Back to Journals » Medical Devices: Evidence and Research » Volume 11
ebb® Complete Tamponade System: effective hemostasis for postpartum hemorrhage
Authors McQuivey RW , Block JE , Massaro RA
Received 5 December 2017
Accepted for publication 2 February 2018
Published 1 March 2018 Volume 2018:11 Pages 57—63
DOI https://doi.org/10.2147/MDER.S158944
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ross W McQuivey,1 Jon E Block,2 Robert A Massaro3
1Clinical Innovations, Salt Lake City, UT, USA; 2Independent Clinical Consultant, San Francisco, CA, USA; 3Monmouth Medical Center, Long Branch, NJ, USA
Abstract: As a leading cause of maternal death, postpartum hemorrhage (PPH) remains a worldwide obstetrical problem. However, in most cases, mortality and morbidity can be averted if efforts are immediately undertaken to achieve hemostasis. Uterine balloon tamponade has been shown to provide effective control of PPH and avoid more invasive surgical procedures and even the emergency peripartum hysterectomy. Recent clinical recommendation suggests that balloon tamponade should be considered earlier in the treatment cascade in conjunction with uterotonic agents to ensure hemostasis in the most timely fashion and maximize clinical outcomes. This paper profiles the ebb® Complete Tamponade System, a unique dual-balloon single-use device that was developed specifically for hemostatic management of PPH. The ebb system combines a uterine conforming balloon that can be rapidly deployed with a vaginal balloon that eliminates the need for vaginal packing. The description, indications for use, procedural steps, and clinical characterization of this device are presented.
Keywords: postpartum hemorrhage, balloon tamponade, hemostasis, maternal bleeding
Introduction
Globally, a woman dies every 4 minutes as a result of postpartum hemorrhage (PPH), resulting in 140,000 deaths annually.1 PPH, when not deadly, is also associated with serious morbidity and sequelae including adult respiratory distress syndrome, coagulopathy, shock, and loss of fertility due to the emergent necessity for operative hysterectomy to control bleeding.2 Consequently, PPH represents a worldwide obstetrical challenge that is particularly problematic among low-resource communities.3
However, in the vast majority of cases, PPH is preventable, and death is avoidable if an effective management plan can be initiated early and rapidly to affect hemostasis.4 In fact, in a large state-wide review of pregnancy-related deaths, Berg et al5 concluded that almost all deaths due to hemorrhage were potentially preventable.
Uterine atony, retained placental tissue, trauma, and coagulopathy are all implicated separately or in combination in the development of PPH.6 The highest risk of mortality and severe morbidity occurs when uncontrolled bleeding develops within the first 24 hours following delivery, underscoring the need for rapid deployment of hemostatic interventions.2
Because it can be safely and expeditiously deployed in a minimally invasive fashion, balloon tamponade has quickly emerged as a front-line intervention for control of severe PPH. Indeed, numerous studies utilizing various balloon tamponade systems have documented a hemostatic success rate in excess of 80% in PPH, irrespective of etiology or type of delivery (ie, vaginal, cesarean section).7–10 Herein, we describe the only dual-balloon tamponade system specifically designed to quickly and effectively treat PPH.
Device description
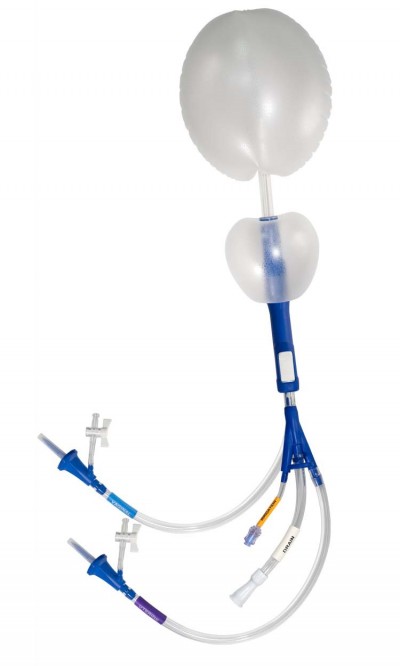
The ebb® Complete Tamponade System (ebb, Clinical Innovations, Salt Lake City, UT, USA) is a disposable, multiple lumen catheter attached to an inflatable balloon system designed to provide tamponade for controlling hemorrhage from the uterus and vagina (Figure 1). The ebb is a dedicated, condition-specific device cleared by the US Food and Drug Administration for temporary control or reduction of postpartum uterine bleeding in the management of PPH (510(k), K153591).
  | Figure 1 ebb® Complete Tamponade System. Note: Courtesy of Clinical Innovations. |
The device consists of two inflatable balloons: the upper uterine balloon is inflated inside the uterus and the lower vaginal balloon is inflated inside the vagina. Inflation is accomplished with isotonic intravenous (IV) fluid such as normal saline or Ringer’s lactate. The uterine balloon catheter has separate lumens to enable inflation/deflation, irrigation, and drainage. The vaginal balloon catheter also has a lumen to enable inflation/deflation. The uterine and vaginal balloons are permanently assembled and are not to be separated. The device may be retained in position for up to 24 hours in the postoperative mode of treatment. The ebb is supplied sterile in peel open pouches for one-time use for a single patient.
Unique to the ebb is the malleable polyurethane balloon material which allows the balloons to conform to the patient’s anatomy better than the traditional silicone used in other PPH balloon devices. The malleability of the uterine balloon allows the device to better occupy the available space inside the uterus, regardless of the patient’s anatomy or existing pathology (eg, fibroids, uterine horn, etc). The malleable vaginal balloon anchors the uterine balloon, effectively removing the need of a vaginal pack – reducing balloon expulsion while also providing treatment for vaginal bleeding, if present. The maximum recommended fill volumes are 750 mL for the uterine balloon and 300 mL for the vaginal balloon. The uterine balloon volume may be exceeded if clinically necessary, such as treating global atony vs a lower-uterine segment (focal) atony. Importantly, the balloons can be inflated directly from any IV bag without the need of a syringe or stopcocks.
Indications for use
The ebb is indicated for use in providing temporary control or reduction of postpartum uterine bleeding. Inflation of the vaginal balloon anchors the uterine balloon and decreases blood flow to the uterus via the collateral pelvic vasculature. The ebb should be used in the setting of postpartum uterine bleeding when conservative management is warranted. Box 1 summarizes the contraindications, warnings, and precautions associated with the ebb system.
  | Box 1 Contraindications, warnings, and precautions ebb® |
Procedural details
Catheter placement
Only physicians trained to perform vaginal/cervical examinations and who have experience in the placement of an intrauterine catheter should perform the procedure. The ebb may be used after either a vaginal delivery or cesarean section. It is recommended that catheter placement should be performed with concomitant ultrasound guidance to ensure insertion safety and reduce risk of iatrogenic injury with the device. Trendelenberg positioning of the patient has also been recommended prior to placement to assist in the insertion of the device. The clinical scenario of a PPH requires accurate assessment of the patient’s hemodynamic status, and thus a quantified blood loss methodology is recommended along with close monitoring of urine output with the placement of a urinary catheter.
Methods
- Ensure the stopcocks remain open prior to placement to allow any residual air in the system to escape during placement. Collapse the uterine balloon loosely around and over the tip of the catheter and gently insert the ebb by cupping the uterine balloon end of the catheter and directly inserting it through the dilated cervix to the fundus. In cases where the cervix is not completely dilated, insert the ebb with the clinician’s finger tips at the cervical os and then gently advancing the catheter tip to the fundus.
- Advance the ebb, under ultrasound guidance, until the entire uterine balloon is within the uterine cavity and the vaginal balloon is positioned within the vaginal cavity. The space between the uterine and vaginal balloons should be within the cervical os.
- Once the balloons are properly placed, close the stopcocks and begin instilling the fluid into the uterine balloon – which is clearly labeled “uterine”. The one-way, “rapid-fill spikes” on the ebb system allow use of standard IV bags for immediate inflation. If a catheter is attached to an IV fluid bag prior to insertion, make sure to leave the fluid bag at or below the level of the balloon to prevent premature filling. Warmed IV fluids have been shown to be advantageous to the clotting cascade,11 but are not a requirement.
- The fill procedure begins with one hand in the vagina and the clinician’s fingers at the level of the cervical os, the other hand is placed on the patient’s abdomen to determine the level of the fundus and verify uterine expansion as the balloon is filled. The uterine balloon should be filled beginning with ~250 mL and increased incrementally until bleeding is stopped or it is determined by the clinician that further fill volume may result in unsafe overdistention of the uterus. The clinician’s examining fingers should remain at the level of the cervical os to ensure that the intrauterine balloon has not been overinflated, which may cause the balloon to prolapse into the vagina. If this is felt, the balloon should be deflated and repositioned, then reinflated to assure proper positioning.
- During and after each incremental fill, the clinician should determine whether additional fill is warranted and safe, based on repeated/continued evaluations of bleeding, volume dispensed, resistance to fill, and patient characteristics such as uterine wall thickness and surgical history. Dildy et al12 demonstrated that 45% of patients require >500 mL. Unless clinically indicated, it is recommended that filling of the uterine balloon should not exceed a maximum of 750 mL.
- Once tamponade is achieved, one may mark the height of the fundus on the abdomen to allow for the detection of increasing uterine size in the absence of vaginal bleeding, a sign of a dangerous concealed hemorrhage. One can also evaluate the space between the balloon and fundus periodically with ultrasound, if necessary, to rule out a concealed hemorrhage.
- The position of the vaginal balloon with respect to the uterine balloon can be adjusted for patient anatomy by increasing or decreasing the distance between the two balloons using the white locking mechanism found on the blue handle of the device.
- The vaginal balloon may be left uninflated or may be inflated as desired. A maximum of 300 mL can be infused into the vaginal balloon, although clinical experience has demonstrated that 120–150 mL is adequate in most cases.
- An irrigation lumen is provided which allows for irrigation of the uterus distal to the uterine balloon should the clinician determine there is a need to ensure drainage lumen patency. To irrigate, connect a suitable syringe to the one-way luer connector on the irrigation lumen and flush with a small amount (20–30 mL) of sterile fluid until drainage is visualized. If no drainage is visualized after instilling 20–30 mL of sterile fluid, a clotted system should be suspected and one should evaluate the patient for a potential concealed hemorrhage (eg, rising fundal height, vital sign decompensation in the absence of continued blood loss via the drainage lumen). Irrigation should not be initiated for the purpose of dislodging or removing clots.
- The drainage lumen of the ebb should be connected to a graduated collection container and monitored for signs of persistent bleeding. If bleeding continues, further investigation into the cause of the bleeding should be immediately initiated.
- The stopcocks on each of the uterine and vaginal balloon lumens can be used to reduce or increase the volume of fluid as required for effective tamponade.
- External traction may be applied at the physician’s discretion for cases that require individualization of care to increase tamponade effect by securing a 500 g weight up to a maximum of 1,000 g (a 1/2 L or 1 L IV bag) to the traction ring feature below the vaginal balloon.
Post-cesarean delivery catheter placement
In PPH cases following completed cesarean section delivery, surgical wound disruption may occur with use of any device. Thus, catheter placement should occur under ultrasound guidance to minimize the risk to the uterine scar. It is imperative that the proper insertion procedures detailed earlier are followed to maximize clinical benefit and minimize hysterotomy repair disruption.
Intraoperative cesarean catheter placement
Catheter placement at the time of laparotomy during cesarean section may also be performed. However, it is recommended to avoid the retrograde application (insertion of the device though the hysterotomy to the cervix and vagina from above) and instead utilize the normal vaginal placement once the hysterotomy is closed. The uterus should be clear of any retained placental fragments, arterial bleeding, or lacerations, and the hysterotomy should be closed immediately. This reduces the chance of inadvertently damaging the balloon during the hysterotomy repair and reapproximates the myometrium as quickly as possible, reducing blood loss. Once the hysterotomy is closed, the ebb system is inserted into the uterus via the vagina in the normal fashion. Insertion should be performed under direct observation of the uterine incision.
The uterine balloon should then be filled using available isotonic fluids directly from any IV bag. Particular attention should be paid to uterine distention and suture integrity as the balloon is filled. The fill process should be incremental, with assessment of volume during and after each incremental fill. The drainage lumen should also be assessed following each incremental fill to determine if bleeding has been arrested. The vaginal balloon is then positioned and filled in the usual fashion to prevent the system from becoming dislodged, and then the cesarean section is completed in the normal fashion.
ebb removal
The ebb should be deflated once it is no longer required. The timing of this decision is left to the clinician managing the patient. In general, once bleeding has been controlled, the patient’s hemodynamic status stabilized, and any coagulopathy/acidosis/hypothermia/hypoperfusion has been reversed, the catheter should be deflated until it can be atraumatically removed and the area observed for signs of persistent or recurring bleeding.
The traditional recommendation has been to leave balloon tamponade devices in place for 24 hours to assure effective hemostasis. However, recent literature suggests that, in most cases, 4–6 hours of tamponade is adequate to achieve hemostasis.13 In a retrospective cohort study, Einerson et al14 showed no differences in PPH-related outcomes associated with balloon tamponade duration >12 hours compared to removal at 2–12 hours. Indeed, in a postpublication exchange, these authors speculated that it is reasonable to considerable balloon removal before 12 hours if hemostasis is firmly established.15,16
Deflation of the balloon may be achieved by an incremental reduction of uterine volume followed by a period of observation until the uterine balloon is empty; however, an established and proven methodology has not been published. Deflation is facilitated by opening the relevant stopcock and allowing the fluid to drain out of the balloon(s) into a container under gravity drainage. The patient should be carefully monitored for any signs of rebleeding or hemodynamic instability. If this occurs, the volume should be replaced and the system left in place.
Once the balloon drainage has stopped, a syringe may be attached to the stopcock and the remaining fluid may be aspirated until there is none left in the balloon(s). Alternatively, if quicker deflation is desired, the inflation lumen above the spike may be cut. The catheter may then be gently removed. After removal, the patient should be carefully monitored for any signs of rebleeding or hemodynamic instability.
Preclinical laboratory evaluation
Antony et al17 undertook a bench-top preclinical study to characterize the filling capacity and pressure–volume relationship of various tamponade balloons used in PPH. Seven different non-condition-specific (ie, off-label) as well as condition-specific (eg, ebb, bakri) catheter balloons were filled with water ex vivo, and intraluminal pressure was measured incrementally as the balloons were filled until rupture or until 5,000 mL was reached. The ebb system was one of only three balloons to exhibit low intraluminal pressures throughout the experiment. Balloons with low intraluminal pressure–volume ratios are preferred clinically as they fill more easily and rapidly, and to greater volumes. Additionally, the ebb was one of two balloons that remained unruptured at 5,000 mL – well in excess of the labeled capacity, but demonstrating its durability and strength.
Clinical performance characteristics
The clinical performance of the ebb system has been documented in a number of published reports.12, 18–20 Belfort et al19 examined the association between incremental infusion of fluid volume and intraluminal pressure and uterine blood flow using the ebb system in normal postpartum uteri. They concluded that intraluminal pressure increases curvilinearly as volume of the intrauterine tamponade balloon is increased. Thus, they speculated that the hemostatic mechanism of action in PPH with balloon tamponade is related to a reduction in uterine artery perfusion pressure likely resulting from direct compression of the artery in the lower segment and/or wall conformational changes.
Hemostatic success with the ebb system has been documented clinically in women with PPH.12,18 Dildy et al12 reported that bleeding decreased or stopped in 98% (50 of 51) of women with PPH that were refractory to initial administration of uterotonic agents. This effect was noted in women with uterine atony with or without concomitant placentation abnormalities. Dildy et al12 also noted that the ebb vaginal balloon serves to eliminate vaginal packing and provided the clinical advantage of anchoring the device against expulsion and maintaining proper placement. Of important note, in this particular study, 45% of the patients required >500 mL of fluid in the uterine balloon to treat the PPH appropriately.
Balloon tamponade with the ebb system has also been associated with a decrease in maternal morbidity. In a retrospective chart review, Breen20 found that introduction of balloon tamponade into the PPH clinical management algorithm entirely eliminated the need for emergency hysterectomy compared to cases treated with the previous management regimen that did not include balloon tamponade.
Discussion
Balloon tamponade has demonstrated excellent hemostatic effectiveness in the setting of PPH,7,8,12 even in cases of severe bleeding.21 Importantly, the clinical performance of balloon tamponade has been consistent irrespective of bleeding etiology.22 Additionally, compared to other interventions (eg, compression suturing), the safety profile is extremely attractive with a low documented occurrence of adverse events or complications.
Timely and effective hemostasis with balloon tamponade has translated clinically to a noteworthy decrease in the need for invasive procedures. Laas et al9 reported that introduction of balloon tamponade into the standard treatment algorithm for PPH resulted in a large and significant decrease in the rates of arterial embolization and ligation procedures as well as the use of compression sutures. These findings were subsequently confirmed by Gauchotte et al,23 who additionally showed a decrease in the need for operative hysterectomy following the introduction of balloon tamponade in PPH management.
When initially introduced, balloon tamponade for control of PPH was mostly promulgated as a secondary or tertiary intervention in the treatment cascade and, in almost all cases, was recommended after the failure of uterotonic agents.2,24 However, the body of clinical experience and the introduction of dedicated, condition-specific balloon systems such as ebb now recommend that balloon tamponade should be considered much earlier in the treatment course,25 perhaps as a first-line intervention working in concert with prostaglandin administration.10,26 Indeed, Barinov et al27 recently reported that introduction of balloon tamponade as a first-line therapy in combination with treatment of coagulopathy resulted in a significant reduction in the number of peripartum hysterectomies compared to standard treatment that did not include balloon tamponade.
Conclusion
The unique dual-balloon ebb system combines a uterine conforming balloon that can be rapidly deployed with a vaginal balloon that eliminates the need for vaginal packing. Balloon tamponade with the ebb system offers effective hemostasis in women with PPH and has the potential added benefit of providing a safe fertility sparing treatment.
Author contributions
All authors contributed to the conception, execution, and drafting of the manuscript and provided critical revision of the manuscript for intellectual content. All authors read and provided final approval of the version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
Disclosure
RWM is an employee of and JEB is an independent advisor to Clinical Innovations (Salt Lake City, UT, USA). The authors report no other conflicts of interest in this work.
References
American College of Obstetricians, Gynecologists. ACOG practice bulletin: clinical management guidelines for obstetrician-gynecologists number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039–1047. | ||
Walfish M, Neuman A, Wlody D. Maternal haemorrhage. Br J Anaesth. 2009;103(Suppl 1):i47–i56. | ||
Calvert C, Thomas SL, Ronsmans C, Wagner KS, Adler AJ, Filippi V. Identifying regional variation in the prevalence of postpartum haemorrhage: a systematic review and meta-analysis. PLoS One. 2012;7(7): e41114. | ||
Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–1373. | ||
Berg CJ, Harper MA, Atkinson SM, et al. Preventability of pregnancy-related deaths: results of a state-wide review. Obstet Gynecol. 2005;106(6):1228–1234. | ||
Devine PC. Obstetric hemorrhage. Semin Perinatol. 2009;33(2):76–81. | ||
Doumouchtsis SK, Papageorghiou AT, Arulkumaran S. Systematic review of conservative management of postpartum hemorrhage: what to do when medical treatment fails. Obstet Gynecol Surv. 2007;62(8):540–547. | ||
Doumouchtsis SK, Papageorghiou AT, Vernier C, Arulkumaran S. Management of postpartum hemorrhage by uterine balloon tamponade: prospective evaluation of effectiveness. Acta Obstet Gynecol Scand. 2008;87(8):849–855. | ||
Laas E, Bui C, Popowski T, Mbaku OM, Rozenberg P. Trends in the rate of invasive procedures after the addition of the intrauterine tamponade test to a protocol for management of severe postpartum hemorrhage. Am J Obstet Gynecol. 2012;207(4):281 e281–e287. | ||
Revert M, Cottenet J, Raynal P, Cibot E, Quantin C, Rozenberg P. Intrauterine balloon tamponade for management of severe postpartum haemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124(8):1255–1262. | ||
Sobieszczyk S, Breborowicz G. Management recommendations for postpartum hemorrhage. Arch Perinat Med. 2004;10(4):1–4. | ||
Dildy GA, Belfort MA, Adair CD, et al. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210(2):136.e1–136.e6. | ||
Prevention and management of postpartum haemorrhage: green-top guideline no. 52. BJOG. 2017;124(5):e106–e149. | ||
Einerson BD, Son M, Schneider P, Fields I, Miller ES. The association between intrauterine balloon tamponade duration and postpartum hemorrhage outcomes. Am J Obstet Gynecol. 2017;216(3):300.e1–300.e5. | ||
Alouini S. Excessive duration of intrauterine balloon placement. Am J Obstet Gynecol. 2017;217(2):226. | ||
Einerson BD, Son M, Miller ES. Reply. Am J Obstet Gynecol. 2017; 217(2):226–227. | ||
Antony KM, Racusin DA, Belfort MA, Dildy GA, 3rd. Under pressure: intraluminal filling pressures of postpartum hemorrhage tamponade balloons. AJP Rep. 2017;7(2):e86–e92. | ||
Atilgan R, Ozkan ZS, Orak U, Baspinar M. Complete tamponade system for management of severe postpartum vaginal haemorrhage due to uterine atony. BMJ Case Rep. 2014;2014. | ||
Belfort MA, Dildy GA, Garrido J, White GL. Intraluminal pressure in a uterine tamponade balloon is curvilinearly related to the volume of fluid infused. Am J Perinatol. 2011;28(8):659–666. | ||
Breen M. Double balloon tamponade for postpartum hemorrhage. Int J Gynaecol Obstet. 2017;138(1):129. | ||
Ghirardini G, Alboni C, Mabrouk M. Use of balloon tamponade in management of severe vaginal postpartum hemorrhage and vaginal hematoma: a case series. Gynecol Obstet Invest. 2012;74(4):320–323. | ||
Son M, Einerson BD, Schneider P, Fields IC, Grobman WA, Miller ES. Is there an association between indication for intrauterine balloon tamponade and balloon failure? Am J Perinatol. 2017;34(2):164–168. | ||
Gauchotte E, De La Torre M, Perdriolle-Galet E, Lamy C, Gauchotte G, Morel O. Impact of uterine balloon tamponade on the use of invasive procedures in severe postpartum hemorrhage. Acta Obstet Gynecol Scand. 2017;96(7):877–882. | ||
Ahonen J, Stefanovic V, Lassila R. Management of post-partum haemorrhage. Acta Anaesthesiol Scand. 2010;54(10):1164–1178. | ||
Marasinghe JP, Du Plessis J, Epitawela D, Umstad MP. Management of postpartum haemorrhage with uterine balloon tamponade: the way forward. Aust N Z J Obstet Gynaecol. 2015;55(4):315–317. | ||
Viteri OA, Sibai BM. Uterine balloon tamponade for the management of postpartum haemorrhage: a challenge and an opportunity for better evidence. BJOG. Epub 2017 Sep 8. | ||
Barinov SV, Zhukovsky YG, Dolgikh VT, Medyannikova IV. Novel combined strategy of obstetric haemorrhage management during caesarean section using intrauterine balloon tamponade. J Matern Fetal Neonatal Med. 2017;30(1):29–33. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
