Back to Journals » International Journal of Nanomedicine » Volume 13
Dose reduction of bone morphogenetic protein-2 for bone regeneration using a delivery system based on lyophilization with trehalose
Authors Zhang X, Yu Q, Wang YA, Zhao J
Received 5 September 2017
Accepted for publication 8 December 2017
Published 12 January 2018 Volume 2018:13 Pages 403—414
DOI https://doi.org/10.2147/IJN.S150875
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Xiaochen Zhang,1,* Quan Yu,2,* Yan-an Wang,1 Jun Zhao2
1Department of Oral and Maxillofacial-Head and Neck Oncology, 2Department of Orthodontics, College of Stomatology, Ninth People’s Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
*These authors contributed equally to this work
Introduction: To induce sufficient new bone formation, high doses of bone morphogenetic protein-2 (BMP-2) are applied in regenerative medicine that often induce serious side effects. Therefore, improved treatment strategies are required. Here, we investigate whether the delivery of BMP-2 lyophilized in the presence of trehalose reduced the dose of BMP-2 required for bone regeneration.
Materials and methods: A new growth factor delivery system was fabricated using BMP-2-loaded TiO2 nanotubes by lyophilization with trehalose (TiO2-Lyo-Tre-BMP-2). We measured BMP-2 release characteristics, bioactivity, and stability, and determined the effects on the osteogenic differentiation of bone marrow stromal cells in vitro. Additionally, we evaluated the ability of this formulation to regenerate new bone around implants in rat femur defects by micro-computed tomography (micro-CT), sequential fluorescent labelling, and histological analysis.
Results: Compared with absorbed BMP-2-loaded TiO2 nanotubes (TiO2-BMP-2), TiO2-Lyo-Tre-BMP-2 exhibited sustained release, consistent bioactivity, and higher stability of BMP-2, and resulted in greater osteogenic differentiation of BMSCs. Eight weeks post-operation, TiO2-Lyo-Tre-BMP-2 nanotubes, with various dosages of BMP-2, regenerated larger amounts of new bone than TiO2-BMP-2 nanotubes.
Conclusion: Our findings indicate that delivery of BMP-2 lyophilized with trehalose may be a promising method to reduce the dose of BMP-2 and avoid the associated side effects.
Keywords: bone morphogenetic protein-2, dose reduction, delivery system, trehalose, lyophilization, TiO2 nanotubes, BMP-2, regenerative medicine, surface modification
Introduction
Bone morphogenetic proteins (BMPs) are a family of bone growth factors that strongly induce new bone formation.1 Among BMPs, BMP-2 is considered to be the most potent and has been widely used to regenerate bone.2–5 As BMP-2 may be easily lost from implanted sites through diffusion or because of its short in vivo half-life, high doses are often applied for clinical treatment.6,7 However, high dosages of BMP-2 increase the cost of treatment and also cause unexpected serious complications, including ectopic new bone formation, osteolysis, excessive immune responses, postoperative neurological impairment, and compromised airways.8–10 Therefore, novel methods for reducing the dose of BMP-2 are needed to prevent the occurrence or reduce the severity of these adverse effects.
The osteogenic bioactivity, capacity, and optimum dose of BMP-2 for achieving effective bone regeneration depend on the system for BMP-2 delivery.11,12 Therefore, it is necessary to develop an appropriate delivery system for BMP-2 in order to reduce the dose required for bone regeneration. Owing to their good biological performance, TiO2 nanotube layers have been extensively studied and used as carrier materials in regenerative medicine.13–15 Coadministration of growth factors, such as BMP-2, with TiO2 nanotube layers may significantly increase the osteoinductive capacity.16 However, the local delivery of growth factors also has several shortcomings, including uncontrolled flow and potential inactivation by heat, extreme pH, or proteases, thereby shortening the duration of efficacy.17,18 Accordingly, the loading of biological molecules onto scaffolds by coating them via lyophilization has recently gained interest. Lyophilization, also referred to as freeze-drying, is an efficient approach that has been commonly applied to improve the chemical and physical stability of biomolecules.19 However, this process generates various stresses that may lead to the inactivation of biomolecules during the freezing and drying steps.20,21 Hence, protectants are often employed to keep biomolecules from freezing and desiccation stresses.22 Trehalose, a naturally nonreducing disaccharide present in diverse organisms, is the most potent and widely studied stabilizer, exhibiting several advantages over other materials, including reduced rates of fermentation, non-toxicity, and a slower pH response.23,24 Lyophilized calcium-deficient hydroxyapatite scaffolds with trehalose have been shown to enable long-term release of BMP-2 while retaining protein activity and stability.25 Recent studies have used lyophilized BMP-2 with the addition of trehalose (Lyo/Tre) loaded on scaffolds for enhanced osteogenesis.25,26 However, despite these advantages of preserving protein activity and stability, the potential applications of Lyo/Tre for BMP-2 dose reduction have not been studied to date.
In this work, we aim to determine whether the delivery of BMP-2 lyophilized in the presence of trehalose (Lyo/Tre) reduced the BMP-2 dose required for effective bone regeneration. A new growth factor delivery system was fabricated using BMP-2-loaded TiO2 nanotubes by lyophilization following the addition of trehalose (TiO2-Lyo-Tre-BMP-2). We measured BMP-2 release characteristics, bioactivity, and stability, and determined the effects of this BMP-2 formulation on osteogenic differentiation of bone marrow stromal cells (BMSCs) in vitro. Furthermore, we examined the ability of TiO2-Lyo-Tre-BMP-2 to regenerate new bone around implants in rat femur defects. The bone formation around implants induced by TiO2-Lyo-Tre-BMP-2 was compared with that induced by BMP-2-loaded TiO2 nanotubes (TiO2-BMP-2) to determine whether the new delivery system for BMP-2 allows reduction of the dose required for new bone formation.
Materials and methods
Substrate preparation
The TiO2 nanotube layers were fabricated using Ti thin foils (thick, 0.25 mm) or rods (diameter, 2 mm; length, 8 mm) with a purity of 99.5% (Alfa Aesar, Ward Hill, MA, USA) by anodization. First, the Ti foils or rods were immersed in a mixture (2 mL of 48% hydrofluoric acid (HF), 3 mL of 70% HNO3, and 100 mL of deionized water) for 5 min to remove the naturally formed outer oxide layer, followed by rinsing in deionized water and drying under a nitrogen stream. Then, the nanotubes were fabricated in an electrolyte solution containing 0.5% HF (w/v) and 1 M H3PO4 with a potentiostat (voltage, 20 V) for 3 h.27 Finally, the samples were sintered at 500°C for 2 h to crystallize the as-deposited amorphous-structured TiO2 nanotubes. All nanotubes were sterilized in a steam autoclave at 120°C for 30 min before use.
BMP-2 radio-iodination and nanotube loading
To investigate the release kinetics of BMP-2 in vitro, the loaded BMP-2 (Rebone, Shanghai, China) was radiolabeled with 125I and the final concentration of 125I-BMP-2 was adjusted to 0.2 mg/mL in PBS, as previously described.28 For the lyophilization of the BMP-2 group, 125I-BMP-2 solution mixed with 1 M trehalose18 at a fixed volume of 30 μL/sample was dripped onto TiO2 nanotubes prepared on foil (TiO2-Lyo-Tre-BMP-2). Using this method, each foil was loaded with 6 μg of 125I-BMP-2. Then, 125I-BMP-2 solution (0, 1.5, 2.5, or 5 μL) was mixed with 1 M trehalose at a fixed volume of 30 μL and dripped onto nanotubes for preparation of implants. In this manner, each implant was loaded with 0, 0.3, 0.5, or 1.0 μg of 125I-BMP-2. All samples were pre-frozen at −80°C for 3 h prior to being lyophilized for 24 h in a FreeZone freeze-drier (Labconco, Kansas City, MO, USA). For the absorption group, the same 125I-BMP-2 solution without trehalose was physically dripped onto the TiO2 nanotubes of the foil or implant (TiO2-BMP-2), using the same method, for 30 min before use.
Scanning electron microscopy observations and Fourier transform infrared spectroscopy analysis
A Quanta 200 field-emission gun scanning electron microscope (SEM XL-30; Philips, Amsterdam, the Netherlands) was employed to observe the surface morphology of the samples. All samples were sputter-coated with platinum prior to imaging for 1 min. Surface chemical characterization of the samples was performed using Fourier transform infrared spectroscopy (FTIR, Nicolet Avatar 660; Nicolet, Madison, WI, USA), in the wavelength range of 600–4,000 cm−1.
Ethics statement
All experiments were approved by the Animal Care and Experimental Ethics Committee of the Ninth People’s Hospital affiliated with the Shanghai Jiao Tong University School of Medicine and performed in accordance with standard animal research guidelines published by National Institutes of Health.
Cell culture
Six-week-old male Fisher 344 rats were obtained from the Ninth People’s Hospital Animal Center (Shanghai, China). BMSCs were isolated and cultured following established protocols, as previously described.29 BMSCs were passaged twice before use.
Release profiles and bioactivity of BMP-2 in vitro
To investigate the release profiles of BMP-2 from nanotube layers, samples of various formulations (n=4) were washed with PBS and then incubated in 6-well plates containing 1 mL of DMEM (Gibco BRL, Gaithersburg, MD, USA) with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA) per well at 37°C. The medium was sampled at 1 h and 1, 3, 7, 10, 14, 21, and 28 days. At each time point, fresh DMEM was added after the medium was removed. The release of BMP-2 was quantified by analyzing the radioactivity of 125I in the removed DMEM by employing a gamma counter (Shanghai Institute of Nuclear Instrument Factory, Shanghai, China). Finally, all 125I counts were normalized to baseline after applying a correction for decay.
To investigate the bioactivity of BMP-2 released from TiO2 nanotubes, releasates of culture from the first 7 days for each sample were collected together, and analysis of alkaline phosphatase (ALP) activity was performed employing the BMSCs. Cells (2×104 cells/well) were seeded in 48-well plates. The concentration of BMP-2 releasates of each group was adjusted to 100 ng/mL according to the cumulative BMP-2 release of different formulations. Twenty-four hours after seeding, the medium was removed and replaced with DMEM (0.5 mL) containing 10% FBS with BMP-2 releasate (100 ng/mL). The medium alone and the same amount of fresh BMP-2 were employed as the negative (NC) and positive controls, respectively. On days 3, 7, and 14 after incubation, BMSCs were lysed with 0.05% Triton™ X-100 (80 μL). Then, 20 μL of lysate was added to 100 μL of disodium p-nitrophenylphosphate hexahydrate (2 mg/mL) and 2-amino-2-methyl-1-propanol (0.75 M). ALP activity was estimated by monitoring the absorbance at 405 nm after incubation of the mixtures for 30 min at 37°C, and then normalized to the total protein content determined by employing bicinchoninic acid assays, as described previously.30
Preservation of BMP-2 bioactivity from Lyo/Tre nanotubes
Samples (n=24) of TiO2-Lyo-Tre-BMP-2 were stored at −20°C, 4°C, or 25°C. Each sample was kept in a microcentrifuge tube and completely sealed. After storage for 2 or 5 weeks, samples of each group (n=4) were incubated in PBS, and BMP-2 releasate (100 ng/mL) was collected for the first 7 days to evaluate the BMP-2 bioactivity using ALP activity assays. On day 7, ALP activity was estimated as described in the previous section. To determine the change in BMP-2 bioactivity upon storage at different temperatures and durations, fresh BMP-2 without lyophilization was employed as a standard.
Proliferation of BMSCs exposed to different nanotube formulations
The proliferation of BMSCs cultured with different groups (TiO2, TiO2-BMP-2, TiO2-Lyo-Tre-BMP-2) (n=4 per group) was investigated in a 6-well Transwell® system (0.4 μm; Corning Inc., Corning, NY, USA) on days 1, 3, 7, and 14. Cells were seeded in the lower wells at 105 cells/well (0.5 mL), and the different nanotubes were placed in the upper wells. The culture medium was changed every 2 days. At designated time points, the cells were collected to quantify deoxyribonucleic acid (DNA) contents using the Hoechst 33258 dye (Sigma-Aldrich Co., St Louis, MO, USA) as previously described.31 Proliferation of BMSCs cocultured with no nanotubes were also assessed.
Effects of BMP-2-loaded nanotubes on the osteogenic differentiation of BMSCs
The osteogenic differentiation of BMSCs cultured with different formulations (TiO2, TiO2-BMP-2, and TiO2-Lyo-Tre-BMP-2) (n=4 per group) was investigated in 6-well Transwell plates (0.4 μm). The culture medium was changed every 2 days. Total RNA was extracted using TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) on days 3, 7, and 14. Highly purified gene-specific primers for runt-related transcription factor 2 (Runx2), osteopontin (OPN), osteocalcin (OCN), bone sialoprotein (BSP), ALP, and the housekeeping gene, GAPDH, were synthesized by Shengong Co., Ltd. (Shanghai, China; Table 1). The expression levels of each bone-related gene and GAPDH at each time point were determined by real-time polymerase chain reaction (RT-PCR) (7900HT sequence-detection system; Applied Biosystems, Foster City, CA, USA). Data were analyzed using the comparative CT method and the results were expressed as transcript levels using the housekeeping gene GAPDH as the control for normalization. Analysis of endogenous bone-related gene expression was carried out by calculating the relative expression levels of genes compared with those in BMSCs cultured with TiO2 nanotubes.32
  | Table 1 Nucleotide sequences of primers used for real-time quantitative polymerase chain reaction |
Rat femur defect model for evaluating bone regeneration
Surgical procedure
The right femurs of 48 male Fisher 344 rats were implanted with the following 8 groups of implants (n=6 rats per group): TiO2, TiO2-0.3 μg BMP-2, TiO2-0.5 μg BMP-2, TiO2-1.0 μg BMP-2, TiO2-Lyo-Tre, TiO2-Lyo-Tre-0.3 μg BMP-2, TiO2-Lyo-Tre-0.5 μg BMP-2, and TiO2-Lyo-Tre-1.0 μg BMP-2.
Sequential fluorescent labeling
A polychrome sequential fluorescent labeling method was performed to assess new bone formation and mineralization around the implants as described previously.33 Briefly, rats were injected intraperitoneally with 30 mg/kg Alizarin Red S (Sigma-Aldrich Co.) and 20 mg/kg calcein (Sigma-Aldrich Co.) at 2 and 6 weeks after implantation, respectively.
Specimen preparation
Eight weeks after surgery, all rats were sacrificed. The right femurs were harvested and then fixed in 10% buffered formaldehyde for micro-CT and histomorphometric observations.
Micro-CT assay
The fixed specimens were imaged and detected employing a GE eXplore Locus SP Micro-CT (GE Healthcare, Chicago, IL, USA) to visualize the new bone formed around the implants. The parameters were set at 80 μA current and 80 kV voltage, with an exposure time of 3,000 ms. The 3D images were reconstructed using GEHC MicroView software (GE Healthcare, London, UK) after scanning. To obtain the parameters of bone mineral density and bone volume fraction (bone volume/total volume) of the newly formed bone around the implant, a region with a radius of 0.5 mm from the implant surface within the bone marrow cavity was chosen for analysis.
Histomorphometric observations
All samples were dehydrated in ascending concentrations of ethanol 50%–100%, and then embedded in polymethylmetacrylate for undecalcified sectioning. Fully polymerized specimens were sectioned into 150-μm-thick sections using a Leica SP1600 saw microtome (Leica, Wetzlar, Germany) in an orientation nearly parallel to the long axis of the implants. All sections were subsequently ground and polished to a final thickness of ~30 μm for Alizarin Red S and calcein fluorescence imaging. Finally, the samples were stained with van Gieson’s picrofuchsin for subsequent histological analysis. Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA) was used to evaluate the areas of new bone formed around the implants.
Statistical analysis
Statistical comparisons were carried out using one-way analysis of variance and Student–Newman–Keuls test. All statistical analyses were performed using SAS 8.2 (SAS Institute Inc., Cary, NC, USA). All data are reported as mean ± SD. Differences with p-values of <0.05 were considered significant.
Results
Surface observations and characterizations
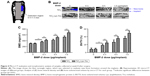
Surface views of TiO2 nanotubes are shown in Figure 1A, and those of TiO2-BMP-2 (left) and TiO2-Lyo-Tre-BMP-2 (right) are shown in Figure 1B. The anodized TiO2 nanotube layer was distributed uniformly over the substrate with a diameter and length of ~100 and 400 nm, respectively. Relatively little amorphous glassy matrix remained on the surface of TiO2-BMP-2, while a much larger amount was observed on the surface of TiO2-Lyo-Tre-BMP-2. The results for surface chemical characterization are shown in Figure 1C. The characteristic infrared (IR) bands were found at 600–710 cm−1 for all the groups; the absorption peaks at 1,690 and 3,400 cm−1 were only seen in groups of TiO2 and TiO2-BMP-2.
In vitro release profiles of BMP-2 from TiO2 nanotubes
The in vitro release kinetics of BMP-2 for different groups are shown in Figure 1D. BMP-2 was released from TiO2-Lyo-Tre-BMP-2 nanotubes in a sustained manner during the experiment; however, TiO2-BMP-2 nanotubes exhibited an initial burst of BMP-2 release, followed by a slow release over days 3–28. Approximately 73% of the BMP-2 loaded on TiO2 nanotubes was released over the first 3 days, whereas the equivalent amount of BMP-2 was released from TiO2-Lyo-Tre nanotubes over a period of 14 days.
Bioactivity of BMP-2 released from TiO2 nanotubes
The bioactivities of BMP-2 released from different formulations are shown in Figure 1E. The equivalent amount of BMP-2 releasates from TiO2-BMP-2 and TiO2-Lyo-Tre-BMP-2 nanotubes and from fresh BMP-2 exhibited significantly enhanced ALP activity compared with the NC (p<0.01) on days 3, 7, and 14. However, no significant differences were found in the bioactivities of BMP-2 among the releasates from the 3 formulations at each time point.
Stability of BMP-2 with Lyo/Tre
The bioactivity of BMP-2 loaded on TiO2-Lyo-Tre-BMP-2 nanotubes under different storage temperatures and times are shown in Figure 1F. After 2 weeks of storage at −20°C and 4°C and after 5 weeks of storage at −20°C, over 90% of the bioactivity of BMP-2 remained compared with that of fresh BMP-2. After 2 weeks of storage at 25°C and 5 weeks of storage at 4°C, BMP-2 retained about 85% of its bioactivity. After 5 weeks of storage at 25°C, the bioactivity of BMP-2 decreased to about 75%. The bioactivities of BMP-2 loaded on the TiO2-Lyo-Tre-BMP-2 nanotubes stored at 25°C for 2 or 5 weeks were significantly lower than those of the TiO2-Lyo-Tre-BMP-2 nanotubes stored at −20°C and 4°C during the same time frame (p<0.01).
Effects of culture with nanotubes on the proliferation of BMSCs
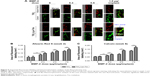
The proliferation of BMSCs cultured with medium only, TiO2, TiO2-BMP-2, or TiO2-Lyo-Tre-BMP-2, on days 1, 3, 7, and 14, was analyzed with a DNA-detection assay (Figure 2). The number of BMSCs following coculture with different groups increased over time and was nearly equal at each time point, indicating that the various nanotube formulations have no cytotoxicity.
Effects of culture with nanotubes on the osteogenic differentiation of BMSCs
The osteogenic differentiation of BMSCs cultured with different nanotube formulations (TiO2, TiO2-BMP-2, and TiO2-Lyo-Tre-BMP-2) was determined by RT quantitative PCR (Figure 3A–E). For BMSCs cultured with TiO2-Lyo-Tre-BMP-2 nanotubes, the expression level of Runx2 mRNA was first upregulated at day 3 (9.7-fold), which increased continuously from days 7 (16.2-fold) to 14 (19.8-fold) (Figure 3A); OPN expression showed a dramatical rise from days 3 (24.8-fold) to 7 (56.2-fold) and then increased slightly from days 7 to 14 (64-fold) (Figure 3B); OCN, BSP, and ALP mRNA levels increased slightly at day 3 (4.9-, 16.9-, and 4.2-fold, respectively), followed by a marked increase between days 7 (11.4-, 38.7-, and 7.6-fold, respectively) and 14 (41.5-, 135.1-, and 18.3-fold, respectively) (Figure 3C–E). For BMSCs cultured with TiO2-BMP-2 nanotubes, the expressions of Runx2, OPN, OCN, BSP, and ALP mRNAs were similar to those in BMSCs cultured with TiO2-Lyo-Tre-BMP-2 nanotubes; however, the fold increases were significantly lower on day 14 (12.2-, 39.9-, 20.1-, 68.3-, and 9.9-fold, respectively) (p<0.01). The expression levels of Runx2 (4.9- and 9.4-fold-, respectively) and OPN (16.2- and 33.5-folds, respectively) mRNAs were also significantly lower than those in BMSCs cultured with TiO2-Lyo-Tre-BMP-2 nanotubes on days 3 and 7 (p<0.01). Overall, the expression levels of osteogenic markers were significantly lower in BMSCs cultured with TiO2 nanotubes than in those cultured with the other 2 types of nanotubes at each time point (p<0.01).
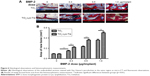
In vivo bone formation around implants
Next, we examined the ability of BMP-2 delivered employing lyophilized TiO2 nanotubes with additional trehalose (TiO2-Lyo-Tre) to reduce the BMP-2 dose needed for in vivo bone regeneration (Figures 4–6). The ability of BMP-2 to induce bone regeneration depends on the delivery system used. Micro-CT analysis (Figure 4), sequential fluorescent labeling analysis (Figure 5), and histological analysis (Figure 6) all revealed that various doses of BMP-2 delivered employing TiO2-Lyo-Tre led to significantly greater new bone formation around the implants than that achieved employing TiO2 alone. The formation of new bone increased with the increase in BMP-2 dose in both groups. The newly formed bone areas in rats implanted with TiO2-Lyo-Tre-BMP-2 nanotubes containing 0.3 or 0.5 μg BMP-2 were approximately equal to those implanted with TiO2-BMP-2 nanotubes containing 0.5 or 1.0 μg BMP-2, respectively, indicating that delivery of a lower dose of BMP-2 by TiO2-Lyo-Tre nanotubes led to new bone formation similar to that of delivery of a higher dose of BMP-2 by TiO2 nanotubes alone.
Discussion
BMP-2 has been used extensively to induce new bone formation;2–5 however, clinical application of BMP-2 requires high doses,6,7 which are associated with increased treatment costs and unwanted side effects, such as excessive immune responses, wound complications, and bone resorption.8–10,34 Therefore, an appropriate delivery system for reducing the dose of BMP-2 for bone regeneration is required. Recent studies have mainly focused on organic, inorganic, and synthetic carrier scaffolds. These carrier scaffolds include collagen sponges,35 calcium phosphate-based cements,36 polylactic acid, polyglycolide, and their copolymer.37 Although there are many suitable delivery carriers available, the optimal scaffold for BMP-2 delivery remains to be identified. Furthermore, our previous works have shown that BMP-2 or Lenti-BMP-2 lyophilized on scaffolds with trehalose enable long-term release of BMP-2 while retaining protein activity, thereby enhancing osteogenesis.25,26 However, it was not known whether delivery of BMP-2 based on Lyo/Tre could reduce the dose of BMP-2 required for effective bone regeneration. Here, we hypothesize that delivery systems for BMP-2 based on Lyo/Tre may be applied as an alternative method to develop a novel delivery system for the reduction of BMP-2 dose. In this study, we developed a novel delivery system based on Lyo/Tre to achieve efficient bone formation. The effects of these nanotubes on BMSC differentiation in vitro were demonstrated, and their implantation in rat femurs yielded significantly enhanced bone formation in vivo. Thus, our data provide important insights into the applications of TiO2 nanotubes lyophilized with trehalose in bone regeneration and delivery of BMP-2.
A much larger amount of amorphous glassy matrix was observed on the surface of TiO2-Lyo-Tre-BMP-2 than on that of TiO2-BMP-2. This discrepancy between the 2 formulations under SEM observation might be attributed to trehalose. Each foil (TiO2-BMP-2, TiO2-Lyo-Tre-BMP-2) was loaded with 6 μg of BMP-2, while for TiO2-Lyo-Tre-BMP-2, 3×10–5 mol (1.1×104 μg) trehalose was added as a protectant.
As shown in the FTIR spectra of different formulations, the characteristic IR bands were found at 600–710 cm−1 for all the groups, proving the existence of Ti–O bonds in the TiO2 lattice. However, the absorption peaks at 1,690 and 3,400 cm−1 were only found in groups of TiO2 and TiO2-BMP-2, which were attributed to the bending mode of OH and the stretching vibration of surface hydroxyl and absorbed water,38 indicating that no water molecules existed on the surface of TiO2-Lyo-Tre-BMP-2 nanotubes. These may be attributed to the lyophilization process.
As a BMP-2 delivery system, TiO2-Lyo-Tre-BMP-2 nanotubes facilitated sustained release, persistent bioactivity, and higher stability of BMP-2; these features are important for enhanced bone regeneration. Compared with the bolus release of BMP-2 from TiO2-BMP-2 nanotubes, TiO2-Lyo-Tre-BMP-2 nanotubes were associated with slow, prolonged BMP-2 release. This release kinetics of difference between the 2 formulations could be due to the high binding affinity of BMP-2 with titanium-based materials, caused by lyophilization.39 Additionally, we found that the bioactivity of BMP-2 released from TiO2-Lyo-Tre-BMP-2 nanotubes was preserved, similar to that of fresh BMP-2. Although adsorbed BMP-2 also maintained its bioactivity, the osteogenesis of BMSCs cultured with TiO2-BMP-2 was statistically lower than that of BMSCs cultured with TiO2-Lyo-Tre-BMP-2. This result indicates that the BMP-2 release profiles play a key role in promoting osteogenic differentiation of cells. For promotion of the osteogenesis of BMSCs, release of BMP-2 in a sustained manner is more effective. Accordingly, sustained release of BMP-2 with maintained bioactivity has been shown to improve the osteogenic ability of BMP-2.40,41 Moreover, long-term delivery of BMP-2 over a period of 4 weeks was shown to induce a significantly higher amount of newly-formed bone than the same quantity of BMP-2 released over 3 days.42,43 Other research studies have found that BMP-2 does not induce a significant amount of new bone formation when released over <7 days.44,45 These results may be partially explained by the observation that sustained release of BMP-2 can enhance the long-term osteogenesis and migration of progenitor cells or mesenchymal stem cells for several weeks; these processes are necessary for successful new bone formation.46
Growth factors are inactivated easily by storage at different temperatures and for different durations; however, few studies have been performed to assess the bioactivities of growth factors that were loaded onto delivery systems for bone regeneration. In 1 study, 60% of the bioactivity of growth factors absorbed on a Ca-P cement scaffold was preserved after storage at room temperature for 5 weeks. However, the release profiles of the loaded growth factors were not explored.47 In the present study, our delivery system based on Lyo/Tre was found to preserve the bioactivity of BMP-2 during the preparation process of the implant, as well as to facilitate the maintenance of over 75% of BMP-2 bioactivity at 25°C for at least 5 weeks. By retaining the stability of bioactive growth factors, new delivery systems based on Lyo/Tre may improve the capacity for regeneration of new bone tissue in various clinical applications.
Compared with TiO2-BMP-2 nanotubes, TiO2-Lyo-Tre-BMP-2 nanotubes promoted the sustained release, maintained bioactivity, and stability of BMP-2; these effects were thought to be conferred by Lyo/Tre. Lyophilization is an effective method for increasing the physical and chemical stability of biomolecules. However, some growth factors may undergo irreversible structural changes during the freezing and drying process, resulting in inactivation of the biomolecule.19 In order to prevent such inactivation, we and others have used trehalose as a protectant.22 The ionic interactions between positively charged cavities in BMP-2 molecules and negatively charged polysaccharide chains in trehalose molecules increase the binding affinity between BMP-2 and trehalose. The lyophilization process further generates an enhanced high-binding affinity between BMP-2/Tre and Ti-based materials. This synergistic effect may be responsible for the sustained release of BMP-2 observed from TiO2-Lyo-Tre-BMP-2 nanotubes.
The bone formation efficacy of BMP-2 delivered by TiO2-Lyo-Tre-BMP-2 nanotubes was superior to that of BMP-2 delivered by TiO2-BMP-2 nanotubes. Doses of 0.3 and 0.5 μg BMP-2 delivered by TiO2-Lyo-Tre-BMP-2 elicited bone formation efficacies similar to those of 0.5 and 1.0 μg BMP-2 delivered by TiO2-BMP-2 nanotubes, respectively. Thus, the enhanced bone regeneration capacity of BMP-2 delivered using a system based on Lyo/Tre is expected to permit the use of lower doses of BMP-2 for successful new bone formation, thereby reducing the severity of side effects induced by high doses of BMP-2.
Conclusion
In summary, delivery of BMP-2 using TiO2-Lyo-Tre nanotubes promoted greater osteogenic differentiation of BMSCs in vitro and enhanced bone regeneration in vivo than delivery of BMP-2 using TiO2 nanotubes alone. Importantly, BMP-2 delivery employing the present Lyo/Tre-based system could reduce the dose of BMP-2 needed for effective bone regeneration through the sustained release, persistent bioactivity, and better stability of BMP-2. Therefore, delivery of BMP-2 using the Lyo/Tre-based system may be an effective method for reducing the required dose of BMP-2 for bone regeneration and suppressing unwanted side effects induced by the administration of high doses of BMP-2.
Acknowledgment
Financial support from the National Natural Science Foundation of China (81200815, 81371164, and 81470765), Scientific and Technological Innovation Action Plan of Shanghai Science and Technology Commission (17441902000).
Disclosure
The authors report no conflicts of interest in this work.
References
Reddi AH. BMPs: from bone morphogenetic proteins to body morphogenetic proteins. Cytokine Growth Factor Rev. 2005;16(3):249–250. | ||
Termaat MF, Den Boer FC, Bakker FC, Patka P, Haarman HJ. Bone morphogenetic proteins. Development and clinical efficacy in the treatment of fractures and bone defects. J Bone Joint Surg Am. 2005;87(6):1367–1378. | ||
Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A(12):2123–2134. | ||
Kanakaris NK, Giannoudis PV. Clinical applications of bone morphogenetic proteins: current evidence. J Surg Orthop Adv. 2008;17(3):133–146. | ||
Poynton AR, Lane JM. Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine. 2002;27(16 Suppl 1):S40–S48. | ||
Shields LB, Raque GH, Glassman SD, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31(5):542–547. | ||
Mont MA, Ragland PS, Biggins B, et al. Use of bone morphogenetic proteins for musculoskeletal applications. An overview. J Bone Joint Surg Am. 2004;86(A Suppl 2):41–55. | ||
James AW, LaChaud G, Shen J, et al. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng Part B Rev. 2016;22(4):284–297. | ||
Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471–491. | ||
Lewandrowski KU, Nanson C, Calderon R. Vertebral osteolysis after posterior interbody lumbar fusion with recombinant human bone morphogenetic protein 2: a report of five cases. Spine J. 2007;7(5):609–614. | ||
Yang HS, La WG, Bhang SH, Jeon JY, Lee JH, Kim BS. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng Part A. 2010;16(4):1225–1233. | ||
Yamamoto M, Takahashi Y, Tabata Y. Enhanced bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 2006;12(5):1305–1311. | ||
Oh S, Daraio C, Chen LH, Pisanic TR, Finones RR, Jin S. Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J Biomed Mater Res A. 2006;78(1):97–103. | ||
Bjursten LM, Rasmusson L, Oh S, Smith GC, Brammer KS, Jin S. Titanium dioxide nanotubes enhance bone bonding in vivo. J Biomed Mater Res A. 2010;92(3):1218–1224. | ||
Sun S, Yu W, Zhang Y, Zhang F. Increased preosteoblast adhesion and osteogenic gene expression on TiO2 nanotubes modified with KRSR. J Mater Sci Mater Med. 2013;24(4):1079–1091. | ||
Ma Y, Zhang Z, Liu Y, et al. Nanotubes functionalized with BMP2 knuckle peptide improve the osseointegration of titanium implants in rabbits. J Biomed Nanotechnol. 2015;11(2):236–244. | ||
Mozhaev VV. Mechanism-based strategies for protein thermostabilization. Trends Biotechnol. 1993;11(3):88–95. | ||
Arakawa T, Prestrelski SJ, Kenney WC, Carpenter JF. Factors affecting short-term and long-term stabilities of proteins. Adv Drug Deliv Rev. 2001;46(1–3):307–326. | ||
Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. 2000;203(1–2):1–60. | ||
Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58(15):1688–1713. | ||
Griebenow K, Klibanov AM. Lyophilization-induced reversible changes in the secondary structure of proteins. Proc Natl Acad Sci U S A. 1995;92(24):10969–10976. | ||
Kasper JC, Schaffert D, Ogris M, Wagner E, Friess W. Development of a lyophilized plasmid/LPEI polyplex formulation with long-term stability–a step closer from promising technology to application. J Control Release. 2011;151(3):246–255. | ||
Lins RD, Pereira CS, Hünenberger PH. Trehalose–protein interaction in aqueous solution. Proteins. 2004;55(1):177–186. | ||
Neta T, Takada K, Hirasawa M. Low-cariogenicity of trehalose as a substrate. J Dent. 2000;28(8):571–576. | ||
Zhao J, Wang S, Bao J, et al. Trehalose maintains bioactivity and promotes sustained release of BMP-2 from lyophilized CDHA scaffolds for enhanced osteogenesis in vitro and in vivo. PLoS One. 2013;8(1):e54645. | ||
Zhang X, Zhang Z, Shen G, Zhao J. Enhanced osteogenic activity and anti-inflammatory properties of Lent-BMP-2-loaded TiO2 nanotube layers fabricated by lyophilization following trehalose addition. Int J Nanomedicine. 2016;11:429–439. | ||
Yu WQ, Jiang XQ, Zhang FQ, Xu L. The effect of anatase TiO2 nanotube layers on MC3T3-E1 preosteoblast adhesion, proliferation, and differentiation. J Biomed Mater Res A. 2010;94(4):1012–1022. | ||
Xie G, Sun J, Zhong G, Liu C, Wei J. Hydroxyapatite nanoparticles as a controlled-release carrier of BMP-2: absorption and release kinetics in vitro. J Mater Sci Mater Med. 2010;21(6):1875–1880. | ||
Zhao J, Hu J, Wang S, et al. Combination of β-TCP and BMP-2 gene-modified bMSCs to heal critical size mandibular defects in rats. Oral Dis. 2010;16(1):46–54. | ||
Li B, Yoshii T, Hafeman AE, Nyman JS, Wenke JC, Guelcher SA. The effects of rhBMP-2 released from biodegradable polyurethane/microsphere composite scaffolds on new bone formation in rat femora. Biomaterials. 2009;30(35):6768–6779. | ||
Rago R, Mitchen J, Wilding G. DNA fluorometric assay in 96-well tissue culture plates using Hoechst 33,258 after cell lysis by freezing in distilled water. Anal Biochem. 1990;191(1):31–34. | ||
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods. 2001;25(4):402–408. | ||
Zhang W, Wang G, Liu Y, et al. The synergistic effect of hierarchical micro/nano-topography and bioactive ions for enhanced osseointegration. Biomaterials. 2013;34(13):3184–3195. | ||
Sciadini MF, Johnson KD. Evaluation of recombinant human bone morphogenetic protein-2 as a bone-graft substitute in a canine segmental defect model. J Orthop Res. 2000;18(2):289–302. | ||
Friess W, Uludag H, Foskett S, Biron R, Sargeant C. Characterizaiton of absorbable collagen sponges as recombinant human bone morphogenetic protein-2 carriers. Int J Pharm. 1999;185(1):51–60. | ||
Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements as bone drug delivery systems: a review. J Control Release. 2006;113(2):102–110. | ||
Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regenenration and repair. Part B: delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol Lett. 2009;31(12):1825–1835. | ||
Huang DG, Liao SJ, Zhou WB, et al. Synthesis of samarium- and nitrogen-co-doped TiO2 by modified hydrothermal method and its photocatalytic performance for the degradation of 4-chlorophenol. J Phys Chem Solids. 2009;70(5):853–859. | ||
Jansen JA, Vehof JW, Ruhe PQ, et al. Growth factor-loaded scaffolds for bone engineering. J Control Release. 2005;101(1–3):127–136. | ||
Jeon O, Song SJ, Kang SW, Putnam AJ, Kim BS. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly (L-lactic-co-glycolic acid) scaffold. Biomaterials. 2007;28(17):2763–2771. | ||
La WG, Jin M, Park S, et al. Delivery of bone morphogenetic protein-2 and substance P using grapheme oxide for bone regeneration. Int J Nanomedicine. 2014;9(Suppl 1):107–116. | ||
La WG, Kang SW, Yang HS, et al. The efficacy of bone morphogenetic protein-2 depends on its mode of delivery. Artif Organs. 2010;34(12):1150–1153. | ||
Jeon O, Song SJ, Yang HS, et al. Long-term delivery enhances in vivo osteogenic efficacy of bone morphogenetic protein-2 compared to short-term delivery. Biochem Biophys Res Commun. 2008;369(2):774–780. | ||
Uludag H, Gao T, Porter TJ, Friess W, Wozney JM. Delivery systems for BMPs: factors contributing to protein retention at an application site. J Bone Joint Surg Am. 2001;83(A Suppl 1):S128–S135. | ||
Boerckel JD, Kolambkar YM, Dupont KM, et al. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials. 2011;32(22):5241–5251. | ||
Quinlan E, Thompson EM, Matsiko A, O’Brien FJ, Lopez-Noriega A. Long-term controlled delivery of rhBMP-2 from collagen–hydroxyapatite scaffolds for superior bone tissue regeneration. J Control Release. 2015;207:112–119. | ||
Le Nihouannen D, Komarova SV, Gbureck U, Barralet JE. Bioactivity of bone resorptive factor loaded on osteoconductive matrices: stability post-dehydration. Eur J Pharm Biopharm. 2008;70(3):813–818. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.