Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 14
CYP2C9*3/*3 Gene Expression Affects the Total and Free Concentrations of Valproic Acid in Pediatric Patients with Epilepsy
Authors Wu X, Dong W, Li H, Yang X, Jin Y, Zhang Z, Jiang Y
Received 14 January 2021
Accepted for publication 16 March 2021
Published 9 April 2021 Volume 2021:14 Pages 417—430
DOI https://doi.org/10.2147/PGPM.S301893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Xikun Wu,1 Weichong Dong,1 Haoran Li,1 Xiuling Yang,1 Yiran Jin,1 Zhiqing Zhang,1 Ye Jiang2
1The Second Hospital of Hebei Medical University, Shijiazhuang City, People’s Republic of China; 2Pharmacy College, Hebei Medical University, Shijiazhuang City, People’s Republic of China
Correspondence: Zhiqing Zhang
The Second Hospital of Hebei Medical University, Department of Pharmacy, 215 Hepingxi Road, Shijiazhuang City, 050000, People’s Republic of China
Email [email protected]
Ye Jiang
Pharmacy College, Hebei Medical University, 361 Zhangshandong Road, Shijiazhuang City, 050017, People’s Republic of China
Email [email protected]
Purpose: To perform therapeutic drug monitoring (TDM) of total and free plasma valproic acid (VPA) concentrations in pediatric patients with epilepsy and to analyze related factors.
Patients and Methods: Pediatric epileptic patients treated in 2015– 2019 in our hospital were assessed. Total and free plasma VPA concentrations were obtained by UPLC and LC-MS/MS, respectively. Regression analysis was performed to examine the associations of free plasma VPA with total plasma VPA and plasma protein binding rate. The impacts of individual situation, CYP2C9 genotype, and drug combination on VPA concentration were examined.
Results: Of the 251 patients, 81 had lower total concentrations than effective therapeutic levels; 86 and 31 patients had infections and central nervous system dysplasia, respectively. VPA’s daily doses and free drug concentrations were significantly lower in the CYP2C9 *3/*3 genotype group versus the CYP2C9 *1/*3 and CYP2C9 *1/*1 groups (P< 0.05). Free and total VPA concentrations were linked by Y = 0.0004 X2 + 0.042 X + 0.3035 (r=0.6981); VPA plasma protein binding rate and free VPA concentration were related by Y = 0.0003 X2 - 0.0127 X + 0.9777 (r=0.8136). Both total and free VPA concentrations were significantly decreased in patients simultaneously administered phenobarbital, meropenem and biapenem (P< 0.05), with therapeutic failure after meropenem/biapenem co-administration.
Conclusion: Free VPA amounts have nonlinear relationships with total VPA amounts and plasma protein binding rate in epileptic children. Additionally, CYP2C9 *3/*3 expression affects VPA metabolism. Since phenobarbital affects VPA metabolism, TDM is recommended. Meanwhile, carbapenem-co-administration with VPA should be prohibited.
Keywords: valproic acid, pediatric patient, free concentration, CYP2C9 gene expression, TDM
Plain Language Summary
What is the current knowledge on the topic?
Valproic acid (VPA) is widely used in pediatric patients with epilepsy. Its pharmacokinetics shows obvious individual differences, and TDM is recommended during treatment.
What question did this study address?
This work quantitatively characterized the relationship between total and free VPA concentrations, analyzed the effect of drug metabolic genotype on total and free VPA concentrations in pediatric patients with epilepsy.
What does this study add to our knowledge?
The relationship between free amounts and total VPA concentration or plasma protein binding rate can be characterized with quadratic equations. CYP2C9 *3/*3 gene expression affects the total and free concentrations of VPA.
How might this change clinical pharmacology or translational science?
TDM of free VPA concentration may contribute to improving VPA therapy in pediatric patients. Additionally, dosage adjustment can be recommended according to CYP2C9 gene expression in patients during treatment.
Introduction
Valproic acid (VPA) is a broad-spectrum antiepileptic drug. It can be administered either alone or in combination with other antiepileptic drugs. The effective therapeutic concentrations of VPA range from 50 to 100µg/mL,1–3 while higher plasma levels may increase the risk of adverse effects.4,5 Therefore, therapeutic drug monitoring (TDM) is highly recommended during treatment with VPA. According to previous reports, the plasma protein binding rate of VPA is approximately 90%-95%,3,6 which is consistent with nonlinear dynamics as there is no linear relationship between VPA’s total and free concentrations.7–9 As drugs are biologically active in the unbound status, it is necessary to perform TDM of free VPA concentration.
Meanwhile, hepatic microsomal cytochrome oxidase polymorphisms may affect the metabolic rates and levels of substrate drugs, and drug concentrations differ among patients with distinct genotypes. Although other VPA metabolizing pathways such as microsomal glucuronide conjugation and mitochondrial beta-oxidation might also contribute to the heterogeneity of VPA-PK, VPA is metabolized by hepatic enzymes, including CYP2C9, CYP2C19, CYP2A6 and CYP2B6, whose activities influence the pharmacokinetics of VPA.10–12 Genome encoded CYP2C9 is located on the 10th chromosome in humans and highly polymorphic, which could affect CYP2C9 activity, alter the rate of VPA metabolism and impact its efficacy and safety profiles. The wild type allele of CYP2C9 is termed CYP2C9*1; CYP2C9*3, a mutant allele, is found in 10% of Asians. Patients with the CYP2C9*1 genotype have normal metabolic rate, while CYP2C9*3 mutations may lead to a decrease in encoded protein activities, alongside delayed VPA metabolism, which results in increased risk of dose-dependent adverse drug reactions.13–15 In addition, CYP2C9*3 is possibly associated with increased 4-ene-VPA, a hepatotoxic metabolic product.16 Thus, classifying CYP2C9 genotypes in patients with epilepsy may alter the efficacy and safety profiles of VPA depending on genes encoding pharmacodynamic and pharmacokinetic elements, which could be beneficial in initiating dose adjustment17,18.
In this study, 251 pediatric patients administered VPA were enrolled in TDM for total and free VPA concentrations, assessing the relationships between the two parameters. Further analysis of factors (drug dose, disease type, drug metabolism genotype, drug combination, etc.) affecting VPA concentration was performed.
Patients and Methods
Chemicals and Instruments
The chemicals were purchased as follows: VPA standard (Lot 100963–201101, National Institute for Food and Drug Control, ≥ 99.0%); α-bromoacetophenone (LA50L09, J&K Scientific, Switzerland); ammonium acetate (Lot G1319026, Aladdin, Shanghai, ≥ 99.0%); methanol (Lot 178338, Fisher, USA); acetonitrile (Lot G1319026, Fisher, USA). Blank human plasma was provided by The Second Hospital of Hebei Medical University. Triethylamine, sulfuric acid, and other reagents were of analytical grade.
UPLC was performed on a Waters UPLC H-CLASS (Waters, USA) equipped with a quaternary pump, an online degasser, an autosampler, a column heater, a UV detector, and the Empower 3 workstation, on an ACQUITY UPLC BEH C18 column (2.1 mm×50 mm, 1.7 µm; Waters, USA). The LC-MS/MS system comprised an LC-20AD HPLC system (Shimadzu, Japan), an API 4000+ mass spectrometer detector (AB Sciex, USA), the Analyst 1.6 data processing system (AB Sciex, USA), and a Symmetry® C18 column (2.1 mm×100 mm, 3.5 µm; Waters, USA). A high-speed centrifuge (radius 50 mm; Abbott, USA), an electronic analytical balance (XW-80A, Shanghai Medical University Instrument Factory), a low-temperature fridge (MDF-U2086S, Sanyo, Japan), an electronic balance (HZF-B1200/0.1 g, Huazhi, USA), PVC hollow fiber ultrafiltration membranes (internal diameter of 1.0 mm and wall thickness of 0.2 mm, retaining molecular weight 15000 Da and above; Shanghai Lanjing Membrane Technology Engineering Co., Ltd) were also used.
Clinical Data
Pediatric patients diagnosed with epilepsy between January 2015 and December 2019 in our hospital were included in this study. Inclusion criteria were: ① diagnosis of epilepsy, including generalized epilepsy (absence seizure, myoclonic seizure, atonic seizure, mixed seizure, special type syndrome, status epileptics, etc.), partial seizure (local epilepsy, with or without complete seizure, etc.); ② age ≤ 14 years; ③ single or combined treatment with oral VPA; ④ clinical VPA TDM recommended. Exclusion criteria were: ① continuous treatment with VPA lasting less than 72 h; ② failure to obey the doctor’s advice on VPA treatment within 72 h before TDM.
The study protocol was approved by the Ethics Committee of The Second Hospital of Hebei Medical University (2017-R015) and was conducted in accordance with the Declaration of Helsinki. A written legal guardian informed consent was taken from each participant.
Methods
All samples from patients were acquired for though concentration (before next dose after at least 3 days of VPA therapy). Total plasma VPA concentration was assessed by the pre-column derivation UPLC method. Free VPA concentration was detected by LC-MS/MS after hollow fiber centrifugal ultrafiltration (HFCF-UF). All these methods were modified according to our previous studies.19,20 The CYP2C9 allele was detected by the fluorescence in situ hybridization (FISH) technology.
Total VPA Concentration Determination
About 200 μL of plasma spiked with 200 μL internal standard solution (128 μg/mL of cyclohexane carboxylic acid) was added to 200 μL sulfuric acid (1 M) for acidification. Samples were then placed into a vortex mixer for extraction with 4 mL N-hexane for 2 min. After a 5 min centrifugation at 6 ×103 g, the supernatant was used for derivatization. Then, 3 mL of supernatant spiked with 20 μL α-bromoacetophenone (25 μg/mL) and 20 μL triethylamine was added to a 50°C water bath for 15 min derivatization, followed by drying under nitrogen gas. The sample was redissolved with 500 μL mobile phase, and 1 µL was injected into the UPLC system for analysis.
For VPA separation, a BEH C18 column (50 mm × 2.1 mm, 1.7 μm) on a Waters Acquity UPLC H-Class was used. The mobile phase consisted of acetonitrile and water (80:20, V/V) at a flow rate of 0.2 mL/min. The detection wavelength was 254 nm, and the column temperature was maintained at 30°C. The injection volume was 1 μL.
Free VPA Concentration Determination
The HFCF-UF device is shown in Figure 1. Briefly, 400 µL of plasma was collected into a glass centrifuge tube. Then, a PVC hollow fiber tube was folded, immersed into the plasma sample, and capped. Next, the sample was centrifuged for 5 min at 1224 g; the ultrafiltrate was collected inside the hollow fiber tube, and 10 µL thereof was injected into the LC-MS/MS system for analysis.
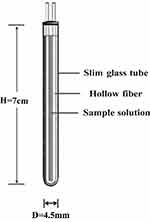 |
Figure 1 Hollow fiber centrifugal ultrafiltration device (HFCF-UF). |
The following chromatographic conditions were used. A Symmetry® C18 column (2.1 mm×100 mm, 3.5 µm) was selected, with the column temperature maintained at 40°C. The mobile phase consisted of 0.05 mg/mL sodium acetate and acetonitrile (40:60 V/V) at a flow rate of 1 mL/min. The injected volume was 10 µL.
Mass spectrometry was performed under the following conditions: ion source, electron spray ionization (ESI) in the negative ion mode; declustering potential (DP), 70 V; collision energy (CE), 10 eV. VPA was detected by selected ion monitoring mode (m/z: 142.9).
Patient Data Acquisition
From the hospital information system (HIS), inpatient data, including name, gender, age, times of admission and discharge, clinical diagnosis, height and body weight, were retrieved according to admission number. VPA dosage, drug combination and drug metabolism-gene test results were retrieved via the electronic medical record system. Laboratory test results for total protein, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, total bilirubin, direct bilirubin and indirect bilirubin, among others, were retrieved through the laboratory information system (LIS). All the collected data were input into the database and statistically analyzed.
Data Processing
Research data were expressed as arithmetic mean and standard deviation (mean ± SD). The SPSS 21.0 software (IBM, USA) was used for statistical analysis. P<0.05 was considered statistically significant (power above 80%). Continuous data with normal distribution were assessed by the t-test. Categorical data were compared by the χ2 test. The correlation between two variables with continuous independent data (free and total VPA concentration, and free VPA concentration and plasma protein binding rate) was evaluated by linear-regression analysis.
Results
Demographic Characteristics of Pediatric Patients with Epilepsy
Of the 251 pediatric patients involved in VPA TDM, 176 were male (70.12%) and 75 were female (29.88%); the observed gender difference was statistically significant. Other demographic characteristics are shown in Table 1.
 |
Table 1 Demographic Characteristics of 251 Pediatric Patients with Epilepsy |
TDM of Total and Free VPA Concentrations
The standard equation for total plasma VPA concentration detected by UPLC was Y = 0.0092X - 0.0051 (r2 = 0.9993), with a linear range of 5–150 µg/mL. The regression equation for free VPA concentration was Y = 2013459.6 X + 116740.6 (r2 = 0.9999), with a linear range of 0.2–20 µg/mL.
TDM results of the 251 patients are shown in Table 2. A total of 150 cases (59.76%) had values within the effective therapeutic range (50–100 µg/mL); 81 cases (32.37%) had concentrations lower than 50 µg/mL, and 20 (7.97%) had amounts higher than 100 µg/mL. In addition, 154 cases (61.35%) received doses in line with the recommended range (20–30 mg/kg), while 74 (29.48%) and 23 (9.16%) patients received doses below 20 mg/kg and above 30 mg/kg, respectively.
 |
Table 2 TDM Results of 251 Pediatric Patients with Epilepsy |
Clinical Diagnosis
The clinical diagnoses of the pediatric patients with epilepsy are listed in Table 3. Infectious diseases were found in 114 cases (45.42%), and no significant differences were observed in laboratory test results and basic features between the infection and non-infection groups. Neurodevelopmental disorders were found in 37 cases (14.74%). Analysis of basic information in pediatric patients according to the presence or absence of neurodevelopmental disorders was summarized. BMI in pediatric epileptic patients with neurodevelopmental disorders was obviously lower compared with the control value (15.86 ± 2.62 kg/m2 vs 16.91 ± 3.00 kg/m2, P=0.033). There were no significant differences between the two groups in terms of VPA’s daily dose and total concentration; however, mean free VPA concentration in pediatric epileptic patients with neurodevelopment disorders was only 78.41% that of the control group (3.92 ± 2.57µg/mL vs 4.99 ± 3.49µg/mL), indicating a statistically significant difference (P=0.049).
 |
Table 3 Clinical Diagnoses of 251 Pediatric Patients with Epilepsy |
Effects of CYP2C9 Gene Expression on VPA Concentration and Plasma Protein Binding Rate
Three CYP2C9 genotypes are involved in VPA metabolism, including CYP2C9 *1/*1, CYP2C9 *1/*3 and CYP2C9 *3/*3. Of the 112/251 pediatric patients who underwent the CYP2C9 genotype test, 91 had the CYP2C9 *1/*1 genotype; 15 and only 6 had the CYP2C9 *1/*3 and CYP2C9 *3/*3 genotypes, respectively.
The patients were grouped by metabolic genotype, and daily drug dose, total and free VPA concentrations, and plasma protein binding rate were analyzed. There were no significant differences in total VPA concentrations among the three groups. In the CYP2C9 *3/*3 group, the daily dose was lower than that of the CYP2C9 *1/*3 (P=0.049) or CYP2C9 *1/*1 (P=0.001) group. Free VPA concentration was lower in the CYP2C9 *3/*3 group compared with the CYP2C9 *1/*1 group (P=0.019); meanwhile, plasma protein binding rate was higher in the CYP2C9 *3/*3 group compared with the CYP2C9 *1/*3 (P=0.016) and CYP2C9 *1/*1 (P=0.002) groups. There were no significant differences between the CYP2C9 *1/*3 and CYP2C9 *1/*1 groups in the above parameters. These results are shown in Table 4.
 |
Table 4 VPA Levels and Plasma Protein Binding Rates in Pediatric Patients with Different CYP2C9 Genotypes |
The recommended VPA’s daily dose is 20–30 mg/kg. The daily doses in the 3 groups of patients with different metabolic genotypes were analyzed based on actual and recommended dose conditions. Our results revealed significant differences among the 3 pediatric patient groups whose doses deviated from the recommended value. Mean daily dose in the CYP2C9 *3/*3 group was 16.12 ± 2.34 mg/kg, and all 6 cases had doses lower than the recommended range; 67 out of 91 cases (73.63%) in the CYP2C9 *1/*1 group met the reference dose requirements, while 15 and 9 cases had insufficient and excessive doses, respectively. No significant differences in total VPA concentration deviation under the above-specified drug doses were found among the three groups (Table 5).
 |
Table 5 Deviation Cases Regarding Daily Dose and VPA Levels in Different Metabolic Genotypes |
Association of Free VPA Concentration with Plasma Protein Binding Rate
Based on TDM results of the 251 included pediatric epilepsy patients, a regression equation of free VPA concentration (Y) and total VPA concentration (X) was determined as Y = 0.0004 X2 + 0.042 X + 0.3035 (r = 0.6981). Meanwhile, plasma protein binding rate (Y) and free VPA concentration (X) were bound by the equation Y = 0.0003 X2 - 0.0127 X + 0.9777 (r = 0.8136). These results are shown in Figures 2 and 3, respectively.
 |
Figure 2 Correlation of free VPA concentration and total VPA concentration. |
 |
Figure 3 Correlation of free VPA concentration and plasma protein binding rate. |
Combined Medications
A total of 118 out of 251 pediatric inpatients with epilepsy (47.01%) were treated with VPA monotherapy; meanwhile, 58 (23.11%), 44 (17.53%) and 31 (12.35%) patients were simultaneously administered two, three and four 4 or more antiepileptic drugs, respectively. Compared with the VPA monotherapy group, the combined medication group showed lower total protein in the patient plasma (P=0.046; Table 6).
 |
Table 6 Treatment Summary of the Monotherapy and Combined Medication Groups |
Totally 40 combined medication cases were treated by oral, intramuscular, or intravenous administration of phenobarbital. There was no significant difference in daily doses between the VPA monotherapy and combined medication groups. In the combined medication group, total and free VPA concentrations were lower, representing only 87.14% and 68.31% of those found in the VPA monotherapy group, respectively; the differences were statistically significant (P=0.046 and P=0.006). Combined medication resulted in changes of liver function: ALT and AST amounts in the combined medication group were 110.84% and 121.72% those found in the monotherapy group, respectively, indicating a statistically significant difference (P=0.049) in AST. These results are shown in Table 7.
 |
Table 7 Effects of Phenobarbital and Carbapenem Co-Administration on VPA Concentrations and Liver Function |
Six pediatric patients were treated with carbapenems due to serious infections such as severe pneumonia, upper respiratory tract infection and sepsis, including meropenem (5 cases) and biapenem (1 case). Daily doses of carbapenem in the combined medication groups were similar to those of the VPA monotherapy group, whereas total and free VPA concentration dramatically dropped to only 25.03% (P=0.000) and 27.69% (P=0.038) of those found in the VPA monotherapy group, respectively.
Discussion
TDM Results
Epilepsy could be well controlled if VPA concentration fit the recommended range. A daily dose of 20–30 mg/kg is recommended to achieve effective VPA concentration. According to TDM results of the 251 pediatric patients with epilepsy, only 59.75% had levels within the effective therapeutic range (50–100 µg/mL). In contrast, VPA amounts in 32.27% of patients failed to reach 50 µg/mL, and epilepsy could not be effectively controlled. The main reason was the low administration dosage of VPA. Out of 251 patients, 154 (61.35%) met the recommended daily dose, while 74 had doses below the lower limit of the recommended dose (20 mg/kg). Clinicians in our hospital tend to select a lower dosage of VPA at the beginning of epilepsy treatment, and adjust the dosage based on TDM and clinical outcomes. Therefore, TDM plays an important role in this case.
Neurodevelopmental Disorders and Epilepsy
Epilepsy is a complex nervous system disease often accompanied by intellectual disability and developmental disorders. The risk of epilepsy is higher in pediatric patients with developmental disorders compared with the healthy pediatric population.21,22 In this study, 14.74% (37 of 251) of the pediatric patients with epilepsy had neurodevelopmental disorders, which was much higher than the incidence found in the healthy pediatric population. According to previous findings, excessive excitatory neurotransmitter activity might lead to epilepsy, and a specific type of epilepsy might be related to developmental disorders.23 Copy number variation (CNV), especially microdeletion, is an essential factor in familial epilepsy. A previous study revealed a new rare gene microdeletion as an important pathogenetic mechanism of mental/developmental disorders.24 Besides, genetic mutation is also one of the pathogenetic factors of epilepsy and mental/developmental disorders, with ion channel and synapse related genes being most commonly mutated.25–27
Infectious Diseases and Epilepsy
A total of 114 pediatric patients with epilepsy and infectious diseases (viral encephalitis, upper respiratory tract infection, pulmonary infection, Epstein-Barr virus infection, central nervous system infection, and urinary tract infection) were assessed in this study, representing 45.41% of all patients. Multiple infections might increase the risk of epilepsy, suggesting that a systemic inflammatory process might occur during epilepsy. According to a Danish correlation study on epilepsy and infection risk in 25,825 children and young adults,28 32% of all patients had infections and an inpatient history. The risk of epilepsy increased by 78% due to infection (HR=1.78, 95% CI 1.73–1.83), with central nervous system infection showing the highest risk (HR=4.97, 95% CI 4.42–5.59). A study performed in Taiwan29 indicated that central nervous system infection is an independent risk factor for epilepsy, increasing its odds by 10.7 times versus the control group. Furthermore, when infection-related epilepsy in children is not well cured, the in-hospital mortality rate could reach 16%.30 Children tend to be the main targets of human herpesvirus-6 (HHV-6), Epstein-Barr virus,31,32 central nervous system infection, respiratory syncytial virus infection33 and urinary tract infection,34 which all may increase the risk of epilepsy. Accordingly, in children with a history of epilepsy or a family history, special attention should be paid to the prevention of infectious diseases. In case of epilepsy-induced infections, effective treatment measures should be taken once infections occur since this might reduce the frequency of epilepsy seizure and clinical symptoms.
Effects of CYP2C9 Gene Expression on VPA Concentrations and Plasma Protein Binding Rate
CYP2C9 is one of the metabolic enzymes of VPA. It has three subtypes: CYP2C9 *1/*1, the most common, is the wild homozygous type belonging to the fast metabolism type; CYP2C9 *1/*3, a mutant heterozygote, belongs to the intermediate metabolism type; CYP2C9 *3/*3 found in the least proportion of individuals is a homozygous mutant type that belongs to the slow metabolism type.
A total of 112 pediatric patients administered VPA were grouped by CYP2C9 genotype. There were no significant differences among groups with different genotypes in terms of total VPA concentrations; however, daily dose was significantly lower in the CYP2C9 *3/*3 group compared with the CYP2C9 *1/*3 and CYP2C9 *1/*19 groups. This indicated that clinicians had already adjusted the dosages for pediatric patients according to the clinical symptoms or TDM results. The average daily dose in the CYP2C9 *3/*3 group was 16.12 ± 2.34 mg/kg, representing 73.44% and 68.92% of those found in the CYP2C9 *1/*3 and CYP2C9 *1/*1 groups, respectively. These findings suggest that patients with CYP2C9 *3/*3 gene expression have a lower metabolic rate, and total VPA concentration was maintained within the effective range by reducing the drug dose. Earlier research also discovered that heterozygous genotype CYP2C9*3 had higher mean plasma VPA concentrations than did those subjects with the wild-type genotype [(3.9±0.4) µg kg mL(−1) mg(−1) vs (3.4±0.4) µg kg mL(−1) mg(−1), p=0.0001] when patients from Northern Han Chinese population were administrated with same VPA dose.13 This conclusion significantly supported what we found.
Different drug metabolism types might lead to distinct VPA concentrations in patient; therefore, the recommended VPA dose should differ according to CYP2C9 genotype. Previous research indicated that 15–20% of the total VPA dose is metabolized by CYP enzymes including CYP2C9,12,36 and it is even more important among children rather than adults with significant PK affection.15 The normal recommended dose of 20–30 mg/kg fitted for CYP2C9 *1/*1 genotype patients; in individuals with CYP2C9 *3/*3 expression, the daily dose should be reduced by approximately 50% due to delayed VPA metabolism. CYP2C9 variation induced VPA metabolize discrepancy were reported among different populations such as Urkarian,37 Japanese38 and Turkish,39 however, CYP2C9 genotype was found irrelevant with VPA exposure within Norwegians.40 This might indicated a CYP2C9 expression diversity based on different race. Other CYP enzymes including CYP2A6,12 CYP2B612 and CYP2C1940 were discovered to be related with VPA pharmacokinetics.
Besides, the plasma protein binding rate was higher in the CYP2C9 *3/*3 group (97.70% ± 1.05%) compared with the other groups (93.58% ± 3.70% and 95.10% ± 2.68%). Because a high proportion of VPA binds to plasma proteins, free VPA concentration was only 1.29 ± 0.92 µg/mL in the CYP2C9 *3/*3 group, which was lower than those of the other groups (4.39 ± 3.99 µg/mL and 3.68 ± 2.42 µg/mL). Total VPA concentrations in 2 cases with CYP2C9 *3/*3 approached the higher limit of effective therapeutic concentration (83.80 µg/mL and 95.14 µg/mL, respectively), but with unsatisfactory clinical outcomes. Epilepsy symptoms were finally controlled in these patients by passively increasing the doses of other antiepileptic drugs. VPA has relatively high plasma protein binding rates, while only unbound drugs are effective. Thus, monitoring of the total VPA concentration cannot fully reflect the effectiveness of clinical therapy. Instead, free drug concentrations should be simultaneously monitored, and the relationship between free drug amounts and clinical outcomes should be assessed.
Associations of VPA Free Concentration with Total Concentration and Plasma Protein Binding Rate
The TDM results of the 251 pediatric patients with epilepsy showed that the relationship between free concentration and VPA total concentration was nonlinear, following a quadratic equation, corroborating previous findings.41 Nonetheless, the correlation was not high enough (r=0.6981), and the data partially deviated from the 95% confidence interval, especially when total VPA concentration exceeded 100 µg/mL, as shown in Figure 2. A quadratic equation showed a better correlation (r=0.8136) between free VPA concentration and plasma protein binding rate, with the majority of test points within the 95% confidence interval.
Meanwhile, free VPA concentration and plasma protein binding rate may vary with race, age, physiological features, and pathological condition. In the present study, the average VPA plasma protein binding rate in children aged 6 months to 14 years old was 92.51% ± 4.00%, and mean free VPA concentration was 4.91 ± 3.39 µg/mL. A previous study41 reported plasma protein binding rates in 52 inpatient and outpatient children under 14 years old of 86.59% ± 2.89% and 90.69% ± 3.69%, respectively. A Japanese study42 found a total VPA concentration of 71.2 ± 32.3 µg/mL in epilepsy patients, with a free concentration of 7.05 ± 5.84 µg/mL and a plasma protein binding rate of 90.1%. Wassim et al35 reported a VPA protein binding rate of around 83.0% in 902 Arabians (10–75 years old), while the average free drug concentration was 18.3 µg/mL. Drisaldi et al43 investigated 174 American adults and determined a free VPA concentration range of 7–23 µg/mL; the free VPA concentration range in an intensive care unit (ICU) of Miami was 5–17 µg/mL, while plasma protein binding levels were 48% on average.44
Effects of Combined Medication on VPA
Comparing cases administered VPA combined with phenobarbital and those without phenobarbital treatment, the daily doses of VPA were similar (23.07 ± 6.52 mg/kg and 22.53 ± 5.51 mg/kg). In contrast, total and free VPA concentrations in the combined medication group were lower, representing only 87.14% and 68.31% of those of individuals not administered phenobarbital. Phenobarbital, an inducer of liver microsomal enzymes, could accelerate VPA metabolism and reduce its plasma concentration, thus affecting the antiepileptic effect of VPA. Consequently, increasing the dose of VPA is required when co-administrated with phenobarbital. On the other hand, the inhibitory effects of VPA on liver metabolizing enzymes could increase the concentration of phenobarbital;45 thus, both drugs should be subjected to TDM in order to adjust their doses.
ALT and AST amounts were higher in pediatric patients of the combined medication group compared with those not administered phenobarbital, and AST difference was statistically significant; 6 cases were considered to show drug-related hepatic insufficiency, including 1 treated with 5 antiepileptic drugs, 4 administered 3 drugs and 1 treated with a single antiepileptic drug. This indicated that antiepileptic drug combination not only provides effective therapy for refractory and complex epilepsy, but also increases the risk of hepatic metabolic damage.
Six pediatric patients were treated with carbapenems due to serious infection, leading to decreased total and free VPA concentrations, which were 25.03% and 27.69% of those found in individuals not administered carbapenems, respectively. VPA concentrations in 2 pediatric patients dropped to 6.90 µg/mL and 12.71 µg/mL, respectively, hence failing to effectively control epilepsy. One of them had status epilepticus, and diazepam, phenobarbital or phenytoin sodium were added to the subsequent treatment. The above conditions occurred because clinicians did not pay attention to drug-drug interactions between VPA and carbapenems. Therefore, when they received the TDM results showing VPA concentrations below the effective range, they did not substitute them with other antibiotics. A total of 5 pediatric patients were kept under carbapenem treatment according to infection conditions; in 3 of them, no changes were made in relation to dosage; in 1, the daily dose of meropenem was increased from 42 mg/kg to 53 mg/kg, while in another case, the dose of biapenem was reduced from 30 mg/kg to 22.5 mg/kg. Since epilepsy was not well-controlled, the 6 pediatric patients were switched to other antiepileptic drugs or received increased doses of other antiepileptic drugs co-administrated with VPA.
Carbapenems, such as meropenem, imipenem, ertapenem and biapenem, inhibit VPA absorption and increase its transport to erythrocytes, decrease the decomposition of glucuronyl valproate into free VPA, and reduce plasma drug concentrations by approximately 60%. Moreover, drug interactions could not be alleviated by adjusting VPA dosage.46,47 Thus, VPA combined with carbapenems should be avoided, and other antiepileptic drugs such as carbamazepine and phenytoin sodium could be considered as substitutions for compulsory carbapenems used against serious infections during antiepileptic treatment.
Conclusions
This study showed that appropriate VPA dosage in pediatric epilepsy was affected by various CYP2C9 genotypes, with the daily dose required for achieving similar VPA concentrations significantly lower in patients with the CYP2C9 *3/*3 genotype compared with those harboring CYP2C9 *1/*1 and CYP2C9 *1/*3, respectively. Thus, different VPA dosages should be suggested based on drug metabolic genotype. Non-linear associations were found of free VPA amounts with total VPA levels and plasma protein binding rate, both reflected by quadratic equations. Accordingly, a combination of phenobarbital might result in reduced VPA total and free concentrations, which is why TDM is recommended. Carbapenems should be avoided during epilepsy treatment with VPA because they can significantly decrease total and free VPA concentrations, leading to therapeutic failure.
Funding
This work was financially supported by the Key Research and Development Project of Hebei Province.
Disclosure
The authors declare that there is no conflicts of interest regarding the publication of this article.
References
1. Valproic acid [package insert]. Maple Grove, MN: Upsher-Smith Laboratories, Inc; 2016.
2. Goldenberg MM. Overview of drugs used for epilepsy and seizures: etiology, diagnosis, and treatment. P&T. 2010;35:392–415.
3. Hilal-Dandan R, Brunton LL. Pharmacotherapy of the epilepsies. In: Goodman and Gilman’s Manual of Pharmacology and Therapeutics.
4. Beydoun A, Sackellares J, Shu V. Safety and efficacy of divalproex sodium monotherapy in partial epilepsy a double-blind, concentration-response design clinical trial. Neurology. 1997;48(1):182–188. doi:10.1212/WNL.48.1.182
5. Nasreddine W, Beydoun A. Valproate-induced thrombocytopenia: a prospective monotherapy study. Epilepsia. 2008;49(3):438–445. doi:10.1111/j.1528-1167.2007.01429.x
6. Kodama Y, Koike Y, Kimoto H, et al. Binding parameters of valproic acid to serum protein in healthy adults at steady state. Ther Drug Monit. 1992;14(1):55–60. doi:10.1097/00007691-199202000-00009
7. Cramer JA, Mattson RH, Bennett DM, Swick CT. Variable free and total valproic acid concentrations in sole- and multi-drug therapy. Ther Drug Monit. 1986;8(4):411–415. doi:10.1097/00007691-198612000-00005
8. Bellver M, Sánchez M, Gonzalez A, Santos Buelga D, Domínguez-Gil A. Plasma protein binding kinetics of valproic acid over a broad dosage range: therapeutic implications. J Clin Pharm Ther. 1993;18(3):191–197. doi:10.1111/j.1365-2710.1993.tb00612.x
9. Patsalos PN, Zugman M, Lake C, James A, Ratnaraj N, Sander JW. Serum protein binding of 25 antiepileptic drugs in a routine clinical setting: a comparison of free non–protein-bound concentrations. Epilepsia. 2017;58(7):1234–1243. doi:10.1111/epi.13802
10. Ito M, Ikeda Y, Arnez JG, Finocchiaro G, Tanaka K. The enzymatic basis for the metabolism and inhibitory effects of valproic acid: dehydrogenation of valproyl-CoA by 2-methyl-branched-chain acyl-CoA dehydrogenase. BBA-Gen Subjects. 1990;1034(2):213–218. doi:10.1016/0304-4165(90)90079-C
11. Ghodke-Puranik Y, Thorn CF, Lamba JK, et al. Valproic acid pathway: pharmacokinetics and pharmacodynamics. Pharmacogenet Genom. 2013;23(4):236–241. doi:10.1097/FPC.0b013e32835ea0b2
12. Kiang TK, Ho PC, Anari MR, Tong V, Abbott FS, Chang TK. Contribution of CYP2C9, CYP2A6, and CYP2B6 to valproic acid metabolism in hepatic microsomes from individuals with the CYP2C9*1/*1 genotype. Toxicol Sci. 2006;2:261–271. doi:10.1093/toxsci/kfl096
13. Tan L, Yu JT, Sun YP, Ou JR, Song JH, Yu Y. The influence of cytochrome oxidase CYP2A6, CYP2B6, and CYP2C9 polymorphisms on the plasma concentrations of valproic acid in epileptic patients. Clin Neurol Neurosurg. 2010;112(4):320–323. doi:10.1016/j.clineuro.2010.01.002
14. Jiang D, Bai X, Zhang Q, et al. Effects of CYP2C19 and CYP2C9 genotypes on pharmacokinetic variability of valproic acid in Chinese epileptic patients: nonlinear mixed-effect modeling. Eur J Clin Pharmacol. 2009;65(12):1187–1193. doi:10.1007/s00228-009-0712-x
15. Budi T, Toth K, Nagy A, et al. Clinical significance of CYP2C9-status guided valproic acid therapy in children. Epilepsia. 2015;56(6):849–855. doi:10.1111/epi.13011
16. Wang C, Wang P, Yang LP, Pan J, Yang X, Ma HY. Association of cyp2c9, cyp2a6, acsm2a, and cpt1a gene polymorphisms with adverse effects of valproic acid in chinese patients with epilepsy. Epilepsy Res. 2017;132:64–69. doi:10.1016/j.eplepsyres.2017.02.015
17. Gielgens RC, Bok LA. Commentary on clinical significance of CYP2C9-status-guided valproic acid therapy in children. Epilepsia. 2016;57(8):
18. Toth K, Budi T, Kiss A, et al. Phenoconversion of CYP2C9 in epilepsy limits the predictive value of CYP2C9 genotype in optimizing valproate therapy. Pers Mad. 2015;12:201–209.
19. Zhang JF, Zhang ZQ, Dong WC, Jiang Y. A new derivatization method to enhance sensitivity for the determination of low levels of valproic acid in human plasma. J Chromatogr Sci. 2014;52:1173–1180. doi:10.1093/chromsci/bmt167
20. Zhang ZQ, Dong WC, Yang XL, et al. The influence of plasma albumin concentration on the analysis methodology of free valproic acid by ultrafiltration and its application to therapeutic drug monitoring. Ther Drug Monit. 2015;37(6):776–782. doi:10.1097/FTD.0000000000000225
21. Depositario-Cabacar DF, Zelleke TG. Treatment of epilepsy in children with developmental disabilities. Dev Disabil Res Rev. 2010;16(3):239–247. doi:10.1002/ddrr.116
22. Singh BK, White-Scott S. Role of topiramate in adults with intractable epilepsy, mental retardation, and developmental disabilities. Seizure. 2002;11:47–50. doi:10.1053/seiz.2001.0571
23. Salpekar J. Neuropsychiatric effects of epilepsy in developmental disorders. Curr Opin Psychiatry. 2018;31(2):109–115. doi:10.1097/YCO.0000000000000392
24. Gao K, Zhang Y, Zhang L, et al. Large de novo microdeletion in epilepsy with intellectual and developmental disabilities, with a systems biology analysis. Adv Neurobiol. 2018;21:247–266.
25. Lamm WW, Millichap JJ, Soares DC, et al. Novel de novo EEF1A2 missense mutations causing epilepsy and intellectual disability. Mol Genet Genomic Med. 2016;4(4):465–474. doi:10.1002/mgg3.219
26. Kong W, Zhang Y, Gao Y, et al. SCN8A mutations in Chinese children with early onset epilepsy and intellectual disability. Epilepsia. 2015;56(3):431–438. doi:10.1111/epi.12925
27. Zhang Y, Kong W, Gao Y, et al. Gene mutation analysis in 253 Chinese children with unexplained epilepsy and intellectual/developmental disabilities. PLoS One. 2015;10(11):e0141782. doi:10.1371/journal.pone.0141782
28. Ahlers FS, Benros ME, Dreier JW, Christensen J. Infections and risk of epilepsy in children and young adults: a nationwide study. Epilepsia. 2019;60(2):275–283. doi:10.1111/epi.14626
29. Lin CH, Lin WD, Chou IC, Lee IC, Hong SY. Epilepsy and neurodevelopmental outcomes in children with etiologically diagnosed central nervous system infections: a retrospective cohort study. Front Neurol. 2019;10:528. doi:10.3389/fneur.2019.00528
30. Lam SK, Lu WY, Weng WC, Fan PC, Lee WT. The short-term and long-term outcome of febrile infection-related epilepsy syndrome in children. Epilepsy Behav. 2019;95:117–123. doi:10.1016/j.yebeh.2019.02.033
31. Bartolini L, Theodore WH, Jacobson S, et al. Infection with HHV-6 and its role in epilepsy. Epilepsy Res. 2019;153:34–39. doi:10.1016/j.eplepsyres.2019.03.016
32. Bartolini L, Theodore WH, Jacobson S, Gaillard WD. Detection of HHV-6 and EBV and cytokine levels in saliva from children with seizures: results of a multi-center cross-sectional study. Front Neurol. 2018;9:834. doi:10.3389/fneur.2018.00834
33. Cha T, Choi YJ, Oh JW, et al. Respiratory syncytial virus-associated seizures in Korean children, 2011–2016. Korean J Pediatr. 2019;62(4):131–137. doi:10.3345/kjp.2018.07066
34. Cruz-Cruz MDR, Gallardo-Elías J, Paredes-Solís S, Legorreta-Soberanis J, Flores-Moreno M, Andersson N. Factors associated with epilepsy in children in Mexico: a case-control study. Bol Med Hosp Infant Mex. 2017;74(5):334–340. doi:10.1016/j.bmhimx.2017.05.006
35. Nasreddine W, Dirani M, Atweh S, Makki A, Beydoun A. Determinants of free serum valproate concentration: a prospective study in patients on divalproex sodium monotherapy. Seizure. 2018;59:24–27. doi:10.1016/j.seizure.2018.04.012
36. Ho PC, Abbott FS, Zanger UM, Chang TK. Influence of CYP2C9 genotypes on the formation of a hepatotoxic metabolite of valproic acid in human liver microsomes. Pharmacogenomics J. 2003;3:335–342. doi:10.1038/sj.tpj.6500210
37. Monostory K, Nagy A, Tóth K, et al. Relevance of CYP2C9 function in valproate therapy. Curr Neuropharmacol. 2019;17(1):99–106. doi:10.2174/1570159X15666171109143654
38. Goto S, Seo T, Murata T, et al. Population estimation of the effects of cytochrome P450 2C9 and 2C19 polymorphisms on phenobarbital clearance in Japanese. Ther Drug Monit. 2007;29(1):118–121. doi:10.1097/FTD.0b013e318030def0
39. Gunes A, Bilir E, Zengil H, et al. Inhibitory effect of valproic acid on cytochrome P450 2C9 activity in epilepsy patients. Basic Clin Pharmacol Toxicol. 2007;100(6):383–386. doi:10.1111/j.1742-7843.2007.00061.x
40. Smith RL, Haslemo T, Refsum H, Molden E. Impact of age, gender and CYP2C9/2C19 genotypes on dose-adjusted steady-state serum concentrations of valproic acid—a large-scale study based on naturalistic therapeutic drug monitoring data. Eur J Clin Pharmacol. 2016;72:1099–1104. doi:10.1007/s00228-016-2087-0
41. Gu XR, Yu SR, Peng QL, Ma M, Hu Y, Zhou B. Determination of unbound valproic acid in plasma using centrifugal ultrafiltration and gas chromatography: application in TDM. Anal Biochem. 2020;588:113475. doi:10.1016/j.ab.2019.113475
42. Itoh H, Suzuki Y, Fujisaki K, Sato Y, Takeyama M. Correlation between plasma ammonia level and serum trough concentration of free valproic acid in patients with epilepsy. Biol Pharm Bull. 2012;35(6):971–974. doi:10.1248/bpb.35.971
43. Drisaldi A, Weeda E, Meyens R, et al. Accuracy of valproic acid concentration correction based on serum albumin. Neurocrit Care. 2019;30(2):301–306. doi:10.1007/s12028-018-0627-4
44. Riker RR, Gagnon DJ, Hatton C, et al. Valproate protein binding is highly variable in ICU patients and not predicted by total serum concentrations: a case series and literature review. Pharmacotherapy. 2017;37(4):500–508. doi:10.1002/phar.1912
45. Schoemaker R, Wade JR, Stockis A. Brivaracetam population pharmacokinetics in children with epilepsy aged 1 month to 16 years. Eur J Clin Pharmacol. 2017;73(6):727–733. doi:10.1007/s00228-017-2230-6
46. Wu CC, Pai TY, Hsiao FY, Shen LJ, Wu FL. The effect of different carbapenem antibiotics (ertapenem, imipenem/cilastatin and meropenem) on serum valproic acid concentrations. Ther Drug Monit. 2016;38(5):587–592. doi:10.1097/FTD.0000000000000316
47. Šíma M, Hartinger J, Rulíšek J, Šachl R, Slanař O. Meropenem-induced valproic acid elimination: a case report of clinically relevant drug interaction. Prague Med Rep. 2017;118(2–3):105–109. doi:10.14712/23362936.2017.11
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
