconto, Cádiz; Francesca Majoral Masana, Lleida; Ricardo Ramón, Luís Alonso Muñoz, Roberto Arranz, and Patricia Bayo Calduch, Valencia; David Pérez Silguero, Las Palmas de Gran Canaria; Aritz Bidaguren Urbieta, San Sebastián-Donosti, Guipúzcoa; Laura Martínez Pérez, Santiago de Compostela; and Bernardo Muel Rodríguez, Gijón, Asturias, Spain.
Disclosure
This study was supported by Brudy Laboratories, Barcelona, Spain. Neither the author nor the participants cited in the Dry Eye Clinical Study Group (DECSG) list have any conflict of interest to disclose. Brudy Laboratories was not involved in the analysis of data and interpretation of the result. The author reports no other conflicts of interest in this work.
References
Perry HD. Dry eye disease: pathophysiology, classification, and diagnosis. Am J Manag Care. 2008;14(Suppl 3):S79–S87. | |
Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28(3):155–177. | |
Behrens A, Doyle JJ, Stern L, et al; Dysfunctional tear syndrome study group. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25:900–907. | |
No authors listed. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):93–107. | |
No authors listed. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):75–92. | |
Butovich IA. Cholesteryl esters as a depot for very long chain fatty acids in human meibum. J Lipid Res. 2009;50(3):501–513. | |
Holly FJ, Lemp MA. Tear physiology and dry eyes. Surv Ophthalmol. 1977;22(2):69–87. | |
Rand AL, Asbell PA. Nutritional supplements for dry eye syndrome. Curr Opin Ophthalmol. 2011;22(4):279–282. | |
de Lorgeril M, Salen P. New insights into the health effects of dietary saturated and omega-6 and omega-3 polyunsaturated fatty acids. BMC Med. 2012;10:50. | |
Swanson D, Block R, Mousa SA. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr. 2012;3(1):1–7. | |
Nichols PD, Petrie J, Singh S. Long-chain omega-3 oils-an update on sustainable sources. Nutrients. 2010;2(6):572–585. | |
Jenkins DJ, Josse AR. Fish oil and omega-3 fatty acids. CMAJ. 2008;178(2):150. | |
McCabe E, Narayanan S. Advancements in anti-inflammatory therapy for dry eye syndrome. Optometry. 2009;80(10):555–566. | |
Kangari H, Eftekhari MH, Sardani S, et al. Short-term consumption of oral omega-3 and dry eye syndrome. Ophthalmology. 2013;120(11):2191–2196. | |
Pinazo-Durán MD, Galbis-Estrada C, Pons-Vázquez S, Cantú-Dibildox J, Marco-Ramírez C, Benítez-del-Castillo J. Effects of a nutraceutical formulation based on the combination of antioxidants and ω-3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disorders. Clin Interven Aging. 2013;8:139–148. | |
Rosenberg ES, Asbell PA. Essential fatty acids in the treatment of dry eye. Ocul Surf. 2010;8(1):18–28. | |
Galbis-Estrada C, Pinazo-Durán MD, Cantú-Dibildox J, Marco-Ramírez C, Díaz-Llopis M, Benítez-del-Castillo J. Patients undergoing long-term treatment with antihypertensive eye drops responded positively with respect to their ocular surface disorder to oral supplementation with antioxidants and essential fatty acids. Clin Interv Aging. 2013;8:711–719. | |
He J, Bazan HE. Omega-3 fatty acids in dry eye and corneal nerve regeneration after refractive surgery. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4–6):319–325. | |
Wojtowicz JC, Butovich I, Uchiyama E, Aronowicz J, Agee S, McCulley JP. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011;30(3):308–314. | |
Roncone M, Bartlett H, Eperjesi F. Essential fatty acids for dry eye: a review. Cont Lens Anterior Eye. 2010;33(2):49–54; quiz 100. | |
Surette ME. The science behind dietary omega-3 fatti acids. CMAJ. 2008;178(2):177–180. | |
Oleñik A, Jiménez-Alfaro I, Alejandre-Alba N, Mahillo-Fernández I. A randomized, double-masked study to evaluate the effect of omega-3 fatty acids supplementation in meibomian gland dysfunction. Clin Interv Aging. 2013;8:1133–1138. | |
Regulation (EU) No. 432/2012 of the European Parliament and of the Council of 16 May 2012. Available from http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:136:0001:0040:en:PDF. Accessed December 10, 2013. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


