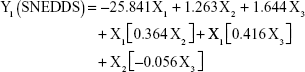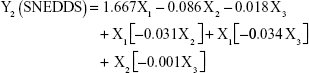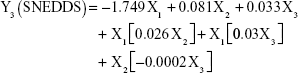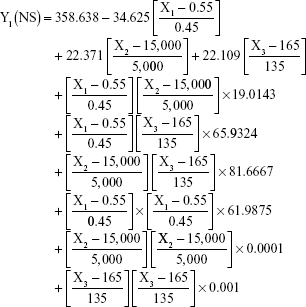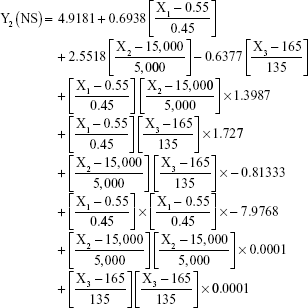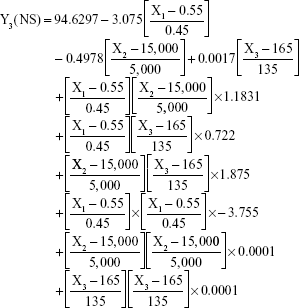Back to Journals » Drug Design, Development and Therapy » Volume 9
Custom fractional factorial designs to develop atorvastatin self-nanoemulsifying and nanosuspension delivery systems – enhancement of oral bioavailability
Authors Hashem F, Al-Sawahli M , Nasr M , Ahmed OAA
Received 5 April 2015
Accepted for publication 24 April 2015
Published 19 June 2015 Volume 2015:9 Pages 3141—3152
DOI https://doi.org/10.2147/DDDT.S86126
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Shu-Feng Zhou
Fahima M Hashem,1 Majid M Al-Sawahli,2 Mohamed Nasr,1 Osama AA Ahmed3,4
1Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Helwan University, Cairo, Egypt; 2Holding Company for Biological Products and Vaccines (VACSERA), Giza, Egypt; 3Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia; 4Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Minia University, Minia, Egypt
Abstract: Poor water solubility of a drug is a major challenge in drug delivery research and a main cause for limited bioavailability and pharmacokinetic parameters. This work aims to utilize custom fractional factorial design to assess the development of self-nanoemulsifying drug delivery systems (SNEDDS) and solid nanosuspensions (NS) in order to enhance the oral delivery of atorvastatin (ATR). According to the design, 14 experimental runs of ATR SNEDDS were formulated utilizing the highly ATR solubilizing SNEDDS components: oleic acid, Tween 80, and propylene glycol. In addition, 12 runs of NS were formulated by the antisolvent precipitation–ultrasonication method. Optimized formulations of SNEDDS and solid NS, deduced from the design, were characterized. Optimized SNEDDS formula exhibited mean globule size of 73.5 nm, zeta potential magnitude of -24.1 mV, and 13.5 µs/cm of electrical conductivity. Optimized solid NS formula exhibited mean particle size of 260.3 nm, 7.4 mV of zeta potential, and 93.2% of yield percentage. Transmission electron microscopy showed SNEDDS droplets formula as discrete spheres. The solid NS morphology showed flaky nanoparticles with irregular shapes using scanning electron microscopy. The release behavior of the optimized SNEDDS formula showed 56.78% of cumulative ATR release after 10 minutes. Solid NS formula showed lower rate of release in the first 30 minutes. Bioavailability estimation in Wistar albino rats revealed an augmentation in ATR bioavailability, relative to ATR suspension and the commercial tablets, from optimized ATR SNEDDS and NS formulations by 193.81% and 155.31%, respectively. The findings of this work showed that the optimized nanocarriers enhance the oral delivery and pharmacokinetic profile of ATR.
Keywords: nanostructures, optimization, experimental design, fractional factorial design
Introduction
Reduction of mortality and morbidity of hyperlipidemia requires the development of promising delivery systems in order to enhance the oral delivery and bioavailability of hyperlipidemia drugs.1 Atorvastatin (ATR) is a cholesterol-lowering drug which belongs to the class of synthetic hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, known as statins. Blood levels of cholesterol, low-density lipoprotein, triglyceride, and very low-density lipoproteins are reduced after ATR treatment. On the other hand, ATR increases high-density lipoproteins in patients with a wide variety of dyslipidemias.2,3
Recently, attention has been drawn toward self-nanoemulsifying drug delivery systems (SNEDDS) and solid nanosuspensions (NS) for improving the oral bioavailability of Biopharmaceutics Classification System (BCS) class II drugs via enhancing their solubility. SNEDDS are uniform preconcentrate of oils, surfactants, and co-surfactants that form nanoemulsion with 20–200 nm size range upon dilution with no need to perform a dissolution step. SNEDDS spread instantly in the gastrointestinal tract (GIT) fluids. GIT fluids and their motility provide the necessary dispersion of the nanoemulsion.4–9 The anhydrous nature of SNEDDS allows for the oral administration of the drug in soft or hard gelatin capsules. These nano-sized droplets offer enhancement in dissolution rates and hence bioavailability of poorly water soluble and lipophilic drugs.4,10,11
Solid NS is a colloidal dispersion of drug with suitable stabilizer(s) that shows particle sizes below 1 μm to maintain long-term colloidal stability.12,13 Solid NS are usually produced in liquid media, but stability issue is still one of the critical aspects to be considered.14–17 Dispersed system stabilizers act by accumulation at the interface of drug particles to reserve steric or ionic barriers. Accordingly, NS physical stability and in vivo behavior rely significantly on the type and amount of stabilizer used in the preparation of NS.18 The aim of this study was to improve ATR oral bioavailability by using custom fractional factorial design to formulate and optimize nanocarriers of ATR using SNEDDS and NS technologies.
Materials and methods
Materials
ATR (CAS number 134523-03-8) was kindly supplied by Egyptian International Pharmaceutical Industry Company, Egypt. Oleic acid (99.99%), polyoxyethylene sorbitan monooleate (Tween 80), propylene glycol 99.5%, poloxamer 407, maltose, and other solvents were obtained from Sigma-Aldrich® (St Louis, MO, USA).
Formulation and characterization of SNEDDS-loaded ATR
Construction of ternary phase diagram
Self-emulsifying systems were prepared with varying concentrations of oleic acid, Tween 80 (surfactant), and propylene glycol. Two grams of each mixture was prepared by mixing SNEDDS components in a 10 mL capped glass vial. The components were mixed by vortex mixer for a minute. Nanoemulsification formation efficiency was assessed by adding 100 mg of each mixture to 20 mL double distilled water, followed by mixing using a magnetic stirrer. Only clear or slight bluish dispersions with no precipitation of the drug, as inspected visually, were considered in the nanoemulsion region of the diagram.19,20 The area of nanoemulsion formation was identified and phase diagram was plotted using TriDraw® software.21–24
Formulation of ATR SNEDDS
After identification of the self-emulsifying region, formulations that form nanoemulsion within 1 minute and have a clear or slight/transient bluish appearance after dissolving 10 mg of ATR were considered. The selected formulations were included in the factorial design after passing thermodynamic stability studies such as centrifugation, heating–cooling and freeze–thaw cycles as previously reported.25
Formulation of ATR NS
ATR solid NS were prepared using the antisolvent precipitation–ultrasonication method.26 ATR was dissolved completely in methanol and the methanolic solution was then passed through a 0.45 μm filter (Millipore®, Massachusetts, MA, USA) to remove the possible impurities. The antisolvent phase was prepared by dispersing a specific concentration of stabilizer in distilled water. At room temperature, 3 mL of methanolic solution was quickly injected by a syringe into 50 mL of antisolvent and mixed using Ultra Turrax® high shear homogenizer (Ika®, Staufen, Germany) at a specific speed, according to the design, for 10 minutes at room temperature. Drug particles precipitated from the antisolvent were also sonicated for a designed period. During the process, the temperature was controlled at 4°C–8°C using an ice bath. Then, the NS samples thus prepared were stirred using magnetic stirrer, Cimarec® (Thermoscientific, NJ, USA), at room temperature for 12 hours to remove methanol. The formulations were subjected to centrifugation at 20,000 rpm at 8°C and followed by freeze drying for 72 hours using mannitol as a cryoprotectant.
Factorial design of SNEDDS and NS formulations
Custom fractional factorial design was constructed in this work using statistical package JMP 7.1 software (SAS; SAS Institute, Cary, NC, USA). For ATR SNEDDS, the factors studied were: oil percent (X1), surfactant percent (X2), and co-surfactant percent (X3). For ATR NS, the factors studied were: stabilizer concentration percent (X1), homogenization speed in rpm (X2), and sonication time in minutes (X3). Levels for each formulation variable were designed. The selected responses for both designs were mean globule or particle size in nm (Y1) and zeta potential in mV (Y2). The third response (Y3) for SNEDDS formula was conductivity (μs/cm) and for NS was the yield percent. The factor levels of each design are presented in Table 1. Fourteen and 12 experimental runs were formulated for ATR SNEDDS and NS, respectively.
  | Table 1 ATR SNEDDS and NS experimental runs showing factor combinations |
Characterization of the optimized formulations
Particle size and zeta potential measurement
Laser diffraction technique using Zetatrac® particle size analyzer (Microtrac Inc, PA, USA) was used to measure mean particle size and zeta potential of optimized SNEDDS and NS samples. Hundred milligrams of optimized SNEDDS or NS samples were mixed with 10 mL of purified water at 25°C.
Electron microscope investigation
The morphology of the optimized SNEDDS globules and solid NS was visualized by transmission electron microscope (TEM) (Jeol® JEM-100CX electron microscope, Japan Electron Optics lab., Tokyo, Japan) and scanning electron microscope (SEM) (Jeol® JXA-840A, Japan), respectively.
In vitro ATR release
In vitro dissolution test was conducted in a dissolution apparatus United States Pharmacopeia (USP) type 2, (Erweka®, Heusenstamm, Germany). The temperature was maintained at 37°C±0.5°C, and the stirring rate was 100 rpm. Size one soft gelatin capsules, each containing 500 mg of optimized SNEDDS formula loaded by 10 mg ATR, were used. Solid NS optimized formula equivalent to 10 mg of ATR was used. Optimized SNEDDS and NS formulae were dispersed in 900 mL of phosphate buffer saline (pH 6.8) (dissolution medium). Aliquots, each of 3 mL, were withdrawn from the dissolution medium at selected time intervals and replaced with an equivalent amount of fresh dissolution medium. Samples were then filtered through a 0.45 μm syringe filter (Millipore®) immediately before dilution, or when necessary. Concentrations of ATR were determined using a validated high performance liquid chromatography (HPLC) method reported by Kurakula et al.27 The dissolution experiments were carried out in triplicate.
Pharmacokinetic study of the optimized formulations
Animals used for pharmacokinetic study were adult female Wistar albino rats weighing 200–250 g. All animals had free access to food, pellet diet, and tap water ad libitum, and were maintained at a relative humidity of 65%–85%, a temperature of 23°C–25°C, and in a schedule of 12 hours light/dark cycle. The use of animals was approved by the local Institutional Review Board for Preclinical and Clinical Research (approval date December 23, 2014) who ensured the care and use of animals conformed to Guiding Principle in Care and Use of Animals (DHEW publication NIH 80-23) and stick to the “Principles of Laboratory Animal Care” (NIH publication #85-23, revised in 1985).28,29 Animals were divided into four groups, with six animals in each group. The first group was orally administered with ATR suspension. The second group was orally administered with SNEDDS optimized formula. The third group was orally administered with optimized NS formula. The fourth group was orally administered with suspension of commercial ATR tablets. The animal groups under study were administered ATR dose equivalent to 25 mg ATR/kg body weight. All groups were administered orally using a ball-tipped feeding needle. Blood samples (0.25 mL) were withdrawn at 0.0, 0.25, 0.5, 1, 2, 4, 6, 8, 12 and 24 hours in micro-centrifuge tubes. Samples were analyzed using reported HPLC method.27
Results and discussion
Mathematical experimental design and formulation of ATR SNEDDS and NS
Enhancing the poor oral bioavailability of antihyperlipidemic drugs is strongly recommended in order to overcome the rising problems associated with increasing levels of low-density lipoprotein and total serum cholesterol in hypercholesterolemia and mixed dyslipidemia cases. This work mainly focused on improving the oral delivery of the poorly water soluble drug ATR utilizing SNEDDS and NS technologies using custom fractional factorial design.
For SNEDDS formulations, highest solubility of ATR in all components of nanoemulsion, which were a variety of oils, surfactants, and co-surfactants, were examined (data not shown). It is reported that the highest solubility of ATR in the oil phase is more imperative for the nanoemulsion formulation than drug solubility in surfactant or co-surfactant due to the prospective dilution of the entire formula in GIT and possible reduction of surfactants or co-surfactants leading to drug precipitation.30–33 Results showed that oleic acid recorded the highest ATR solubility. For surfactants, Tween 80 recorded the highest ATR solubility. This could be due to the high hydrophilic-lipophilic balance (HLB) of Tween 80 (HLB 15). This value enhances nanoemulsion formation efficiency. For co-surfactants, the inclusion of co-surfactant in nanoemulsion provides the interfacial film with sufficient flexibility for different curvatures required to form nanoemulsion over a wide range of composition.34 Propylene glycol recorded the highest ATR solubility. Accordingly, the designed emulsifying system contained oleic acid as an oil component, Tween 80 as a surfactant, and propylene glycol as a co-surfactant. Ternary phase diagrams were designed in order to demonstrate regions of nanoemulsion formation. Variable proportions of oil, surfactant, and co-surfactant were tested. The shaded regions reflect the nanoemulsification behavior (Figure 1). More shaded areas indicate enhanced ability of self-nanoemulsification.19,22 All these formulae contain sufficient content of oil components to ensure that appropriate ATR dose is achieved in the solubilized form. This guarantee is a must as the focus is about enhancing the bioavailability of the drug that shows poor aqueous solubility. The range of oil content was 5%–25%, for all ternary phase diagrams series. Surfactant content was in the range of 20%–90%. For co-surfactant, the range extended from 5% to 75%.
According to custom fractional factorial design of SNEDDS and solid NS, the factor combinations yielded various values of observed mean responses. The observed responses are shown in Table 2. According to custom fractional factorial design, the factor combinations yielded various values of observed mean responses. SNEDDS results for Y1 were in the range 67.7–170.9 nm for runs 1 and 3. Zeta potential (Y2) showed range values of −11.3 (run 13) to −26 mV (run 1). The highest value for conductivity (Y3) was achieved by runs 3 and 8. Solid NS results for Y1 were in the range 247.8–712.9 nm for runs 7 and 12. Zeta potential (Y2) showed the highest value 8 (run 7) and the lowest value −8.61 mV (run 1). The highest value for yield (Y3) was achieved by run 1.
Analysis of variance (ANOVA) according to the data of the responses (Y1–Y3) for both SNEDDS and solid NS is shown in Table 3. According to ANOVA, evidence of a regression effect is considered for P-value of 0.05 or less. All P-values listed indicated a significant effect of the independent factors on all responses. The estimated effects and associated P-values for all the three responses are shown in Table 4. Most of the P-values showed a significant effect of the independent factors and combined factors on the responses (Table 4).
Quantile–quantile relationships of plotting the measured against the predicted parameters showed linear correlations, with R2 ranging from 0.71 to 0.87 for SNEDDS and from 0.94 to 0.99 for NS. These values indicate method validity for prediction of the investigated dependant variables within the selected space of the design (Figure 2). Pareto charts, shown in Figure 3, are used to demonstrate the effect of the independent variables and their interactions on the dependant variables. These effects were arranged in decreasing order of importance. Pareto chart was marked with a vertical reference line at the critical P-value of 0.05. Statistical significance is considered when the effect passes the vertical reference line. A positive sign illustrates a direct relationship of the variable with the response. On the other hand, a negative sign showed inverse relationship. SNEDDS charts showed that increasing oil percent (X1) increases mean particle size and conductivity significantly. The variation in the droplet size may be attributed to variations in penetration of oil molecules into the surfactant alkyl chain region that affects film flexibility, which influences surface curvature, and consequently globule size.35 In addition, zeta potential is significantly related inversely to oleic acid percent. This is could be attributed to the unionized nature of oleic acid (pKa=9.85) at the investigated pH (7.2), which has a prominent effect in reducing SNEDDS surface charge with the increase in oleic acid percent.36
  | Figure 2 Quantile–quantile plots for predicting the dependent variables. |
NS revealed that the mean particle size is significantly affected by the magnitude of X2·X3. Also, there is proportional relation between zeta potential and the magnitude of 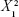 . In the absence of stabilizer, particles do not possess sufficient zeta potential and aggregation is spontaneous.37 Aggregation is attributed to the absence of sufficient steric barrier or electric repulsion that are responsible to maintain stability of the nanostructured formulations. Yield is affected inversely by stabilizer concentration. This may be related to the fact that increasing in stabilizer concentration over the optimized value in the media will hinder the loading of more ATR inside.
. In the absence of stabilizer, particles do not possess sufficient zeta potential and aggregation is spontaneous.37 Aggregation is attributed to the absence of sufficient steric barrier or electric repulsion that are responsible to maintain stability of the nanostructured formulations. Yield is affected inversely by stabilizer concentration. This may be related to the fact that increasing in stabilizer concentration over the optimized value in the media will hinder the loading of more ATR inside.
The prediction equations for SNEDDS (Equations 1–3) and NS (Equations 4–6) including the individual effects of the independent variables and their interaction terms were generated using the mathematical regression models for the observed responses Y1, Y2, and Y3, respectively. Theoretical values of the responses were obtained by the substitution of X1–X3 values in Equations 1–6.
|
|
|
|
|
|
|
|
|
|
|
|
After analyzing the effect of independent variables (X1–X3) on the responses (Y1–Y3) for both SNEDDS and solid NS, levels of these factors were specified by utilizing optimization process and desirability function. Accordingly, SNEDDS predicted values of Y1, Y2, and Y3 were 70.7 nm, −25.3 mV, and 13 μs/cm, respectively. Solid NS predicted values of Y1, Y2, and Y3 were 253.2 nm, 7.2 mV, and 95.4%, respectively. These predicted values were deduced for SNEDDS at X1 of 24.7%, X2 of 54.4%, and X3 of 20.9%. For solid NS, these predicted values were deduced at X1, X2, and X3 levels of 0.6%, 12,000 rpm, and 50 seconds, respectively. To confirm the output predictions, fresh formulations of ATR SNEDDS were prepared with the specified predicted values, ie, optimized levels of factors, oil percent of 24.7%, surfactant percent of 54.4%, and co-surfactant percent of 20.9%. For ATR solid NS, stabilizer concentration of 0.6%, homogenization speed of 12,000 rpm, and sonication time of 50 seconds were the specified predicted values for NS formulation. These optimized levels yielded SNEDDS formulation with globule size of 73.5 nm, zeta potential of −24.1 mV, and conductivity of 13.5 μs/cm. For solid NS, 260.3 nm as mean particle size, 7.4 mV as zeta potential and 93.2% as yield were obtained. The close agreement of observed and predicted values demonstrated the reliability of the optimization procedure in predicting the characteristics of ATR nanocarriers.
Smaller sizes of dispersed drug in oil globules offer a greater surface area and faster release of ATR and enhanced absorption and bioavailability. Nanoemulsions are suggested to penetrate deep into tissues through fine capillaries due to their sub-cellular size. This will offer enhanced delivery of therapeutic agents to target sites in the body.38,39 Zeta potential of the optimized formulation exhibited −25.3 mV, which indicates stability of the system. For submicron particles, the value of zeta potential is an indication of the degree of electrostatic repulsion of the dispersed phase, and hence its stability in the dispersion medium. Higher zeta potential values, either negative or positive, will indicate the stability of the dispersion as it will resist aggregation.25,30 Electrical conductivity gives an indication about stability and nature of formulation (o/w or w/o).25,34 The results indicate that the formulation was o/w type. Electrical conductivity is directly related to water percentage in the SNEDDS formula. The higher the electrical conductivity, the more will be the percentage of water, which allows more freedom for mobility of ions.34 In case of w/o systems, the external phase is oil, which hinders the mobility of ions and no electrical conductivity is recorded.
The optimized poloxamer 407 stabilized NS was successfully formulated and assayed for mean particle size and zeta potential. The lyophilized formula showed white color, markedly friable in comparison to raw ATR, with more flowable and brittle consistency. Optimized solid NS formula exhibited mean particle size of 253.2 nm, 7.2 mV of zeta potential, and 95.4% as yield. The relatively low zeta potential value could affect the stability of ATR NS formulation, which may affect the storage of the prepared NS formula. This may require the fresh preparation of NS before use. The environment where NS are spontaneously formed, due to the technique used, is fully critical. This may be correlated to the theory of crystallization and steps of particle size formation that include particle nucleation, molecular growth, and agglomeration or aggregation. Each step has its rate that critically affects the size of the final particle. Also, the driving force of this process is supersaturation which affects nucleation rate and diffusion-controlled growth rate.40
The globules of SNEDDS formula appeared as discrete spheres as shown in TEM images of Figure 4A. All droplet sizes taken were consistent with data analyzed using particle sizing apparatus. The SEM image of the solid NS formula showed flaky irregular shapes (Figure 4B). It is clear that the antisolvent precipitation–ultrasonication method is able to produce ATR in nano-sized particles.
In vitro ATR release
The release behavior of the optimized formulations was graphically plotted (Figure 5). Dissolution of hard gelatin capsules filled with the optimized SNEDDS and solid NS formulations was performed.
  | Figure 5 In vitro release of optimized SNEDDS and NS formulations. |
The percent cumulative ATR release from SNEDDS formula was 56.78%, 82.92%, and 86.53% after 10, 30, and 60 minutes, respectively. Results showed almost graphical plateau after 10 minutes. In general, it was concluded that the dissolved ATR in SNEDDS of nano-sized globule size facilitates the release of ATR from the optimized formula and increased dissolution rate. This finding may enhance oral bioavailability of ATR due to enhanced dissolution rate. The greater dissolution of ATR in the oily phase may be due to the reduced droplet size and subsequently can enhance surface area that attains an enhanced release rate. Solid NS formula showed lower rate in the first 30 minutes compared with ATR SNEDDS formula (Figure 5). The formula of solid NS was able to release 53.3% of drug within 60 minutes.
The potential of nanoparticles in enhancing dissolution rate and subsequently bioavailability is attributed to pronounced reduction in particle size with increased surface area, enhanced solubility, and amorphous nature of the drug in the preparation.41 Nanonization process improves both the solubility and the dissolution rate.41 According to the Noyes–Whitney equation only the dissolution rate increases with surface area.42 According to previous studies, the Ostwald–Freundlich and Kelvin equations show that this no longer applies at the nanoscale particle size, below 1 μm or preferably less than 0.1 μm, where the extreme curvature of the particles leads to an increase in dissolution pressure and hence solubility.18,43,44
Pharmacokinetic study of the optimized formulations
Means of plasma concentrations-time profiles of the optimized formulations of SNEDDS and solid NS were displayed in Figure 6. The pharmacokinetic parameters were calculated using Kinetica software (Kinetica 5.0.11, Thermo Fisher Scientific Inc., Waltham, MA, USA). The pharmacokinetic study showed significant rise in maximum plasma level (Cmax) and area under curve (AUC[0–∞]) of the investigated optimized formulations versus ATR suspension and marketed ATR tablets (P<0.05). Cmax of ATR was 1,793.74, 8,099.21, 5,909.89, and 1,780.82 ng/mL from suspension, optimized SNEDDS, optimized NS, and commercial tablets, respectively (Figure 6, inset table). AUC[0–∞] values exhibited significant enhancement of oral ATR bioavailability of optimized SNEDDS formula and optimized NS by 170.28% and 71.80%, respectively, in comparison with the oral suspension. Accordingly, these results indicated that optimized ATR SNEDDS and NS formulations improved the pharmacokinetic parameters of the water insoluble drug ATR.
Conclusion
Custom fractional factorial design was utilized to optimize the clear region area of SNEDDS components ternary phase and NS formulations parameters. The development of optimized ATR SNEDDS and NS formulae can significantly improve the pharmacokinetic profile and bioavailability of ATR by 93.81% and 55.31%, respectively, relative to ATR suspension and the commercial tablets. Formulation of ATR SNEDDS showed significant improvement to pharmacokinetic parameters and bioavailability compared with NS formula. The results in this study concluded that optimized ATR nanocarriers enhanced dissolution characters and oral delivery of ATR compared with ATR suspension and commercial ATR tablets.
Acknowledgments
The authors would like to thank Dr Usama Fahmy, Faculty of Pharmacy, King Abdulaziz University, for valuable help in HPLC assay. The authors are also very grateful to Dr Ahmed Zidan, Faculty of Pharmacy, King Abdulaziz University, for useful and constructive discussions on experimental design.
Disclosure
The authors report no conflicts of interest in this work.
References
Ahmed OAA, Hosny KM, Al-Sawahli MM, Fahmy UA. Optimization of caseinate coated simvastatin-zein nanoparticles: improved bioavailability and modified release characteristics. Drug Des Devel Ther. 2015;9:655–662. | ||
Malhotra HS, Goa KL. Atorvastatin: an updated review of its pharmacological properties and use in dyslipidaemia. Drugs. 2001;61: 1835–1881. | ||
Lea AP, McTavish D. Atorvastatin. A review of its pharmacology and therapeutic potential in the management of hyperlipidaemias. Drugs. 1997;53:828–847. | ||
Fatouros DG, Deen GR, Arleth L, et al. Structural development of self nano emulsifying drug delivery systems (SNEDDS) during in vitro lipid digestion monitored by small-angle X-ray scattering. Pharm Res. 2007; 24:1844–1853. | ||
Nazzal S, Smalyukh I, Lavrentovich O, Khan M. Preparation and in vitro characterization of a eutectic based semisolid selfnanoemulsified drug delivery system (SNEDDS) of ubiquinone: mechanism and progress of emulsion formation. Int J Pharm. 2002;235:247–265. | ||
Preshita P, Abhijit AD, Vandana BP. Overcoming poor oral bioavailability using nanoparticle formulations opportunities and limitations. Drug Discov Today Technol. 2002;9:e87–e95. | ||
Date A, Desai N, Dixit R, Nagersanker M. Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances. Nanomedicine (Lond). 2010;5:1595–1616. | ||
Elkasabgy NA. Ocular supersaturated self-nanoemulsifying drug delivery systems (S-SNEDDS) to enhance econazole nitrate bioavailability. Int J Pharm. 2014;460:33–44. | ||
Cuine JF, McEvoy CL, Charman WN, et al. Evaluation of the impact of surfactant digestion on the bioavailability of danazol after oral administration of lipidic self-emulsifying formulations to dogs. J Pharm Sci. 2008;97:995–1012. | ||
Balakumar K, Raghavan CV, Selvan NT, Prasad RH, Abdu S. Self nanoemulsifying drug delivery system (SNEDDS) of rosuvastatin calcium: design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf B Biointerfaces. 2013;112:337–343. | ||
Gursoy R, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–182. | ||
Shegokar R, Müller R. Nanocrystals: industrially feasible multifunctional formulation technology for poorly soluble actives. Int J Pharm. 2010;399:129–139. | ||
Junghanns J, Müller R. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomedicine. 2008;3:295–309. | ||
Patravale VB, Date A, Kulkarn RM. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2010;56:827–840. | ||
McKee J, Rabinow B, Cook C, Gass J. Nanosuspension formulation of itraconazole eliminates the negative inotropic effect of SPORANOX in dogs. J Med Toxicol. 2010;6:331–336. | ||
Patel GV, Patel VB, Pathak A, Rajput SJ. Nanosuspension of efavirenz for improved oral bioavailability: formulation optimization, in vitro, in situ and in vivo evaluation. Drug Dev Ind Pharm. 2014;40:80–91. | ||
Patel VR, Agrawal YK. Nanosuspension: an approach to enhance solubility of drugs. J Adv Pharm Technol Res. 2011;2:81–87. | ||
Dolenc A, Kristl J, Baumgartner S, Planinsek O. Advantages of celecoxib nanosuspension formulation and transformation into tablets. Int J Pharm. 2009;376:204–212. | ||
Basalious EB, Shawky N, Badr-Eldin SM. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: development and optimization. Int J Pharm. 2010;391:203–211. | ||
Date AA, Nagarsenker MS. Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int J Pharm. 2007;329:166–172. | ||
Zhang P, Liu Y, Feng N, Xu J. Preparation and evaluation of self-microemulsifying drug delivery system of oridonin. Int J Pharm. 2008; 355:269–276. | ||
Elnaggar Y, El-Massik M, Abdallah O. Self-nanoemulsifying drug delivery systems of tamoxifen citrate: design and optimization. Int J Pharm. 2009;380:133–141. | ||
Zhao Y, Wang C, Chow AH, et al. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: formulation and bioavailability studies. Int J Pharm. 2010;383:170–177. | ||
Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: design, characterization, in vitro and in vivo evaluation. Drug Deliv. Epub 2014 Feb 10. | ||
Talegaonkar S, Mustafa G, Akhter S, Iqbal Z. Design and development of oral oil-in-water nanoemulsion formulation bearing atorvastatin. J Disper Sci Technol. 2010;31:690–701. | ||
Liu D, Xu H, Tian B, et al. Fabrication of carvedilol nanosuspensions through the anti-solvent precipitation-ultrasonication method for the improvement of dissolution rate and oral bioavailability. AAPS Pharm Sci Tech. 2012;13:295–304. | ||
Kurakula M, Sobahi T, El-Helw A, Abdelaal M. Development and validation of a RP-HPLC method for assay of atorvastatin and its application in dissolution studies on thermosensitive hydrogel-based nanocrystals. Trop J Pharm Res. 2014;13:1681–1687. | ||
Legislation for the protection of animals used for scientific purposes [webpage on the Internet. Belgium: European Commission; 2014. Available from: http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm. Accessed December 15, 2014. | ||
Guiding Principles for the Care and Use of Vertebrate Animals in Research and Training [webpage on the Internet]. Bethesda: American Phsiological Society; 2014. Available from: http://www.the-aps.org/mm/SciencePolicy/About/Policy-Statements/Guiding-Principles.html. Accessed December 15, 2014. | ||
Mustafa G, Khan Z, Bansal T, Talegaonkar S. Preparation and characterization of oil in water nano-reservoir systems for improved oral delivery of atorvastatin. Current Nanoscience. 2009;5:428–440. | ||
Dodiya SS, Chavhan SS, Sawant KK, Korde AG. Solid lipid nanoparticles and nanosuspension formulation of saquinavir: preparation, characterization, pharmacokinetics and biodistribution studies. J Microencapsul. 2011;28:515–527. | ||
Dodiya S, Chavhan S, Korde A, Sawant KK. Solid lipid nanoparticles and nanosuspension of adefovir dipivoxil for bioavailability improvement: formulation, characterization, pharmacokinetic and biodistribution studies. Drug Dev Ind Pharm. 2013;39:733–743. | ||
Shanmugam S, Baskaran R, Balakrishnan P, Thapa P, Yong CS, Yoo BK. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) containing phosphatidylcholine for enhanced bioavailability of highly lipophilic bioactive carotenoid lutein. Eur J Pharm Biopharm. 2011;79:250–257. | ||
Parveen R, Baboota S, Ali J, Ahuja A, Vasudev S, Ahmad S. Oil based nanocarrier for improved oral delivery of silymarin: in vitro and in vivo studies. Int J Pharm. 2011;413:245–253. | ||
Wang LJ, Dong JF, Chen J, Eastoe JL, Li XF. Design and optimization of a new self-nanoemulsifying drug delivery system. J Colloid Interface Sci. 2009;330:443–448. | ||
Kanicky JR, Shah DO. Effect of degree, type, and position of unsaturation on the pKa of long-chain fatty acids. J Colloid Interface Sci. 2002;256:201–207. | ||
Liu G, Zhang D, Jiao Y, et al. Comparison of different methods for preparation of a stable riccardin D formulation via nano-technology. Int J Pharm. 2012;422:516–522. | ||
Bali V, Ali M, Ali J. Nanocarrier for the enhanced bioavailability of a cardiovascular agent: in vitro, pharmacodynamic, pharmacokinetic and stability assessment. Int J Pharm. 2011;403:46–56. | ||
Tamilvanan S. Formulation of multifunctional oil-in-water nanosized emulsions for active and passive targeting of drugs to otherwise inaccessible internal organs of the human body. Int J Pharm. 2009;381:62–76. | ||
Xia D, Quan P, Piao H, et al. Preparation of stable nitrendipine nanosuspensions using the precipitation-ultrasonication method for enhancement of dissolution and oral bioavailability. Eur J Pharm Sci. 2010;40:325–334. | ||
Filippos K, Santipharp P, Yunhui W. Nanosizing – Oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59:631–644. | ||
Dressman J, Reppas C. In vitro-in vivo correlations for lipophilic, poorly water-soluble drugs. Eur J Pharm Sci. 2000;11:73–80. | ||
Muller RH, Keck C. Challenges and solutions for the delivery of biotech drugs – a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol. 2004;113:151–170. | ||
Colombo I, Grassi G, Grassi M. Drug mechanochemical activation. J Pharm Sci. 2009;98:3961–3986. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.