Back to Journals » Clinical Ophthalmology » Volume 14
Demography, Risk Factors, and Clinical and Microbiological Features of Microbial Keratitis at a Tertiary Eye Hospital in Nepal
Authors Bajracharya L , Bade AR , Gurung R, Dhakhwa K
Received 20 June 2020
Accepted for publication 19 August 2020
Published 12 October 2020 Volume 2020:14 Pages 3219—3226
DOI https://doi.org/10.2147/OPTH.S266218
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Leena Bajracharya,1 Asta Ram Bade,2 Reeta Gurung,1 Kavita Dhakhwa1
1Department of Cornea, Tilganga Institute of Ophthalmology, Kathmandu, Nepal; 2Department of Microbiology, Tilganga Institute of Ophthalmology, Kathmandu, Nepal
Correspondence: Leena Bajracharya
Tilganga Institute of Ophthalmology, G.P.O Box: 561 Gaushala, Kathmandu, Nepal
Tel +977 1 4493775
Fax +977- 1- 4474937
Email [email protected]
Background: Infective keratitis is the most common corneal pathology in developing countries. Updated knowledge is needed for its control and proper management.
Methodology: All cases of presumed microbial keratitis that presented in an 18-month period from October 2013 to March 2015 were enrolled. Data collected were demographic profile, risk factors, clinical features, and organisms isolated and their sensitivities.
Results: A total of 602 cases of microbial keratitis were enrolled. Mean age of subjects (598 patients) was 47.9 years with 53.8% male. 64.1% worked in agriculture. 38.3% gave history of trauma followed by history of herpetic eye diseases (17.9%) and topical steroid use (14.2%). A total of 473 who were referred came at an average of 21.5 days of symptoms. 14.9% (n=90) of cases were either perforated or impending to perforate at presentation. 69.6% had infiltrate in the visual axis. A total of 516 (85.7%) underwent diagnostic corneal culture. A total of 256 (49.6%) yielded a positive result. Pure bacterial growth was seen in 111 (43.4%), pure fungal growth in 138 (53.9%), and mixed microbial growth was present in 7 (2.7%) cases. Out of 121 bacterial isolates, 95.0% were Gram positive. Streptococcus pneumoniae (45.5%, n=55) was the most common bacterial isolate followed by Staphylococcus aureus (20.6%, n=25). Out of 145 fungal isolates, Aspergillus and Fusarium species were found in equal numbers (n=41, 28.3% each). Over 85% of Gram-positive organisms isolated in the study were sensitive to vancomycin, cefazolin, moxifloxacin, and gatifloxacin. Over 80% of Gram-negative organisms were sensitive to gentamicin, tobramycin, and amikacin.
Conclusion: Microbial keratitis and associated risk factors occurring in farmers implies a lack of awareness and prevention programs. Delay in reaching tertiary care is resulting in complicated cases. Training of local health workers for prophylaxis, updated guidelines for treating keratitis, and timely referral to higher centers are all important in a chain to decrease the incidence of microbial keratitis.
Key words: microbial keratitis, infective keratitis, corneal ulcer
Introduction
Diseases affecting the cornea are the major cause of blindness in developing countries and infective keratitis is the main cause for this.1 According to the Nepal Blindness survey (1981), corneal pathology accounted for 9% of total blindness, which was second to cataract.2 Factors like lack of awareness and preventive measures, delay in treatment, inaccessibility of eye care, improper treatment, and resistant microorganisms all contribute to persistence of infective keratitis in the community.
The burden of corneal ulceration, risk factors, microorganisms, and susceptible antibiotics vary from region to region and also vary with time. So, an update of the information is important especially for a tertiary eye care hospital which treats a huge number of infective corneal ulcers. The current study is a large hospital-based study done for the same purpose. It is being conducted in Tilganga Institute of Ophthalmology (TIO), a tertiary eye care for subspecialty service, located in Kathmandu, Nepal. Comparison with past study of a similar kind is made for any differences in the findings. This study aims to find out the latest sensitivity pattern of the microorganisms to guide in the choice of antibiotics for doctors and paramedics involved in treating corneal ulcers.
Methodology
This is a descriptive, non-interventional type of study in which all consecutive patients visiting TIO with a clinical or laboratory diagnosis of presumed microbial keratitis from October 2013 to March 2015 (18-month period) were enrolled. Pure viral keratitis like dendritic, geographic, kerato-uveitis, and meta-herpetic ulcers are excluded. Viral keratitis and autoimmune keratitis were included only if they had features of secondary microbial infection. For each patient, a proforma was filled to collect data about demography, risk factors, duration of symptoms, characteristics of ulcer (infiltrate size, location, perforated, or impending to perforate), and microbiological profile. With some exceptions, all microbial keratitis had undergone diagnostic corneal scraping. In our institute, cases do not undergo scraping: 1) when ulcers are perforated or impending to perforate at presentation and if they are intended to manage conservatively ; 2) when infiltrate is deep in the cornea without epithelial defect; 3) in ulcers occurring in children and mentally challenging patients; and 4) when ulcer size is less than 2 mm. We do not stop the antimicrobials which patients were using prior to performing corneal scraping (microbial keratitis, infective keratitis, and corneal ulcer are used interchangeably in the text).
Laboratory Procedures
Specimen collection, inoculation, and identification of the organism had been done through standard protocol. Kimura’s spatula was used to obtain material from the leading edge and base of the ulcer. The specimen was inoculated directly onto blood agar, chocolate agar, Sabouraud dextrose agar (SDA), and thioglycollate broth. Separate glass slides were used for Gram stain, for Giemsa stain, and for potassium hydroxide (KOH) wet mount. For suspected amoebic corneal cases, the specimen was inoculated onto non-nutrient agar overlaid with Escherichia coli. All bacterial cultures were incubated aerobically at 37 °C. To ensure 3 to 5% carbon dioxide, incubated chocolate agar plates were put inside a candle jar. Cultures on blood agar, chocolate agar, and broth were evaluated daily up to 72 hours and then discarded if there was no growth. Fungal cultures in SDA were incubated at 25 °C and looked for any growth till 1 week and discarded thereafter. Similarly, cultures on non-nutrient agar overlaid with E. coli were discarded at 1 week if there was no growth. Microbial culture was considered significant if any/all of the following criteria were satisfied: 1) growth of the same organism was demonstrated on two or more media; 2) confluent growth was seen at the site of inoculation on one solid medium; 3) growth of one medium was consistent with direct microscopy findings (appropriate staining and morphology with Gram stain); and 4) the same organism was grown from repeated scraping material. Identification of bacteria was based on microscopic morphology, staining characteristics, and biochemical properties. Antimicrobial susceptibility testing was performed using the agar disk diffusion technique according to Clinical Laboratory Standard Institute (CLSI) guidelines using S. aureus (ATCC 25923), E. coli (ATCC 25922), and P. aeruginosa (ATCC 27853) as reference strains. Fungi were identified by their colony characteristics on SDA and by their microscopic appearance in lactophenol cotton blue. Using the culture method as gold standard, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of Gram stain and KOH wet mount were determined for bacteria and fungus respectively.
Ethical Consideration
Approval for the study was taken from the Institutional review board of Tilganga Institute of Ophthalmology. Informed consent was taken from all patients and the study complied with the Declaration of Helsinki. For patients aged less than 18 years, a parent or legal guardian provided informed consent on the patient’s behalf.
Results
A total of 602 new corneal ulcer cases in 598 patients were registered in the study period of 18 months (average of 33.4 new cases per month). Two patients had bilateral infiltrate and two patients had infection in the same eye twice. Average age of patients was 47.9 years (Figure 1) with 53.8% male patients. The majority of the subjects (386, 64.1%) were engaged in agriculture, followed by business (73, 12.1%), housewife (43, 7.1%), service (40, 6.6%), and students (19, 3.0%). Overall, 332 (55.1%) were illiterate, 81 (13.5%) were just literate, 14.9% had primary or secondary level education, and 9.6% had high school or above level of education. 41 (6.8%) subjects were children aged ≤15 years. (Education and occupation were not taken into account for the pediatric age group.)
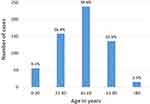 |
Figure 1 Age distribution of the subjects with microbial keratitis. |
A total of 477 (79.2%) cases were from rural areas, 87 (14.4%) from cities, and 38 (6.3%) from the neighboring country India. Microbial keratitis cases presented at TIO throughout the year but slightly more in winter (Figure 2)
 |
Figure 2 Distribution of cases of microbial keratitis with respect to seasons of the year. |
53.1% of 602 ulcers were first treated by either a trained eye health worker in primary eye care or by an ophthalmologist in an eye hospital (including TIO); 33.8% got treatment from pharmacy shop, 6.1% from general health post, and 3.6% were seen by traditional healers at first hand. Overall, people had sought medical help at an average of 5.6 days of onset of symptoms, but those who were referred to TIO (n=473) came at an average of 21.5 days.
69.6% of 602 ulcers had infiltrate partly or fully covering the visual axis (central 4 mm of the cornea). 67% of the eyes with ulcer had best corrected vision of less than 3/60. 26% (n=156) of cases had infiltrate size more than one quadrant of the corneal surface. 278 (46.1%) had hypopyon. 90 cases (14.9%) were either already perforated (n=57, 9.4%) or impending to perforate (n=33, 5.5%), most of which would necessitate emergency surgical intervention. In total, there were 11 cases of endophthalmitis associated with microbial keratitis.
A total of 459 (76.2%) had at least one risk factor for corneal ulcer. History of corneal trauma was present in 231 (38.3%) of ulcers (Table 1). 88.3% (n=204) of trauma was due to organic matter.
 |
Table 1 Risk Factors of Microbial Keratitis |
Among total cases, 516 (85.7%) had undergone diagnostic corneal culture. 14.3% did not undergo corneal culture for reasons as mentioned in “Methodology”. 49.6% (256 out of 516) of culture showed microbial growth. Pure bacterial growth was seen in 111 (43.4%) cases, pure fungal growth in 138 (53.9%) cases, and mixed microbial growth was present in 7 (2.7%) cases (Table 2). A total of 266 microorganisms (121 bacterial and 145 fungal) were isolated from 256 culture-positive cases (Tables 3 and 4).
 |
Table 2 Microbial Growth Pattern in Culture-Positive Cases |
 |
Table 3 Bacterial Isolates |
 |
Table 4 Fungal Isolates |
Out of 121 bacterial isolates, 115 (95.0%) were Gram-positive bacteria (GPB) and 6 isolates were Gram-negative bacteria (GNB) (Table 3). The most common bacterial isolate was Streptococcus pneumoniae (45.5%, n=55), followed by Staphylococcus aureus (20.6% n=25) and Streptococcus viridians (11.6%, n=14).
Out of 145 fungal isolates, 99.3% were filamentous type. Aspergillus and Fusarium species were found in equal numbers, each isolated in 41 cases (28.3%), followed by Curvularia species (12.4%) (Table 4). There was only one case of yeast (Candida albicans) and no cases of Acanthamoeba.
The sensitivity of Gram stain in the detection of bacteria was 82.2% and its specificity was 96.5%. Similarly, for KOH wet mount preparation they were 80.0% and 93.5% respectively in the detection of fungus (Table 5). PPV and NPV of Gram stain and KOH were as shown in Table 5. The antimicrobial sensitivity test for all bacterial isolates is shown in Tables 6 and 7.
 |
Table 5 Correlation Between Direct Microscopic (Gram Stain and KOH Wet Mount) Findings and Culture-Based Diagnosis |
 |
Table 6 Antimicrobial Susceptibility Test: Percentage of Gram-Positive Bacteria Sensitive to the Listed Antibiotic |
 |
Table 7 Antimicrobial Susceptibility Test: Percentage of Gram-Negative Bacteria Sensitive to the Listed Antibiotic |
Discussions
In our study, mean age of patients was 47.9 years which corresponds to the working age group (Figure 1). This was similar to the average age, 40 to 55 years, reported by studies within Nepal and neighboring countries. 3–6 Developed countries had a bimodal age distribution in the incidence of microbial keratitis; first peak in young adults and second peak in the old age group.7–9 This was because of use of contact lenses by young people.7–9 Like in our study, other studies had also shown a male predominance of 52 to 64%. 3–6,10 This could be because males are involved in outdoor or labor-based jobs. Female predominance was seen in Hong Kong8 and the ratio of males and females was equal in France. 9 This was because of more females using contact lenses.8,9 In our study, the majority of patients were farmers (64.1%) compared to 37 to 56% in Bangladesh and South India.3,11 55.1 % of our subjects were illiterate, correlating with the occupation. Like in our study, Sitoula et al5 also found a higher number of keratitis in winter which is the harvesting season of crops.
43.5% of our patients had their initial treatment from people (pharmacist, general nurse, or traditional health worker) who are not trained primary eye caretakers. A significant number (33.8%) presented to the pharmacy in the beginning. This might have been due to convenience, proximity to location of injury or patient’s home, lack of eye care facility, or expected cost or waiting time in eye hospital. Late presentation in tertiary care hospital (average 21.5 days) was the reason why certain ulcers are larger and complicated. In other eye hospitals of Nepal, 76 to 82% of cases had presented after 7 days.5,13 In South India, 60% of ulcer patients presented to an ophthalmologist within 7 days which was a better situation there .3 In France, ulcers due to contact lens and trauma cases had consultation done at an average of 48 hours.9 In our study, perforation was seen in 9.4% at presentation. Lavaju et al12 reported perforated ulcers in 11.3% in their study done in Nepal. Bourcier et al9 mentioned perforation in 0.6% in France.
In the present study, 38.3% gave history of ocular trauma, most of which were from vegetative matter (Table 1). Other studies in Nepal reported trauma in 60 to 82.7%.13,14 In South India and Thailand, trauma was reported as 65% and 43.9% respectively.3,15 In developed countries, trauma accounted for 10 to 16% as a risk factor.7–9,16 In our study, 17.9% of the patients had underlying herpetic eye disease (HED). HED can predispose to secondary microbial infection through decreased sensation, neurotrophic ulcer, dry eye, presence of epithelial defect like dendrite, and topical use of steroids for treatment.17 Sometimes HED with stromal necrosis can mimic microbial keratitis.17 In our study, HED as a risk factor was solely on clinical grounds with past history of recurrent keratitis, significant decrease in corneal sensation, or from past documents.18 Polymerase chain reaction (PCR) test not being done was the limiting factor in our study. Studies from South India and Hong Kong reported HED as a risk factor in 2 to 7%.3,8 There had been mentioning of OSD (which included dry eyes, HED, and others) as a risk factor in 18 to 20% of microbial keratitis in Japan, France, and Australia .7,9,16 In another study in Sydney, HED accounted for 17.4% as a risk factor, of which 7.9% showed a positive herpes simplex viral PCR test.19 There had never been mention of HED disease as a risk factor in any studies in Nepal. Prevalence of herpes simplex virus (HSV) is all over the world and the HSV seropositivity could be as high as 67 to 90% in the population.18 In Nepal, HED as a risk factor was probably underevaluated in previous studies or herpes simplex stromal keratitis might have been incorporated into the microbial keratitis group due to mimicry. In our study, 14.6% of patients were using topical steroids during presentation. The drug had been advised in the local medical shop or had been prescribed in the past for conditions like HED, post-operative keratoplasty, and vernal keratoconjunctivitis. In the studies in India, use of steroid ranged from 7 to 19%.3,4,20 A report5 from Nepal mentioned 2%.
Sitoula et al5 and Puri and Shrestha13 reported 65 to 76.1% cases with vision less than 3/60 which is similar to our study. Our Gram stain and KOH stain sensitivity and specificity were as shown in Table 5. From other reports, the range of sensitivity of Gram stain had been 50 to 100% and for specificity from 77 to 97.6%.4,7,21,22 Similarly for KOH, the reported range of sensitivity had been from 54 to 99% and specificity from 94 to 99%.4,7,21,22 The PPV and NPV for bacteria were greater in our study than those reported by Rai et al22 but for fungus both values were less compared to the same study (Table 5). Bharathi et al4 reported PPV and NPV of more than 97% for bacterial as well as fungus.
In this study, culture was positive in 49.6% of scrapings. In various studies, the rate of positive growth had been 32 to 76%.5,8,9,23 Our study showed slightly more fungus (53.9%) than bacteria. In most studies from India and Nepal the proportion of fungus was usually higher than bacteria (62 to 70%), 3,5,20 but fungal ulcers were quite low in developed countries (6 to 17%).8,16,23 In the present study, Streptococcus pneumoniae (45.5%) was the most common bacteria isolated(Table 3). In Nepal, some studies had reported Streptococcus pneumoniae whereas others reported Staphylococcus aureus or Staphylococcus epidermidis as the predominant bacteria.6,10,14,24 In neighboring countries also, these bacterial isolates were obtained as the commonest.3,11,20,21 But in Australia,7 Taiwan,23 and Thailand,15 Pseudomonas were the most common cause of bacterial keratitis due to contact lenses being the main risk factor. In our study, Aspergillus and Fusarium were found in equal proportion, 28.3% each (Table 4). A decade back, Aspergillus closely followed by Fusarium were the commonest fungi isolated in TIO.6 Aspergillus was also the commonest fungus in Eastern Nepal10 and Bangladesh.11 But in Western Nepal and South India, Fusarium was the commonest.3,24
In our study, vancomycin, gatifloxacin, and cefazoline had >90% sensitivity towards GPB. It also showed that moxifloxacin and gatifloxacin were more potent than other fluoroquinolones for Streptococcus and Staphylococcus(Table 6). Fluoroquinolones were found potent for Pseudomonas (100%), but not for Moraxella (33.3%) (Table 7). In our past study, sensitivities of gentamycin, tobramycin and amikacin to GNB were 40%, 67% and 100% respectively but the current study showed sensitivites of gentamicin and tobramycin to GNB as 100% and that of amikacin as 83.3%.6 Difference in sensitivities in the two studies cannot be inferred due to small number of GNB in past as well as present studies. 6
Conclusion and Recommendation
Persistence of the disease in the past through the present indicates inadequate prevention programs. It has been important to train the first line of defense – the community eye workers and pharmacists who see these patients after their initial trauma – to make sure the patients get correct prophylactic treatment. Focus should be on geographic regions from where many keratitis cases are referred. This study will guide selection of antimicrobials. Facility for culture of corneal specimens should be set up in secondary eye care centers, which should make timely referral for difficult cases. Emphasis should be given for proper management and adequate counseling for patients with recurrent viral keratitis and other conditions needing long-term treatment with topical steroids. These measures would decrease the incidence of microbial keratitis as well the need for more costly keratoplasty which requires long-term follow-up care.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Whitcher JP, Srinivasan M. Corneal ulceration in the developing world – a silent epidemic. Br J Ophthalmol. 1997;81:622–623. doi:10.1136/bjo.81.8.622
2. Brilliant LB, Pokhrel RP, Grasset NC, et al. Epidemiology of blindness in Nepal. Bull World Health Organ. 1985;63:375–386.
3. Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81:965–971. doi:10.1136/bjo.81.11.965
4. Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi P. Aetiological diagnosis of microbial keratitis in South India- a study of 1618 cases. Indian J Med Microbiol. 2002;20:19–24.
5. Sitoula R, Singh S, Mahaseth V, Sharma A, Labh R. Epidemiology and etiological diagnosis of infective keratitis in eastern region of Nepal. Nepal J Ophthalmol. 2015;7:10–15. doi:10.3126/nepjoph.v7i1.13146
6. Feilmeier MR, Sivaraman KR, Oliva M, Tabin GC, Gurung R. Etiologic diagnosis of corneal ulceration at a tertiary eye center in Kathmandu, Nepal. Cornea. 2010;29:1380–1385. doi:10.1097/ICO.0b013e3181d92881
7. Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–27. doi:10.1097/ICO.0b013e318156caf2
8. Ng AL, To KK, Choi CC, et al. Predisposing Factors, Microbial Characteristics, and Clinical Outcome of Microbial Keratitis in a Tertiary Centre in Hong Kong: a 10-Year Experience. J Ophthalmol. 2015;2015:769436. doi:10.1155/2015/769436.
9. Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834–838. doi:10.1136/bjo.87.7.834
10. Amatya R, Shrestha S, Khanal B, et al. Etiological agents of corneal ulcer: five years prospective study in eastern Nepal. Nepal Med Coll J. 2012;14:219–222.
11. Ahmed S, Ghosh A, Hassan SA, Tarafder S, Ruhul Amin Miah M. Predisposing Factors and Aetiologic Diagnosis of Infectious Corneal Ulcer. Bangladesh J Med Microbiol. 2010;04:28–31. doi:10.3329/bjmm.v4i1.8466
12. Lavaju P, Arya SK, Khanal B, Amatya R, Patel S. Demographic pattern, clinical features and treatment outcome of patients with infective keratitis in the eastern region of Nepal. Nepal J Ophthalmol. 2009;1:101–106. doi:10.3126/nepjoph.v1i2.3683
13. Puri LR, Shrestha G. Microbial keratitis: a five years retrospective clinical study in tertiary eye hospital of eastern region of Nepal. J Kathmandu Med Coll. 2015;4:118–125. doi:10.3126/jkmc.v4i4.18252
14. Upadhyay MP, Karmacharya PC, Koirala S, et al. Epidemiologic characteristics, predisposing factors, and etiologic diagnosis of corneal ulceration in Nepal. Am J Ophthalmol. 1991;111:92–99. doi:10.1016/S0002-9394(14)76903-X
15. Tananuvat N, Punyakhum O, Ausayakhun S, Chaidaroon W. Etiology and clinical outcomes of microbial keratitis at a tertiary eye-care center in northern Thailand. J Med Assoc Thailand. 2012;95:S8– S17.
16. Toshida H, Kogure N, Inoue N, Murakami A. Trends in microbial keratitis in Japan. Eye Contact Lens. 2007;33:70–73. doi:10.1097/01.icl.0000237825.98225.ca
17. Suresh PS, Tullo AB. Herpes Simplex Keratitis. Indian J Ophthalmol. 1999;47:155–165.
18. Harris KD. Herpes Simplex Virus Keratitis. Home Healthc Now. 2019;37:281–284. doi:10.1097/NHH.0000000000000791
19. Butler TK, Spencer NA, Chan CC, Singh Gilhotra J, McClellan K. Infective keratitis in older patients: a 4 year review, 1998-2002. Br J Ophthalmol. 2005;89:591–596. doi:10.1136/bjo.2004.049072
20. Basak SK, Basak S, Mohanta A, Bhowmick A. Epidemiological and microbiological diagnosis of suppurative keratitis in Gangetic West Bengal, Eastern India. Indian J Ophthalmol. 2005;53:17–22. doi:10.4103/0301-4738.15280
21. Tewari A, Sood N, Vegad MM, Mehta DC. Epidemiological and microbiological profile of infective keratitis in Ahmedabad. Indian J Ophthalmol. 2012;60:267–272. doi:10.4103/0301-4738.98702
22. Rai PG, Chaudhary M, Sharma AK, Gautam V. Direct microscopy in suppurative keratitis: a report from tertiary level hospital in Nepal. Nepal J Ophthalmol. 2016;8:128–138. doi:10.3126/nepjoph.v8i2.16993
23. Lin TY, Yeh LK, Ma DH, et al. Risk Factors and Microbiological Features of Patients Hospitalized for Microbial Keratitis: a 10-Year Study in a Referral Center in Taiwan. Medicine (Baltimore). 2015;94:e1905. doi:10.1097/MD.0000000000001905
24. Dhakhwa K, Sharma MK, Bajimaya S, Dwivedi AK, Rai S. Nepal .Causative organisms in microbial keratitis, their sensitivity pattern and treatment outcome in western Nepal. Nepal J Ophthalmol. 2012;4:119–127. doi:10.3126/nepjoph.v4i1.5863
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
