Back to Journals » Therapeutics and Clinical Risk Management » Volume 12
Effect of Ascaris lumbricoides infection on T helper cell type 2 in rural Egyptian children
Received 8 August 2015
Accepted for publication 17 November 2015
Published 9 March 2016 Volume 2016:12 Pages 379—385
DOI https://doi.org/10.2147/TCRM.S94019
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Naglaa M Shalaby,1 Nehad M Shalaby2
1Department of Parasitology, Faculty of Medicine, 2Mansoura University Children Hospital, Mansoura University, Mansoura, Egypt
Abstract: Ascaris lumbricoides is a neglected parasite that induces changes in host immune response. This study is conducted to define the serum levels of tumor necrosis factor alpha (TNF-α), interleukin-4 (IL-4), and interleukin-5 (IL-5) in some Egyptian children and their relations to intensity of infection, age, and ascariasis symptoms. Stool samples were examined using formol-ether concentration and Kato-Katz thick smear techniques. Sera of 60 A. lumbricoides-infected children and 20 controls were tested by enzyme-linked immunosorbent assay. The mean sera concentrations of TNF-α, IL-4, and IL-5 were 7.41±2.5 pg/mL, 107.60±18.3 pg/mL, and 389.52±28.0 pg/mL, respectively. The controls had mean serum TNF-α 7.10±2.4 pg/mL, IL-4 25.49±2.6 pg/mL, and IL-5 88.76±22.7 pg/mL. The difference in the concentration of sera cytokines was statistically significant for IL-4 and IL-5 (P<0.01) between A. lumbricoides-infected children and controls. The intensity of infection correlated positively with IL-4 and IL-5 at r=0.959 and r=0.919, respectively. The concentrations of IL-4 and IL-5 correlated positively with the age at r=0.845 and r=0.934, respectively. Asthma and gastrointestinal tract upsets were correlated positively with IL-4 and IL-5. These data indicate that A. lumbricoides infection in our locality is associated with significantly high levels of IL-4 and IL-5.
Keywords: Ascaris lumbricoides, children, rural, Egypt, IL-4, IL-5, TNF-α
Introduction
Infection with gastrointestinal parasites has been globally detected as a serious public health problem in the tropical countries of the world.1,2 The large roundworm Ascaris lumbricoides is a ubiquitous pathogen of humans with worldwide distribution. It is estimated that ~1.5 billion infections are caused by A. lumbricoides alone.3 Such infections are associated with significant morbidity.4
It is reported that the prevalence of soil-transmitted helminth (STH) parasites is commonly high in conditions of poor hygiene and sanitation with more affection in children than adults.5 The mammalian host is infected with A. lumbricoides orally by ingestion of infective ova.6,7 Ascariasis is common in areas where defecation occurs in open latrines and stool is disposed arbitrarily in the surrounding environment.4 Therefore, contamination of food and water is so easy, the most common sources of infection.8
Human infection with A. lumbricoides has been associated with eosinophilia, fever, dyspnea, and cough what is known as Loeffler’s syndrome during the larval migratory phase through the lung and abdominal pain during the intestinal phase.9 Ascariasis is characterized by eosinophilia, mast cell hyperplasia, and high levels of circulating immunoglobin E (IgE).10
On the one hand, numerous experimental animal studies have detected the significance of T helper cell type 2 responses to helminths of gastrointestinal tract and the prominence of certain interleukins (ILs), including IL-4, IL-5, IL-9, and IL-13, in facilitating expulsion of parasite and infection resistance.11,12 On the other hand, few studies have examined human cytokine responses to gastrointestinal helminths, especially those studies searching about cellular response to ascariasis in endemic population.13,14
It should be remembered that individuals living in endemic areas are often infected with more than one STH parasite and many children with A. lumbricoides, particularly those with high parasite burdens, have a high probability of recent or current STH coinfections. This makes it more difficult to attribute specific immunologic effects to ascariasis alone even when blood cells are stimulated with Ascaris antigens in vitro because of the high degree of immunological cross-reactivity between STH antigens.15
It is highlighted that the interaction between different cytokines in the immunity against parasites is not as easy as one could imagine and can differ according to variant situations, including infection duration, sites difference in the host and species, or strains difference of hosts and parasite.16
Therefore, we conducted this study to illustrate the immunological milieu of A. lumbricoides by studying the serum levels of some Th1 cytokines as tumor necrosis factor alpha (TNF-α) and Th2 cytokines as IL-4 and IL-5 in infected children living in endemic rural areas in Mansoura, Egypt, and to check whether there is any relations between these cytokines and various famous clinical symptoms of ascariasis, such as abdominal cramps, diarrhea, and atopy. The relationship of the intensity of infection, age, and the concentration of these cytokines was also highlighted.
Materials and methods
Study area
This study was carried out in slums and destitute poor villages of rural areas of Mansoura, Egypt, where generality of its inhabitants use open latrines and the stool is disposed arbitrarily in the surrounding environment because of the bad habit of children who indiscriminately defecate in their surrounding locality. The mean annual rainfall of the study area is 589–670 mm. The annual minimum and maximum temperatures are 6.5°C and 29.9°C, respectively.
Study design
A cross-sectional study was conducted over 3 months after obtaining ethical permission from Ministry of Health, Mansoura City, Dakahlia Province, Egypt. A medical aid convoy had explained the objectives and all procedures to the children and their families for their agreement. A written consent had been taken from all guardians of participated children.
Subject sampling
This study was performed on 60 children infected with A. lumbricoides known by their positive stool analysis. Their ages were from 4 years to 15 years with mean age 8.53±2.7. The studied group was 45 males and 15 females. A control group was included with 20 healthy children; 15 were males and five were females with matched age and sex. The investigated children were subjected to the following. 1) Complete history, including age, sex, and residence. 2) Clinical history of abdominal cramps and diarrhea. Detailed atopic symptoms, including asthma and skin allergy, were identified using the International Study of Asthma and Allergies in Childhood questionnaire,17 which included ever asthma, ever wheezing, and current wheezing for asthma. With regard to skin allergy, itchy rash ever, eczema ever, and itchy rash in the past year were identified. Ever asthma was defined as “asthma symptoms at any point in the past”. Ever wheezing was defined as “wheezing symptoms at any point in the past”. Current wheezing was defined as “wheezing symptoms within the last 12 months”. 3) Stool samples were collected, processed, and examined using direct saline, iodine, and formol ether concentration techniques. Infection intensity was detected by Kato-Katz thick smear technique as mentioned by Montresor et al18 to count the number of eggs per gram (epg) of feces according to World Health Organization standard, which divides the intensity of infection to light infection with 1–4.999 epg, moderate infection with 5,000–49.999 epg, and heavy infection with ≥50.000 epg. 4) Sera were obtained from blood samples and subjected to enzyme-linked immunosorbent assay (ELISA) for the analysis of TNF-α (Quantikine® ELISA Human TNF-α Immunoassay; R&D Systems, Inc., Minneapolis, MN, USA), IL-4 (Quantikine® ELISA Human IL-4 Immunoassay; R&D Systems, Inc.), and IL-5 (Human Interleukin-5 ELISA; BioVendor – Laboratorni medicina a.s., European Union: BioVendor GmbH) according to the manufacturer’s instructions.
Data management and statistical analysis
Entry of raw data and handling were done using Microsoft Excel spread sheets program and then were transferred to SPSS Version 21 for analysis. Descriptive statistics were computed. The studied data were presented as mean ± standard deviation. Independent samples t-test was used to compare means. Pearson’s correlation was used to evaluate the association between the studied parameters. Chi-square test for independence was used to discover whether there is a relationship between the nominal parameters. At 5% level of significance, P-value <0.05 was considered significant in all analysis.
Results
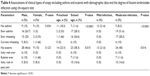
In this study, it was found that school-aged children between 7 years and 15 years had higher parasite load 8,595 epg than preschool-aged children between 1 year and 6 years who showed parasite load 4,482 epg. With regard to the cytokines profile, those children 7–15 years had higher serum IL-5 (411.04±23.0 pg/mL) than preschool-aged children (368.00±14.3 pg/mL) with high significant difference (P<0.01). Also, the mean serum level of TNF-α was significantly higher in school-aged children (8.04±2.7 pg/mL) than preschool-aged children (6.78±1.6 pg/mL; P=0.04). Although IL-4 showed no significant difference in the two age groups (P=0.45), its mean serum level was 105.17±21.8 pg/mL in preschool-aged children and 110.04±17.3 pg/mL in school-aged children, respectively. In all infected children, the mean concentrations of the TNF-α, IL-4, and IL-5 were 7.41±2.5 pg/mL, 107.60±18.3 pg/mL, and 389.52±28.0 pg/mL, respectively, Table 1.
  | Table 1 Relationship between the studied cytokines and infected children age groups (N=60) |
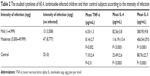
Mild infection was reported among 13 children (3,508 epg), while 47 of them had moderate infection (8,777 epg). The difference between the mean concentration of IL-4 of those with mild infection (82.56±3.8 pg/mL) and moderate infection (116.19±13.4 pg/mL) was statistically significant (P<0.01). In addition, the mean concentration of IL-5 for those with mild infection was 380.95±9.8 pg/mL, and this was statistically different from those with moderate infection of 406.54±29.0 pg/mL (P<0.01). Additionally, the children with mild infection had a mean TNF-α concentration of 6.33±1.3 pg/mL, while those with moderate infection had a mean concentration of 8.14±2.7 pg/mL, and this difference was significant (P=0.002). Comparing the sera cytokines concentration of the A. lumbricoides-infected children with that of the control group, it was found that mean concentration levels of both IL-4 and IL-5 of the infected children had shooting levels of 107.60±18.3 pg/mL and 389.52±28.0 pg/mL, respectively, than control group children in whom the mean concentration levels of IL-4 and IL-5 were 25.49±2.6 pg/mL and 88.76±22.7 pg/mL, respectively, with a high statistically significant difference (P<0.01). Meanwhile, the difference for TNF-α was not significant (P=0.31), as shown in Table 2.
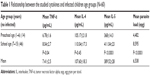
We studied the correlation between the cytokines and the clinical data of the studied groups. We found that the intensity of infection was strongly and significantly (P<0.01) positively correlated with IL-4 and IL-5 at r=0.959 and r=0.919, respectively (Figures 1 and 2), while it was not correlated with TNF-α (r=0.208; P=0.065). Additionally, there was significant positive correlation between IL-4, IL-5, and the clinical symptoms of patients as diarrhea at r=0.607, r=0.579, abdominal cramps at r=0.531, r=0.445, and atopy at r=0.696, r=0.678, respectively (P<0.01). In contrast, TNF-α was not correlated with abdominal cramps, while it was positively correlated with diarrhea and atopy at r=0.320 and r=0.338, respectively, as shown in Table 3. When we studied the associations of different degrees of atopy including that of asthma and eczema with the infection intensity of A. lumbricoides, we found significant associations (P<0.01 and P=0.016), respectively, as shown in Table 4.
  | Figure 1 Correlation between the intensity of infection and IL-4. |
  | Figure 2 Correlation between the intensity of infection and IL-5. |
Discussion
Parasitic helminths infestation is a common condition for billions of humans in different districts of the world where bad sanitation enhances infection and reinfection; children are notably susceptible to heavy infection when ill-health and poor growth result.19
In the current study, we demonstrated that the school-aged children infected with A. lumbricoides produce higher levels of IL-4 and IL-5 than the control subjects. This observation was in agreement with Copper et al and Turner et al20,21 who revealed higher IL-4 profile among the examined subjects. This assertion is proved valid considering the important roles of IL-4 in helminthic infection. Not only IL-4 had a blocking effect on proinflammatory cytokines but also it is implicated in differentiation of B-cell and its activation with the antibodies release, which are specific to the antigen such as IgA and IgE, which could help in clearance of parasite.22,23 In contrast, Goddey et al24 recorded low level of the anti-inflammatory IL-4 and they revealed that the low mean concentration of this IL could be responsible for maintenance of A. lumbricoides in their studied locality in Nigeria.
Moreover, our results with regard to IL-5 are proved valid, considering the major role of IL-5 in immunity against helminthic infection. IL-5 activates eosinophils. Antibodies may act in collaboration with cells such as macrophages and eosinophils by acting as a viaduct bringing the parasite and the activated cell together to assist the toxic molecules release directly onto the parasite surface, a process known as antibody-dependent cell-mediated cytotoxicity.25 Also, the significantly high level of IL-5 concentration among the examined school children embroils IL-5 in immunopathogenesis of A. lumbricoides infection especially during the lung migratory phase. It has been documented that this phase associated clinically with Ascaris pneumonitis and accompanied by cellular infiltration, serous exudates, bronchial irritation and eosinophilia what is known as Loeffler’s syndrome.26,27 The association between A. lumbricoides infection and asthma was initially proven valid by Lynch et al.28
Th1 cells are known by their production of IL-2 and INF-γ. INF-γ stimulates resting macrophages and causing them to secrete an assortment of molecules, as TNF-α, reactive oxygen intermediates, and nitrous oxide. TNF-α has a cooperative effect with nitrous oxide in destroying the intracellular ingested parasites. These molecules, together with other things, intervene with DNA structure, mitochondrial function and membrane integrity and lead to the destruction of target cells. INF-γ has a negative effect on Th2 responses beside its positive role in stimulation of macrophages (Th1 response).29
TNF-α is defined to be a key moderator of pathogenesis in a wide range of inflammatory, infectious, and autoimmune diseases.30 TNF-α is a pleiotropic cytokine. It is necessary for homing of Th2 cells to the place of allergic inflammation. Actually, TNF-α has been exhibited to enhance Th2 cell-mediated phenomena in other systems, such as pathogenesis of allergic inflammation in the gastric mucosa,31 and airway hyper responsiveness suggesting that TNF-α may also be important in mucosal Th2 responses during intestinal helminth infection.32
In our study, although IL-4 and IL-5 levels were predominant, there was no impairment of TNF-α expression in the infected children in comparison to the control group which may explain a mixed Th1/Th2 response. It has been documented the critical role of TNF-α in regulation of Th2 cytokines.33 This data deviates from the observation of Goddey et al24 who revealed a significantly high TNF-α concentration among their investigated subjects. Meanwhile Geiger et al,9 recorded lower level of TNF-α in Ascaris-infected patients than in controls.
Previous studies have proved that in most of the gatrointestinal helminthic infections, the immune responses tend toward the Th2-like responses.34–36 Such responses are associated with significant production of IL-4, IL-5, IL-10, and IL-13 and with consequent strong IgE development, mast cell, and eosinophil responses.37 The end result could be protective immunity. It has been revealed in earlier studies that adult and larval stage antigen of A. lumbricoides in human infection induces Th2 cytokine responses by the greater production of IL-4 in comparison to other cytokines such as IFN-γ.38 This polarization toward Th2 cytokine responses can be emphasized by our study as we reported significantly high level of IL-4 and IL-5 in comparison to TNF-α level production. In addition, we found that the concentration of IL-4 and IL-5 correlated positively with the age at r=0.845 and r=0.934, respectively. This is in agreement with the observation of Goddey et al.24
In the current study, clinical symptoms related to gastrointestinal tract as abdominal cramps and diarrhea were highly and positively correlated with IL-4 and IL-5 altogether with parasitic load and intensity of infection, these findings were agreed by many researchers39,40 who stated that Ascaris infections are usually asymptomatic if infection involved only few worms, but as worm load increases, symptoms of gastrointestinal tract upsets develop. Despite the high prevalence of Ascaris infection in developing countries where diarrhea and other abdominal-related symptoms are dominating, in some infection there is alteration in Ascaris-related abdominal symptoms, which is explained by coinfection by other organisms that alter gastrointestinal tract milieu, leading to interference with penetration of the gut wall by Ascaris worms.41
On studying the relation of IL-4 and IL-5 to atopy in the studied groups, there was highly positive correlation between atopic symptoms, as asthma, eczema and the studied ILs. Prominent elements of Th2 immune response which is evoked to promote worm expulsion from gut, simultaneously lead to high activity of mucosal eosinophils, mucus hyper secretion, and hyperactivity of mucosal muscles giving the full symptoms of asthma and explain the interaction between heavy parasitic infection and atopic symptoms. These relations cope with various studies.42–45
It was stated that there is high risk of asthma due to sensitization to common aeroallergens, such risk is enhanced in Ascaris-infected kids reflexing the complex relationship between Ascaris and asthma susceptibility.46
On the other hand, some researchers deny any association between Ascaris infection and atopy or other allergic disorders.47,48 Such finding might be explained by worm load and timing of infection, which may play role in the relationship between parasitic infection and the host immune response; few worms and/or adult age may protect the hosts against allergy.49
Conclusion
In conclusion, the significantly high levels of IL-4 and IL-5 found in the sera of school-aged children from rural areas of Mansoura, Egypt, embarrasses these cytokines as the main mediators in the host immunity in response to infection with A. lumbricoides in our studied locality. However, further studies on cellular responses and other cytokine production, such as IL-9, IL-10, and IL-13, are needed to define another necessary parameters for obtaining protective immunity.
Acknowledgment
We would like to express our deepest gratitude to our colleagues in the Department of Microbiology and Immunity, Faculty of Medicine, Mansoura University, for their help in this research.
Disclosure
The authors report no conflicts of interest in this work.
References
Nakagawa J, Ehrenberg JP, Nealon J, et al. Towards effective prevention and control of helminth neglected tropical diseases in the Western Pacific Region through multi-disease and multi-sectoral interventions. Acta Trop. 2015;141:407–418. | ||
Omorodion AO, Goddey NOP, Clement IC, Ogbeneovo UD, Oijiangbe AA. Distribution of intestinal parasites among school-age children in delta and Edo states of Nigeria. PUJ. 2012;5(2):121–126. | ||
Chan MS. The global burden of intestinal nematode infections: fifty years on. Parasitol Today. 1997;13:438–443. | ||
Nmorsi OPG, Isaac C, Aashikpelokhai IS, Ukwandu NCD. Geohelminthiasis among Nigerian preschool age children. Int J Med Med Sci. 2009;1(10):407–411. | ||
Anosike JC, Zaccheaus VO, Adeiyongo CM, et al. Studies on the intestinal worm (Helminthiasis) infestation in Central Nigeria Rural Community. J Appl Sci Environ Manage. 2006;10(2):61–66. | ||
Crompton DW, Nesheim MC. National impact of intestinal helminthiasis during the human life cycle. Ann Rev Nutr. 2002;22:35–39. | ||
Eziefula AC, Brown M. Intestinal nematodes: disease burden, deworming and the potential importance of co-infection. Curr Opin Infect Dis. 2008;21(5):516–522. | ||
Damen JG, Luka J, Biwan EI, Lugos M. Prevalence of intestinal parasites among pupils in rural North Eastern Nigeria. Niger Med J. 2011;52:4–6. | ||
Geiger SM, Massara CL, Bethony J, Soboslay PT, Carvalho OS, Corrêa-Oliveira R. Cellular responses and cytokine profiles in Ascaris lumbricoides and Trichuris trichiura infected patients. Parasite Immunol. 2002;24:499–509. | ||
Jackson JA, Turner JD, Rentoul L, et al. Cytokine response profiles predict species-specific infection patterns in human GI nematodes. Int J Parasit. 2004;34(11):1237–1244. | ||
Finkelman FD, Shea-Donohue T, Goldhill J, et al. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. | ||
Bancroft AJ, Grencis RK. Th1 and Th2 cells and immunity to intestinal helminths. Chem Immunol. 1998;71:192–208. | ||
Mahanty S, King CL, Kumaraswami V, et al. IL-4 and IL-5–secreting lymphocyte populations are preferentially stimulated by parasite-derived antigens in human tissue invasive nematode infections. J Immunol. 1993;151(7):3704–3711. | ||
Neva FA, Filho JO, Gam AA, et al. Interferon-gamma and interleukin-4 responses in relation to serum IgE levels in persons infected with human T lymphotrophic virus type I and Strongyloides stercoralis. J Infect Dis. 1998;178(6):1856–1859. | ||
Reina Ortiz M, Schreiber F, Benitez S, et al. Effects of chronic ascariasis and trichuriasis on cytokine production and gene expression in human blood: a cross-sectional study. PLoS Negl Trop Dis. 2011;5(6):e1157. | ||
Allen JE, Maizels RM. Th1-Th2: reliable paradigm or dangerous dogma? Immunol Today. 1997;18:387–392. | ||
Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–491. | ||
Montresor A, Crompton DWT, Bundy DAP, Hall A, Savioli L. Guidelines for the Soil-Transmitted Helminthiasis and Schistosomiasis at the Community Level. WHO/CTD/SIP/98.1. Geneva: World Health Organisation; 1998. | ||
Karagiannis-Voules DA, Odermatt P, Biedermann P, et al. Geostatistical modelling of soil-transmitted helminth infection in Cambodia: do socioeconomic factors improve predictions? Acta Trop. 2015;141:204–212. | ||
Copper PJ, Chico ME, Sandoval C, et al. Human infection with Ascaris lumbricoides is associated with a polarized cytokine response. J Infect Dis. 2000;182(4):1207–1213. | ||
Turner JD, Faulkner H, Kamgno J, et al. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. J Infect Dis. 2003;188(11):1768–1775. | ||
Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine mediated regulation of chronic intestinal helminth infection. JEM. 1994;179(1):347–351. | ||
Wright VJ, Ame SM, Haji HS, et al. Early exposure of infants to GI nematodes induces Th2 dominant immune responses which are unaffected by periodic anthelminthic treatment. PLoS Negl Trop Dis. 2009;3(5):e433. | ||
Goddey NOP, Osagie ID, Maliki A. Serum cytokines profiles in Nigerian children with Ascaris lumbricoides infection. Asian Pac J Trop Med. 2010;3:288–291. | ||
Souza V, Medeiros D, Sales I, et al. Ascaris lumbricoides infection in urban schoolchildren: specific IgE and IL-10 production. Allergol Immunopathol (Madr). 2014;42(3):206–211. | ||
Gill GV, Beeching NJ. Tropical Medicine. 4th ed. Oxford: Blackwell Publishing Company; 2007:352. | ||
Kouro T, Takatsu K. IL-5 and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21(12):1303–1309. | ||
Lynch NR, Hagel IA, Palenque ME, et al. Relationship between helminthic infection and IgE response in atopic and non atopic children in a tropical environment. J Allergy Clin Immunol. 1998;101(2 pt 1):217–221. | ||
Kotb M, Calandra T, editors. Cytokines and Chemokines in Infectious Diseases Handbook. Totowa, NJ: Humana Press; 2003. | ||
Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. | ||
Furuta GT, Schmidt-Choudhury A, Wang MY, et al. Mast cell-dependent tumor necrosis factor alpha production participates in allergic gastric inflammation in mice. Gastroenterology. 1997;113(5):1560–1569. | ||
Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production By T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. 1997;186(10):1737–1747. | ||
Artis D, Humphreys NE, Bancroft AJ, Rothwell NJ, Potten CS, Grenis RK. Tumor necrosis factor (alpha) is a critical component of interleukin 13-mediated protective T helper cell type 2 responses during helminth infection. JEM. 1999;190:953–962. | ||
Gause WC, Urban JF, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–277. | ||
Turner JD, Jackson JA, Faulkner H, et al. Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. J Infect Dis. 2008;197(8):1204–1212. | ||
Figueiredo CA, Barreto ML, Rodrigues LC, et al. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect Immun. 2010;78(7):3160–3167. | ||
King EM, Kim HT, Dang NT, et al. Immuno-epidemiology of Ascaris lumbricoides infection in a high transmission community: antibody responses and their impact on current and future infection intensity. Parasite Immunol. 2005;27(3):89–96. | ||
Kullberg MC, Pearces EJ, Hieny SE, Sher A, Berzifsky JA. Infection with Ascaris lumbricoides alter Th1/Th2 cytokine responses to parasite antigen. J Immunol. 1992;148:3264. | ||
Kosek M, Bern C, Guerrant RL. The global burden of diarrheal disease as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81(3):197–204. | ||
Dold C, Holland CV. Ascaris and ascariasis. Microbes Infect. 2011;13(7):632–637. | ||
Wood JD. Effects of bacteria on the enteric nervous system: implications for the irritable bowel syndrome. J Clin Gastroenterol. 2007;41(suppl 1):7–19. | ||
Peisong G, Yamasaki A, Mao XQ, et al. An asthma associated genetic variant of STAT6 predicts low burden of Ascaris worm infestation. Genes Immun. 2004;5(1):58–62. | ||
Hagel I, Cabrera M, Hurtado MA, et al. Infection by Ascaris lumbricoides and bronchial hyper reactivity: an outstanding association in Venezuelan school children from endemic areas. Acta Trop. 2007;103(3):231–241. | ||
Pereira MU, Sly PD, Pitrez PM, et al. Non atopic asthma is associated with helminth infection and bronchiolitis in poor children. Eur Respir J. 2007;29(6):1154–1160. | ||
Bragagnoli G, Silva MT. Ascaris lumbricoides infection and parasite load are associated with asthma in children. J Infect Dev Ctries. 2014;8(7):891–897. | ||
Palmer LJ, Celedon JC, Weiss ST, Wang B, Fang Z, Xu X. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am J Respir Crit Care Med. 2002;165(11):1489–1493. | ||
Yazdanbakhsh M, Kremsner PG, Van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296(5567):490–494. | ||
Cooper PG. Intestinal worms and human allergy. Parasite Immunol. 2004;26(11–12):455–467. | ||
Alcântara-Neves NM, Badaró SJ, dos Santos MC, Pontes-de-Carvalho L, Barreto ML. The presence of serum anti Ascaris lumbricoides IgE antibodies and of Trichuris trichiura infection are risk factors for wheezing and/or atopy in preschool-aged Brazilian children. Respir Res. 2010;23(11):114. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.