Back to Journals » Medical Devices: Evidence and Research » Volume 8
Detectability and acceptability of continuous pulse signals for the MemoPatch® device, an electronic skin patch intended to deliver tactile medication reminder signals
Authors Abraham I , De Geest J, De Geest W, De Troy E, MacDonald K
Received 17 August 2014
Accepted for publication 25 September 2014
Published 5 February 2015 Volume 2015:8 Pages 119—129
DOI https://doi.org/10.2147/MDER.S72806
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Ivo Abraham,1–3 Jan De Geest,2 Wim De Geest,2 Elke De Troy,4 Karen MacDonald3
1Center for Health Outcomes and PharmacoEconomic Research, University of Arizona, Tucson, AZ, USA; 2TheraSolve, Diepenbeek, Belgium; 3Matrix45, Tucson, AZ, USA; 4Jessa Ziekenhuis, Hasselt, Belgium
Background: Unintended forgetfulness is the most common cause of medication nonadherence. MemoPatch® is an electronic skin patch intended to deliver discreet tactile medication reminder stimuli. This study aimed 1) to evaluate, within an experimental setup, the detectability and acceptability of fifteen continuous bipolar pulse signals; 2) to identify variables, if any, associated with differential perception of the candidate reminder signals; and 3) to collect safety data as reported by subjects or observed by staff.
Methods: This was a laboratory experiment involving 147 healthy adult volunteers (55.1% female, 98.0% Caucasian, with age 41.8±16.0 years, body mass index [BMI] 24.7±4.4, upper body adiposity 28.5%±8.3% body fat, and skin impedance 367.6±140.8Ω) and using an experimental version of the MemoPatch®. Following four training signals administered in fixed order, subjects were exposed to a set of fifteen randomly sequenced signals varying in rise and fall time, width, and current, to be rated in terms of detectability ("too weak", "appropriate", or "too strong") and acceptability.
Results: Ratings of "appropriate" were virtually independent of such variables as sex, BMI, upper body adiposity, and skin impedance at the patch location. Five signals were rated as "appropriate" by ≥67% of subjects and acceptable by ≥95% of subjects, virtually independently of the indicators of interest, and were retained as candidate signals for use in next stages of development and commercialization. Nine adverse events, none serious, were observed in six subjects.
Conclusion: This study yielded five effective and safe candidate signals for potential use in the MemoPatch® device, all equally considered to be of appropriate detectability and high acceptability, in an experimental context. The signals were independent from, and therefore highly robust relative to, sex, BMI, upper body adiposity, and skin impedance at the patch site, lending additional generalizability to the signals and hence their potential relevance to broad commercial application.
Keywords: adherence, satisfaction, persistence, forgetfulness, medication
Introduction
The World Health Organization (WHO) defines adherence as “the extent to which a person’s behavior – taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a health care provider”.1 Nonadherence, the behavior of not following treatment recommendations proposed to and agreed to by a subject, has been estimated at 24.8% across 17 disease conditions, with a low of 11.7% in subjects with human immunodeficiency virus (HIV) disease and a high of 34.5% in the treatment of sleep disorders.2 The difference in clinical outcomes between high and low adherence is 26%.3 While the determinants of nonadherence are multifaceted and reach beyond the patient, 64.4% of patients cite unintended forgetfulness as the main reason for missing medication doses.4 MemoPatch® is an electronic skin patch, currently in development, intended to deliver discreet, tactile medication reminder stimuli.5 In concept, MemoPatch® devices will be optionally hard- or soft-coded with a regimen of reminder signals that corresponds to the medication regimen prescribed to the patient, with the added possibility of a separate reminder signal to remove the patch. When fully functional, the MemoPatch® technology will consist of a thin flexible self-adhesive dermal patch with an integrated pulse generator application-specific integrated circuit (ASIC), printed battery, and body contact electrodes. An optional printed antenna will enable wireless programming.5
A key early development priority is the identification of pulse signals that are both detectable and acceptable as potential reminder signals to subjects. Two prior studies (TS-101 and TS-102, Therasolve, data on file, 2005) evaluated subjects’ detection of and experiences with signals with variable polarity, form, and pulse duration, burst, and current. Study TS-101 was a feasibility study to determine whether healthy volunteer subjects could detect nontherapeutic tactile signals delivered through a patch-delivered stimulation and to solicit their general appraisal of the signals. Signals were unipolar block pulses. The principal findings were that signals at a nontherapeutic intensity level were detected but with significant variability in subjects’ appraisal of these signals; and that a block form was associated with minor discomfort – both findings being important to the present study (TS-103).
In study TS-102, healthy volunteer subjects were administered signals varying in rise and fall time, width, and current in an effort to technically specify candidate signals to be used in TS-103. TS-102 aimed to reduce the many permutations that are possible under various rise and fall times, widths, and currents by identifying those signals that subjects rated as either neutral or acceptable as reminder signals. This yielded a set of fifteen bipolar continuous signals.
In the first instance, the present study, TS-103, aimed to evaluate, within an experimental setup, the detectability and acceptability of this set of fifteen continuous bipolar pulse signals varying in rise and fall time, width, and current. Second, the study intended to identify variables, if any, associated with differential perception of the candidate reminder signals. Third, TS-103 collected safety data as reported by subjects or observed by staff.
Methods
Aims
The aims of the study were: 1) to evaluate, within an experimental setup, the detectability and acceptability of a set of fifteen continuous bipolar pulse signals varying in rise and fall time, width, and current; 2) identify variables, if any, associated with differential perception of the candidate reminder signals; and 3) to assess safety as reported by subjects or observed by staff.
Design
TS-103 was a laboratory experiment in a sample of consenting healthy adults. During a single standardized experimental session, subjects were exposed to a fixed-order set of four training signals followed by a randomly ordered sequence of fifteen reminder signals varying in rise and fall time, width, and current.
Sample
Eligible were adult (age >18 years) male and female healthy volunteers; as well as volunteers with illnesses that were being treated according to the prevailing standard of care, that did not impair subjects’ ability to detect reminder signals, and that did not predispose them to potential adverse events (AEs). Exclusion criteria were: open or recently healed injuries on upper arms; tattoo(s) received on upper arms in preceding 3 months; scars on upper arm exceeding 10 cm2 in area (cumulative if multiple scars); dermatological conditions on upper arms (current or in the past 6 months); paresthesia or other neurosensory impairments in upper arms (current or in the past 6 months); history of skin or other hypersensitivity to electrical stimulation; history of any major cardiovascular event or cardiovascular disease, including but not limited to myocardial infarct, congestive heart failure, peripheral artery disease, stroke, deep venous thrombosis, pulmonary embolism, and/or transient ischemic attack; history of diabetes with known end-organ disease, including but not limited to neuropathy, retinopathy, nephropathy, and leg ulcers (diabetes without end-organ disease did not preclude participation in this study); history of traumatic brain injury; history of serious mental illness, such as psychotic disorders, major depression, bipolar disorder, obsessive–compulsive disorder, and panic disorder; prior treatment with electroconvulsive therapy; any prior transplant; topical treatments (prescribed medications, over-the-counter medications, or consumer products) applied to upper arms in the past 30 days; topical (upper arms), regional (involving arms), or systemic anesthetics in the past 30 days; treatment with any agents known to potentially cause paresthesia or other neurosensory symptoms in the past 30 days; use of any investigational pharmacological agent in the last 30 days; and pregnancy or potential pregnancy.
Sample size calculations (power =0.80 at α=0.05) indicated a minimum requirement of 102 subjects to permit regression modeling with up to ten determinants. Such a sample size would be able to detect an adverse event with a hypothesized prevalence rate of 0.02 within a 95% confidence interval (CI) of 0.00556 to 0.06928.
Subjects were recruited from the student, faculty, and staff bodies of Hasselt University (Hasselt, Belgium) through an email communication sent independently by the university. Interested persons were provided with access to a website to record general contact information, to complete an online checklist of the inclusion and exclusion criteria to enable initial screening, and to schedule their experimental session.
Subjects completing the experiment received €40 to cover time spent registering for, traveling to and from, and participating in the experiment, and an additional €10 for travel and miscellaneous expenses, for a total of €50.
Signals
The reminder signals consisted of continuous bipolar pulses, combined with fixed pulse intervals into bursts of a fixed length, in turn combined with fixed burst intervals into a reminder signal with a fixed activation of 15 seconds.
As human skin is not only resistive but also, capacitative, under prolonged stimulation, the skin attains a charged state that impedes the flow of current. One option to overcome this impedance, meet required current flow, and thus maintain the efficiency of pulses within a burst is to increase the voltage. However, this occurs at the risk of electroporation, or “electropermeabilization” (a significant increase in the electrical conductivity and permeability of the cell plasma membrane caused by an externally applied electrical field). Another option, and used in the experimental MemoPatch® device tested here, is to use a bipolar pulse format in which an opposite subpulse is integrated into the pulse.
The signals used in this study were located below the threshold curves for motor, pain, and tolerance reactions to monopolar currents.6 Moreover, the signals administered in the present (TS-103) study were bipolar currents in which the current reversal was intended to suppress a developing action potential elicited by the initial phase. The biphasic stimulus had a reduced efficacy for neuromuscular stimulation as compared with a single monophasic pulse of the same phase.
All pulses tested fell well below the European standard, International Electrotechnical Commission (IEC) 60601-2-10 “Medical electrical equipment Part 2–10: Particular requirements for the safety of nerve and muscle stimulators”.7
Technical specifications of apparatus
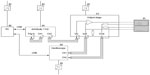
At this stage of development, the experimental MemoPatch® used in the experiment was a 50×50 mm wired patch made of a Kapton® flexible printed circuit board (PCB) substrate equipped with gold-nickel electrode surfaces, and covered with a skin-friendly adhesive. The patch was connected to the test equipment with two leads of approximately 10 cm with rigid ending, which were fit into a zero insertion force (ZIF) connector on the output stage.
Figure 1 depicts the experimental test configuration. Pulses were generated by an ArbStudio 1102 arbitrary waveform generator (LeCroy, Chestnut Ridge, NY, USA), based on parameters specified by the software program ArbStudio Version 3.2.0.2 (LeCroy), running on a Dell Precision M6600 computer (Dell, Round Rock, TX, USA) under the Microsoft Windows® 7 operating system (Microsoft, Redmond, WA, USA). Pulses thus generated were amplified by a custom-designed output stage (Dekimo, Gentbrugge, Belgium) and transmitted to the patches. An oscilloscope (TPS2024B; Tektronix, Beaverton, OR, USA) was used to measure voltage and current.
To prevent leak currents from interfering with equipment, the connection of each of the devices to the electrical grid was regulated by a medical device–certified power supply meeting the IEC 60601-1 international standard.
Procedures and assessments
The experiment was conducted at the Biomed research facility of Hasselt University. The following data were collected after completing eligibility verification and obtaining written informed consent: subject demographics, relevant anthropo- and biometrics, relevant medical history and current clinical status, and skin-related characteristics that could potentially influence the detectability or appraisal of reminder signals. Body mass index (BMI) was calculated as the subject’s weight (in kilograms) divided by the square of his/her height (in meters) without corrections. Upper arm circumference was assessed (in cms) at the midpoint between the tip of the shoulder and the tip of the elbow by means of a tape measure. Pilodensity on the upper arm was determined using the Modified Ferriman–Gallwey score, a visual scale by which observed hair concentration is matched to one of four grades of density.8 Upper body adiposity, expressed as % body fat, was evaluated with the Omron Body Fat Monitor BF306 (Omron Healthcare Co, Ltd, Kyoto, Japan). Skin impedance (in Ω) at the patch location was determined through the MemoPatch® device and associated equipment (Figure 1) at 22 different frequencies ranging from 250 Hz to 250,000 Hz. For purposes of this report, results were focused on the impedance values at 48,267 Hz, which is within range of the recommended 50,000 Hz,9 the slight deviation being due to impedance testing at multiple frequencies on a logarithmic scale.
A fixed training sequence of four signals was administered to train the subjects in the study procedures. This trial sequence consisted of one highly detectable signal, followed by one slightly detectable, one undetectable, and again one highly detectable signal. To avoid an alertness effect, subjects were not told that the first four signals were for training purposes. This was followed by the fifteen experimental signals, which were administered in random order.
Each signal in both the fixed training and the random study sequence was administered in a separate signal event. Subjects were instructed to raise a hand if and when they detected a signal and to rate the signal as “too weak”, “appropriate”, or “too strong”. If no hand was raised, the investigator recorded the signal as “not detected”. For each signal detected, subjects were asked to report any untoward events, rate the acceptability of each signal (defined as “not painful” or “painful”), and provide any additional narrative comments about the signal. Following completion of the signal sequence, subjects were asked to fill out a questionnaire soliciting their evaluation of and satisfaction with the MemoPatch® concept and were offered the opportunity to volunteer additional information. A final examination of the patch site was performed to observe for AEs. Subjects were debriefed, including information as to how to contact the investigators in case of AEs occurring after the study visit.
Pulse selection and validation
We used the following criteria for identifying the preferred continuous bipolar signals for potential use in experimental and commercial versions of the MemoPatch®: a signal rating of “appropriate” by at least 67% of subjects coupled with a positive acceptability rating by at least 95% of subjects; no statistically significant differences in appropriateness and acceptability ratings between women and men; and no statistically significant associations between a rating of “appropriate” with BMI, upper body adiposity, and skin impedance at the patch location. The 67% and 95% cutoff values were chosen by consensus. The sex criterion was intended to identify signals that could be used with both men and women. The weight, upper body adiposity, and skin impedance criteria aimed to identify signals that were independent of upper body mass and potential obesity, and robust relative to variations in skin impedance.
Protection of human subjects
This study was conducted in accordance with the World Medical Association’s Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects. The study protocol was approved by the Ethical Committee of Jessa Ziekenhuis (Hasselt, Belgium) acting on behalf of Hasselt University. All subjects were required to provide written informed consent prior to enrollment in the study.
Results
Subjects
A total of 167 persons who responded to the Hasselt University email communication were screened, of whom three failed the inclusion/exclusion criteria. Of the remaining 164 subjects who consented to participate, one subject experienced a vasovagal reaction prior to administration of any signals and did not proceed with the experiment. In total, 163 subjects completed the experiment; however protocol violations were identified ex post facto in 16 subjects. The analysis sample included 147 subjects.
Table 1 summarizes relevant demographics, anthropo- and biometrics for the sample, stratified by sex. The sample included 81 female (55.1%) and 66 (44.9%) male subjects (P = not significant [ns]) distributed, within statistical parameters, equally across the three age strata (P = ns). The mean age (± standard deviation [SD]) was 41.8±16.0 years, with no statistically significant difference for sex. Forty-four (29.9%) subjects were between the ages of 18 and 30 years, 63 (42.9%) were between the ages of 31 and 55 years, and 40 (27.2%) subjects were between the ages of 56 and 75 years. Most subjects (98.0%) were Caucasian. Men and women differed significantly in mean weight, height (hence also BMI), upper arm circumference, upper body adiposity, and skin impedance at the patch location (all P<0.001). This was confirmed in contingency analyses of sex by categories of these variables (all P<0.001). Note that all women had a pilodensity rating of 1 compared with 66.7% of the men, among whom an additional 24.2% had a rating of 2. Hence, and even though these differences were statistically significant, no further analyses stratified by pilodensity were performed as these would revert back to stratification by sex.
Detectability and acceptability of test signals
The detectability of each of the fifteen experimental signals was classified as “undetected” if subjects did not raise their hand following a stimulus event. If they raised their hand to indicate detection of a signal, they were instructed to rate the signal as “too weak”, “appropriate”, or “too strong”, and also to indicate whether or not the signal was painful. Based on the latter rating, the signal was considered not acceptable or acceptable, respectively. Figure 2 depicts the detectability and acceptability percentages for each signal across all subjects.
As Table 2 shows, on average, women rated fewer signals as “too weak” and more signals as “too strong” compared with men (both P<0.001); however, men and women did not differ statistically in the mean number of signals they rated to be “appropriate” (P = ns) (Figure 3).
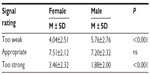  | Table 2 Mean number of signals rated “too weak”, “appropriate”, and “too strong”, stratified by sex |
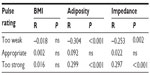
There were no statistically significant differences in detectability and acceptability when stratified by BMI (<25 vs ≥25), upper body adiposity (<25%, 25%–34%, ≥35%), and skin impedance at the patch location (median split <328 Ω and ≥328 Ω, at 48,267 Hz) (all P = ns). Table 3 summarizes the association between detectability ratings and BMI, upper body adiposity, and skin impedance at the patch location. None of the correlation coefficients between BMI and the number of signals rated as “too weak”, “appropriate”, or “too strong” were statistically significant (all P = ns). Upper body adiposity and skin impedance at the patch location correlated negatively with the number of signals rated “too weak” and positively with the number of signals judged “too strong”. These correlations were weak, with absolute values ranging from 0.253 to 0.304. The associated R2 values ranged from 0.064 to 0.092, indicating that minimal proportions of variance in the number of signals rated “too weak” or “too strong” were accounted for by subjects’ adiposity or skin impedance. In contrast, there were no statistically significant correlations between the number of signals rated “appropriate” and adiposity and impedance. Lastly, for validation purposes, we performed a multiple linear regression of the number of signals rated “appropriate” by the subjects as a function of sex, BMI, upper arm circumference, upper body adiposity, skin impedance at the patch location, and pilodensity. No model could be fitted, indicating that jointly, these variables did not predict subjects’ ratings of “appropriate” for the signals administered.
Pulse selection and validation
As noted above, criteria for identifying the preferred continuous bipolar signals for potential use in experimental and commercial versions of the MemoPatch® device included the following: a rating of “appropriate” by at least 67% of subjects coupled with a positive acceptability rating by at least 95% of subjects; no statistically significant differences in appropriateness and acceptability ratings between women and men; and no statistically significant associations between a rating of “appropriate” with BMI, upper body adiposity, and skin impedance at the patch location. Five signals met these criteria (P-06, P-09, P-11, P-13, and P-14).
For validation purposes, we conducted several multivariate and bivariate analyses of these pulses relative to subject characteristics. First, for each of the five signals retained, we performed logistic regressions of a signal’s “appropriate” ratings by subjects as a function of sex, BMI, upper arm circumference, upper body adiposity, skin impedance at the patch location, and pilodensity. No models could be fitted for signals P-06, P-11, and P-14. Skin impedance at the patch location decreased the odds of an “appropriate” rating slightly but significantly for signals P-09 (odds ratio [OR] =0.997, 95% CI 0.994–0.999, P=0.022) and P-13 (OR =0.997, 95% CI 0.995–0.999, P=0.043). Second, for each signal retained, we cross-tabulated the number of subjects who rated the signal as “appropriate” (versus “too weak” or “too strong”) by sex, BMI, upper arm circumference, upper body adiposity, skin impedance at the patch location, and pilodensity (expressed in categories). The 30 contingency analyses performed yielded only two statistically significant results. For signal P-06, sex was significant (P=0.012), with male subjects more frequently scoring this signal as “appropriate”; as was skin impedance (P=0.042), with subjects with higher impedance less frequently scoring this signal as “appropriate”. Upper body adiposity was significant (P=0.001) for signal P-09, with those in the highest category (≥35%) scoring this signal as “appropriate” less frequently than those with less upper body fat.
Appraisal
Subjects were asked to complete a questionnaire evaluating the potential use of, and their likely satisfaction with, a commercial version of the MemoPatch® based on their initial experiences during the study (see Table 4). Without any differences by sex (all P = ns), 78.2% of subjects would use the MemoPatch® as a reminder device; 88.4% would recommend the MemoPatch® as a reminder device to others; 68.0% would prefer a reminder signal not exceeding 15 seconds; 85.7% found the 5×5 cm size acceptable, with 56.8% preferring a square shape and 27.4% expressing no preference for shape; and 89.8% were willing to wear a patch for more than one day at a time. Most subjects were satisfied or very satisfied with the privacy afforded by the MemoPatch® solution (91.8%), its effectiveness as a reminder device (97.3%), and the fact that it is unlikely to interfere with routine daily activities (93.8%).
  | Table 4 Evaluation of and satisfaction with MemoPatch® by all subjects and stratified by sex |
Safety
A total of nine AEs, none serious, were observed in six subjects. One subject became unwell and diaphoretic after application of the patch and during the initial impedance measurement but before any signals were administered – most likely a vasovagal reaction. Symptoms ceased after 15 minutes, and blood pressure was normal. It was decided mutually that the subject would not proceed with the experiment. Another subject became unwell during the experiment, complaining of dizziness, blurred vision, and tinnitus. The experiment was interrupted temporarily; the subject rested for about 30 minutes and following a normal blood pressure reading, chose to continue the experiment without any further problems. Two instances of “small red dots” and five instances of minor red skin rash at the patch location were recorded.
Discussion
The MemoPatch® device, an electronic skin patch intended to deliver discreet tactile medication reminder stimuli, aims to enhance adherence to medication treatment regimens by reducing unintended forgetfulness, the most common cause of patients failing to take their medications as prescribed. MemoPatch® aims to offer an effective alternative to other medication reminder systems by providing a discreet reminder signal perceptible only by the patient and minimally interfering with daily routines. To this end, it is critical that the signals generated are strong enough to be detectable without producing discomfort and pain, and relatedly, that are acceptable to patients. In this signal-finding study, we identified five signals that, in a laboratory setting, were judged to be appropriately detectable in strength and acceptable in experience by most subjects.
However, detectability and acceptability are only base requirements. To develop a commercially usable reminder patch, it is also important to identify signals that are sufficiently generalizable and minimally (if at all) influenced by intersubject variability. The candidate signals were virtually independent of sex, BMI, upper body adiposity, and skin impedance at the patch location. Generalizability of the five signals therefore may be inferred, despite some disparate exceptions across some signals.
Any observed sex differences in detectability were related to undesirable signals, ie, those considered “too weak” or “too strong”. Women tended to rate more signals as “too strong”, while men tended to rate more signals as “too weak”. Critically, men and women did not differ in the number of signals rated “appropriate”. BMI, an overall index of weight, was not associated with signal detectability, whether “too weak”, “appropriate”, or “too strong”. In terms of regional distribution of body fat and weight, upper body adiposity influenced ratings of “too weak” and “too strong”, but not “appropriate”, in the same direction as observed for sex. This is consistent with the differential distribution of body fat among men and women. Skin impedance at the patch location followed a similar pattern: differences in the “too weak” and “too strong” categories but no differences where ratings of “appropriate” were concerned. Skin impedance and adiposity are known to be positively correlated, and women tend to have more body fat in the upper arms.9 Likely, this also explains the multivariate and bivariate findings of a slight but significant effect of skin impedance and adiposity in the five signals retained for potential future use.
The split signals retained in this study have since been used in a follow-on study (TS-104), though now with the alternations of the split signal separated by 5 ms, to improve energy management, and administered with graduated currents starting at 0 mA.10 Like study TS-103, study TS-104 also focused on detectability and appraisal of the signals but in a dynamic fashion. Each signal was tested with a compliance voltage of 70 V and initiated with an electric current of 0 mA, which was increased gradually. Subjects were asked to indicate three transition points: when a signal was detected (T1), when the signal was sufficiently detectable to serve as a reminder signal (T2), and when that signal became too strong as a reminder signal (T3). Selection of candidate signals with alternations separated by 5 ms considered three data points: T1Max and T2Max (defined as, respectively, the maximum current observed at T1 and T2) and T3Pct90 (the T3 current at the 90th percentile). A signal was considered to be usable in future versions of the MemoPatch® device if it met the constraint that (T3Pct90−T2Max) should not be negative. One signal, with T3Pct90 − T2Max = 0.96 mA, met this constraint. Detailed results may be found in Abraham et al.10
In general, the pulses administered in this study were safe and were tolerated well. The vasovagal reactions observed in two subjects were most likely related to the experimental laboratory situation and the anticipation of electrical stimulation of unknown intensity. The skin dots and skin rashes observed were most likely due to the repeated stimulation from the four training and 15 experimental signals and the cumulative exposure over a period of approximately 30 minutes.
Conclusion
This study yielded five effective and safe candidate signals for potential use in the MemoPatch® device, which have since been adapted technically and tested under additional conditions and constraints. While different in the characteristics of their constituent pulses, within margins of statistical significance, the five signals did not differ in the percentage of “appropriate” ratings: all were equally considered to be of appropriate detectability and moreover of high acceptability. The signals were found to be virtually independent from, and therefore highly robust relative to, sex, BMI, upper body adiposity, and skin impedance at the patch site, lending additional generalizability to the signals and hence their potential relevance to broad commercial application.
Acknowledgments
We thank Marleen Missotten and Anne Bogaers for their assistance in conducting the experiments, and Katherine Nelissen, Inge Smolders, and Piet Stinissen for their facilitation of the study.
Author contributions
All authors contributed to the overall study design. Jan De Geest and Wim De Geest designed the device. Jan De Geest developed the experimental setup. Karen MacDonald and Ivo Abraham analyzed the data and drafted the manuscript. Jan De Geest, Wim De Geest, and Elke De Troy revised the manuscript for scientific and technical content.
Disclosure
The authors report no conflicts of interest in this work.
References
World Health Organization. Adherence to Long-Term Therapies – Evidence for Action. Geneva: World Health Organization; 2003. | |
DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. | |
DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes. a meta-analysis. Med Care. 2002;40(9):794–811. | |
Prescription Drug Compliance a Significant Challenge for Many Patients, According to New National Survey [webpage on the Internet]. Harris Interactive; PR Newswire. Available from: http://www.prnewswire.com/news-releases/prescription-drug-compliance-a-significant-challenge-for-many-patients-according-to-new-national-survey-54370352.html. Accessed November 29, 2014. | |
De Geest W, De Geest J, De Geest S, Abraham I. Description, specifications, and ASIC configurations of MEMOPATCH, a transdermal pulse generator medical device to promote patient compliance with medication regimens. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:508–511. | |
Reilly JP. Electrical Stimulation and Electropathology. Cambridge: Cambridge University Press; 1992. | |
European Commission. Commission communication in the framework of the implementation of Council Directive 93/42/EEC concerning medical devices. Official Journal of the European Union. 2005;C 275(08/11/2005):0005–0012. | |
Blume-Peytavi U, Hillmann K, Guarrera M. Hair growth assessment techniques. In: Blume-Peytavi U, Tosti A, Whiting DA, Trüeb RM, editors. Hair Growth and Disorders. Berlin: Springer Berlin Heidelberg; 2008:125–157. | |
Kyle UG, Bosaeus I, De Lorenzo AD, et al; Composition of the ESPEN Working Group. Bioelectrical impedance analysis – part I: review of principles and methods. Clin Nutr. 2004;23(5):1226–1243. | |
Abraham I, De Geest W, De Geest J, De Troy E, MacDonald K. Detectability and appraisal thresholds of split pulse signals for the MemoPatch™ device, an electronic skin patch intended to deliver tactile medication reminder signals (study TS-104). Conf Proc IEEE Eng Med Biol Soc. 2013;2013:914–917. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.