Back to Journals » Research and Reports in Urology » Volume 12
Current Status of Simulation Training in Urology: A Non-Systematic Review
Authors Kozan AA , Chan LH, Biyani CS
Received 8 November 2019
Accepted for publication 20 February 2020
Published 17 March 2020 Volume 2020:12 Pages 111—128
DOI https://doi.org/10.2147/RRU.S237808
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Andrei Adrian Kozan,1 Luke Huiming Chan,2 Chandra Shekhar Biyani3
1Department of Urology, Hull University Teaching Hospitals NHS Trust, Castle Hill Hospital, Cottingham, UK; 2Department of Urology, Sheffield Teaching Hospitals NHS Foundation Trust, Royal Hallamshire Hospital, Sheffield, UK; 3Department of Urology, The Leeds Teaching Hospitals NHS Trust, St James’s University Hospital, Leeds, UK
Correspondence: Chandra Shekhar Biyani
St James’s University Hospital, Department of Urology, Lincoln Wing, Beckett Street, Leeds LS9 7TF, UK
Tel +44 113 2433144
Email [email protected]
Abstract: Simulation has emerged as an effective solution to increasing modern constraints in surgical training. It is recognized that a larger proportion of surgical complications occur during the surgeon’s initial learning curve. The simulation takes the learning curve out of the operating theatre and facilitates training in a safe and pressure-free environment whilst focusing on patient safety. The cost of simulation is not insignificant and requires commitment in funding, human resources and logistics. It is therefore important for trainers to have evidence when selecting various simulators or devices. Our non-systematic review aims to provide a comprehensive up-to-date picture on urology simulators and the evidence for their validity. It also discusses emerging technologies and future directions. Urologists should embed evidence-based simulation in training programs to shorten learning curves while maintaining patient safety and work should be directed toward a validated and agreed curriculum.
Keywords: simulation, education, learning, skills, innovation, urology
Introduction
“I fear not the man who has practiced 10,000 kicks once, but I fear the man who has practiced one kick 10,000 times.” – Bruce Lee
To perform safe and effective surgery, a urologist must undergo countless hours of surgical training in order to overcome learning curves and attain proficiency in a wide range of operations. While surgical training had traditionally been based on Halsted’s apprenticeship model of “see one, do one, teach one”,1 the evolution of the surgical environment over the past few decades have rendered this model obsolete.
Regulations on working hours by the European Working Time Directive have limited the amount of time trainees spend in the operating theatre.2 Shortening of shifts have also led to reduced continuity of the trainer–trainee relationship. Other changes such as an increased emphasis on patient safety, growing patient expectations and increased litigation, all influence the amount of time and opportunity novice trainees receive in the operating theatre.
Simulation has emerged as an effective solution to these challenges. The growing recognition of simulation in urology is reflected by the development of formal simulation training programs across the world. In the United Kingdom, a national simulation-based Urology Bootcamp forms a mandatory part of residency training.3 The European Basic Laparoscopic Urological Skills (E-BLUS)4 program is a validated laparoscopic simulation skills course that is frequently taught across the continent. Similar simulation-based courses have been developed in Asia by the Asian Urological Surgery Training and Education Group (AUSTEG)5 and there is growing interest in Sub-Saharan Africa.6
Simulation is defined as a technique to “replace or amplify real experiences with guided experiences that evoke or replicate substantial aspects of the real world in a fully interactive manner.”7 A simulator is a device or model used for the training of an individual by imitating real-life scenarios. Simulation training is not achieved simply by purchasing a simulator and allowing the trainee to practice unsupervised. To achieve optimal educational outcomes, McGaghie et al outlined that simulation must be integrated into the curriculum and encompass education principles such as feedback, deliberate practice, mastery learning, outcome measurement, skill acquisition and maintenance.8
It is not surprising that a larger proportion of surgical complications occur during the surgeon’s initial learning curve.9 While it is understood that trainees will eventually overcome these learning curves by treating patients, it is our ethical imperative to limit patient harm by using alternative methods of training for skill acquisition. Simulation comes into play by taking the learning curve out of the operating theatre and facilitating training in a safe and consequence-free environment. Errors can be made, learned from and reflected upon without harming a single patient. The simulation that is readily accessible also allows for greater flexibility of training around restricted working hours and limited operating theatre time. There is also evidence for simulation to improve performance when used preoperatively as a warm-up exercise based on a randomized-controlled trial in laparoscopic surgery.10
A simulator should be assessed for its validity before it is integrated into a training curriculum. Studies included in our review classified validity using the following types: face, content, construct, concurrent and predictive (Table 1).11
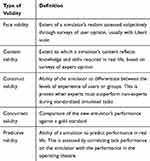 |
Table 1 Types of Validity |
The range of urology simulators has grown rapidly, from low-fidelity bench-top models to high-fidelity virtual reality consoles, covering various subspecialties. While simulation is likely cheaper than running an operating theatre, the cost is not insignificant and requires commitment in funding, human resources and logistics. It is therefore important for trainers to select the best evidence-based simulators for their trainees.
A non-structured search strategy in MEDLINE databases and reference tracking has been performed resulting in a non-systematic review. The studies included were taken from PubMed or references cited therein. The last search was conducted in September 2019. The terms used to perform the search were “urology simulation”. All English titles and abstracts were reviewed and included if they matched with the topic discussed.
This paper aims to provide a comprehensive up-to-date review on urology simulators and the evidence for their validity. We hope for it to serve as a guide for readers who are looking to implement evidence-based simulation in their urological training curriculum.
Open Urology Models
As a specialty, Urology branched from General Surgery with operations mainly done via an open approach in its infancy. However, it is now clear that urology is at the forefront of technology embracing the new and optimizing and updating the old.
Simulation models in open urological surgery are relatively limited in contrast to minimally invasive urology (Table 2). Perhaps seen as the “surgery of the past”, not many simulators have been developed and those available are either bench models or cadaveric ones.
 |
Table 2 Models for Open Urology |
Bench models are more prevalent in open urologic simulation. Basic clinical examination can be practiced using the Male Rectal Examination Trainer (Limbs & Things) where the user learns to differentiate between benign and malignant prostates and evaluate anal tone. The advance model costs £1600. The same company offers an advanced Male (£3920) and Female Pelvic Trainer (£3780) each simulating pathologies according to sex ranging from penile cancer, testicular tumor, cyst, varicocele, epididymo-orchitis, hydrocele, inguinal hernia to vaginal vault, cervix and interchangeable uterine modules (of more interest to the gynecologist) but also important in Urology as all females presenting with visible hematuria should undergo a pelvic exam.25 The female pelvic trainer has shown face and content validity.13
Adult circumcision is easily taught by simulation and is a core skill tested in the UK National Selection for residency training in urology. The model from Limbs & Things, UK comprises a penile model with replaceable foreskin made of synthetic bowel. It costs £170 and comes in a dark or light version with a pack of 5 foreskins, but these can also be bought separately. It has demonstrated face and content validity.15
The No-Scalpel Vasectomy Simulator costing US $225 (Advanced Meditech, USA) is a reusable simulator comprising 2 scrotal skins, 2 testicles and 2 long vas assemblies. The vas assembly can be changed at US $58 per pair. It has shown face validity in a small group.16
There are several suprapubic catheterization bench models available, of low and high fidelity. The main advantages of the low-fidelity ones are the low cost and availability. By contrast, high-fidelity SPC models are limited by their high price. Both have demonstrated various levels of validity. UroEmerge is a low-fidelity model with the highest level of validity, having achieved construct and predictive validity in a group of 36 candidates.17 Cheap and easily reproducible models for SPC have been developed either from a microwave container with a lid and a latex glove26 costing US $2 or from a box and a party balloon simulating the bladder at around AU $2.67.19
Models for suprapubic catheterization with ultrasound guidance have also been advanced. VesEcho consists of an ultrasound compatible gelatin mold that contains a water balloon, a pelvic bone replica and a non-rebreather mask (rectus fascia) that has demonstrated face and content validity among 13 urology residents.20 A cheaper option is US-SCIT (ultrasound-guided suprapubic catheter insertion trainer) constructed from common disposables (glove box, glove, infusion bag, trauma head blocks for stabilization) which showed the same level of validity among 50 participants.21
Another inexpensive, low-fidelity simulator with face and content validity has been created from hot dogs and red vines candy to simulate acute ischemic priapism. It costs US $1.25 and can be assembled in 10 mins.22
Fresh frozen cadavers have been used to teach open common urological operations under the BAUS cadaveric operative modules23 such as circumcision, vasectomy hydrocele repair and testicular fixation, radical orchidectomy and prostate biopsy. The same course offered sling procedures for male and female incontinence, artificial urinary sphincter insertion, colposuspension and rectus sling procedure. Cadaveric training on fresh frozen cadavers was also used for emergency and trauma urology including management of bladder perforation with bladder repair, ureteric reimplantation, emergency nephrectomy, open packing of the pelvis. Face and content validity were shown among 102 participants.23 At the time of this article being written, BAUS only confirmed upcoming dates for the Emergency Urology Cadaveric Course consisting of open cystostomy and SPC insertion, emergency exploration and nephrectomy, ureteric reimplantation, psoas hitch and Boari flap and scrotal exploration, testicular fixation and repair of rupture. It also features andrological emergencies such as penile block, treatment of priapism, dorsal slit, penile fracture repair and Fournier’s gangrene debridement.27
Thiel embalmed cadavers can be used as high-fidelity simulators and indeed a multispecialty evaluation for surgical training found them suitable, realistic, with reduced odor and more cost effective.28 Face validity has been demonstrated in a model for renal transplant proposed by Cabello.24
Endourological Models
The field of endourology with its confined environment lends itself well to simulation training and consequently many simulators have been developed. We have categorized them into nonbiologic (Table 3) and biologic models (Table 4). The nonbiologic ones are bench and virtual reality.
 |
Table 3 Nonbiologic Models for Endourology |
 |
Table 4 Biologic Models for Endourology |
Bench Models
Most of the bench models are high-fidelity and expensive. Cystoscopy and BOTOX injection has proven face and content validity29 on the ETXY-URO simulator. The same device can be used for ureteroscopy and has interchangeable male and female genitalia. It has established face, content and construct validity.30
Several models for ureteroscopy and resection from Limbs and Things, UK such as Uro-Scopic trainer, Bristol TURP and TURBT models with proven validity31–35 are no longer commercially available (AK enquired with the company July 2019).
The Resection Trainer LS10 from Samed, Germany utilizes a substrate for resection similar to human tissue and can be used in conjunction with all resectoscopes. It also has its own irrigation system. The model for TURBT has established face, content and construct validity in study of 76 subjects.36 A synthetic model replicating a hypertrophied prostate developed by Matsuda et al69 was used for HoLEP training (Kansai Medical University, Osaka) and demonstrated face and content validity. The prostate models can be replaced as needed.37
A number of models for ureteroscopy have been developed. The K-Box is a low-fidelity simulator for flexible ureteroscopy that replicates the upper tract. It requires a flexible ureteroscope to be navigated through the device, thus enabling the student to become accustomed to the movements required in flexible URS: in-out, pronation-supination, deflection, grasping-releasing. It has shown content38 and construct39 validity in a group of medical students that outperformed the control group. The Cook URS model was validated during a 2-week flexible URS course for 15 urology trainees. They performed significantly better in skills, time and the simulator demonstrated face, content and construct validity.40 With the EndoUro-Trainer apart from simple URS, stone extraction and basketing the candidate can also destroy the stone through either laser, electrohydraulic or electrokinetic lithotripsy. It has yet to be validated. Mediskills Advanced Scope Trainer is a high-fidelity validated model for face, content, construct and concurrent validity.41 The model is framed in an acrylic case containing a distensible bladder, ureteric orifices and even a distorted ureter designed for rigid and flexible URS. It also has the potential to be paired with a percutaneous access trainer for use in PCNL simulation.
SIM-PCNL is a high-fidelity model that uses 3D-printed molds to respect anatomically correct pelvicalyceal system, kidney and relevant adjacent structures such as thoracolumbar spine, adipose tissue and all layers of the posterior abdominal wall. It has been validated for face, content and construct validity in a group of 15 participants (5 experts and 10 novices) in a full-simulation environment with the greatest realism including percutaneous access, dilatation, lithotripsy and nephroscopy. Ultrasonographic appearances were rated low.42
Construct validity has been demonstrated for iPERC (a radiation-free training model) in 30 participants; however, neither face nor content validity was evaluated in the study.43 The Perc Trainer from Mediskills has been used to teach renal access via ultrasound or fluoroscopy, nephrostomy, tract dilatation, stone extraction/fragmentation and nephrostomy siting. Although reported in the literature it has not yet been validated.70
The C-Arm fluoro-less trainer from SimPORTAL, Minneapolis was designed using 3D printing and accommodates two webcams connected to a computer. The images obtained are processed to give a simulated on-screen image. According to Noureldin and Andonian, it has not yet been validated.71 More recently, Yoshida et al72 have developed an artificial kidney model, the T-box. This proved to be a feasible alternative to the biological porcine model showing similar intrarenal pressure and back-flow rates and that use of a smaller ureteral access sheath 10/12-Fr could result in a more rapid increase in intrarenal temperature during lasering. No endourological experiments have been performed yet on the model.72
Ballistic gel has also been used to create a kidney puncture model with an additional iPAD-guided puncture. This design was evaluated by five novices and three experts, showing good face, content and construct validity.73
Virtual Reality (VR) Models
The UroMentor, 3D Systems (previously Simbionix, USA) remains the most evaluated and validated VR system. Instruments include rigid and flexible cystoscopes, ureteroscopes, guidewires and baskets. A variety of preprogrammed tasks and cases of stones and strictures can be practiced with a real-time simulation of fluoroscopy and C-arm utilization. It has demonstrated face, content, construct and predictive validity, and a randomized controlled trial also showed transfer of skills from VR to OR (operating room).44–54 From the same company, the PERC Mentor is the only VR simulator validated for training and assessment of percutaneous renal access.55–57 It costs around US $100,000.71
Many VR resection simulators have emerged. The Uro Trainer (Karl Storz, Germany) offers modules for TURP with prostate resections increasing in difficulty and ranging from 55 to 90 g, as well as TURBT. Validation exists only for the later module in the form of face, content and construct in a group of 22 participants including residents and consultants.58 The TURP Mentor (3D Systems) offers platforms for TURP, TURBT and laser BPH, the manufacturer advertising it as the most advanced training simulator. The TURP element has been validated for face, content and construct.59,60
SurgicalSIM TURP (HelSim, USA) is a simulator that tracks the learner’s progress and compiles performance data over time issuing a detailed evaluation report. Studies have shown face, content and construct validity.61,62
The UroS platform from VirtaMed offers multiple BPH simulators such as TURP, ThuLEP, HoLEP, Diode PVP and TURBT simulation. The system has eight TURP full cases, four TURBT modules with various locations and difficulties including the risk of bladder perforation. The TURP component has demonstrated face, content and construct validity.65 Out of the laser BPH sections, HoLEP has been validated for face, content and construct validity in 53 participants66 and the diode PVP for construct validity.67 The same VirtaMed platform can be adapted and customized for specific procedures for other businesses and indeed it is being used for training for Rezum and UroLift.74,75
GreenLight SIM is another VR simulator used to teach GreenLight laser prostatectomy. It contains part-task exercises to familiarize the student with the device and technology and six operative procedures. It has shown face, content and construct validity.68
Biologic Models
The BAUS Cadaveric Modules had an established curriculum however future dates have yet to be confirmed. Another fresh frozen cadaveric (FFC) simulation program to teach ureteroscopy was validated for face and content by Huri et al76 in their study group. Twenty-nine obstetric residents confirmed construct validity for cystoscopy on similarly treated cadavers.77
Thiel embalmed cadavers (TEC) have good tissue color, consistency and malleability without the odor or infection risk and thus are used successfully for surgical training. Lower and upper tract endoscopy on Thiel cadavers has been evaluated by 12 urologists demonstrating face and content validity.78
Mains et al79 showed face and content validity in the first designated ureterorenoscopy course on Thiel cadavers with a high level of satisfaction among participants. So far only face validation exists for TURP on TEC.80 Sixty trainees validated URS in both ex vivo and live porcine models while undergoing a two-day program consisting of lectures, dry lab and live porcine training.81 Several animal models for teaching PCNL exist, however, they only have face validity at most. The majority consists of porcine kidney and ureter with skin flaps, subcutaneous tissue and muscles for fluoroscopic and/or ultrasound access. They are relatively simple and cheap to construct.71
More recently face, content and construct validity have been demonstrated for transurethral bulking for stress urinary incontinence. Female porcine bladders were mounted in a modified hysteroscopy trainer. Six experts and six trainees completed the simulator’s evaluation.83 Boar bladder can be used as a high-fidelity tool for teaching core cystoscopic skills in novice residents. Construct validity has been shown for the model.84
Laparoscopic Urology Models
Many simulators exist to teach basic laparoscopic skills. These are either box trainers, commercially available or “handmade” endotrainers, or virtual reality (VR) trainers. However, only few urology procedure-specific simulators have been developed so far (Table 5).
 |
Table 5 Laparoscopic Urology Models |
A bench model for laparoscopic partial nephrectomy made of polyvinyl alcohol incorporated in a SimMan mannequin was used for validation in a theatre environment and also coupled with non-technical skills; interestingly urology residents consistently rated themselves higher for non-technical skills.85,86 The group from Cleveland Clinic, Ohio developed and established face, content and construct validity for a ureteric reimplantation model made of hydrogel in 12 trainees and 5 experts.87 A randomized prospective, controlled study evaluated a latex model and showed face and concurrent validity, concluding that skills learned on their urethrovesical model transfer to a live porcine model. The live pig model was also assessed for face validity.88 Another synthetic model demonstrated face, content and concurrent validity in a group of 22 (10 experts and 12 novices).89
The virtual reality simulators are expensive; LapMentor (US $60-100,000) and LapSim (US $55,000) and procedure-specific modules have not yet been validated. Their basic skills modules, however, have been validated.11
Animal models are the primary teaching vector for urological procedure training. There are ex vivo and in vivo models with main drawbacks being ethical constraints and variances in anatomy. A rabbit model has been used to train for laparoscopic nephrectomy showing a decrease in duration of surgery and complications after 20 procedures.90 Pyeloplasty can be taught on a model made of porcine bladder or chicken crop and esophagus to simulate the renal pelvis and ureter. Both models have been evaluated for construct validity.91,92 A group of 50 participants (30 novices and 20 trained laparoscopists) assessed a chicken model designed for Lich-Gregoire ureteric reimplantation. The trachea simulated the ureter, the esophagus the common iliac and the crop simulated the bladder. The authors demonstrated face, content and construct validity.93 A key step in radical prostatectomy, urethrovesical anastomosis, has been simulated in a chicken chest or skin model and a porcine intestinal model. They all have shown construct validity.94–96
Live pigs are being used for training on full procedures as part of a laparoscopic course teaching radical nephrectomy and cystectomy or for more advanced participants inferior vena cava suture. The models have not undergone any validation.97
A course on Thiel embalmed cadavers for UK trainees is also running at the University of Leeds based on the model validated by Rai at al.98 for laparoscopic radical nephrectomy. Each trainee performs the procedure under supervision after attending a live demonstration in the operating theatre.
Robotic Urology Models
There has been a trend in recent years of developing new models for robotic surgery (Table 6) or refining and establishing a curriculum for training. Virtual Reality (VR) simulators are the mainstay for basic and procedural skills.
 |
Table 6 Robotic Urology Models |
Weiner et al123 suggested 10 hrs of simulator training for basic and advanced skills may be optimal for an acceptable level of surgical ability. Interestingly, however, Mills et al124 concluded in a study of 10 attending surgeons that there was no correlation between basic skills simulator performance and intraoperative performance. The clinical implications of this remain unestablished.
VR simulators are expensive tools in the arsenal of robotic training. The dVSS and dV-Trainer are the most extensively validated VR simulators, demonstrating all levels of validity.99–102 The dVSS costs USD $89,000101 works directly with the da Vinci console but cannot be used if the console is in use for operating thus greatly limiting training time. The Mimic dVT is a stand-alone simulator costing USD $158,000101 that runs MSim software like the dVSS. It is the most validated robotic simulator.11 Tube-3 module to teach urethrovesical anastomosis (UVA) has also been broadly validated.106–108 FlexVR (Mimic Technologies) is a newer flexible and portable training platform design to teach fundamentals of robotic surgery. It has yet to be formally validated.
RobotiX Mentor is also a stand-alone simulator created by 3D Systems. The basic skills modules have shown face, content and construct validity100,101,103 and the prostatectomy module has recently been validated for face, content and construct in a group of 13 novice, 24 intermediate and 8 expert surgeons.104
The Robotic Surgical Simulator (RoSS) by Simulated Surgical Systems is another stand-alone simulator that has basic skills and procedure-specific modules. It costs USD $120,000.101 Studies have demonstrated face, content and construct validity for the fundamental skills.100,101 So far only the augmented reality UVA module has confirmed construct validity.105 The platform also offers robotic-assisted radical prostatectomy (RARP), robotic-assisted radical cystectomy (RARC) and lymph node dissection.
MaestroAR has been designed by Mimic Technologies to provide procedure-specific training in augmented reality media for both dV-Trainer and FlexVR. The partial nephrectomy module has face, content, construct and concurrent validity.109 Mimic also developed the Xperience Team Trainer which functions as a complementary hardware unit for the dV-Trainer. It is aimed at assistant simulation, verbal communication and reaction to one another’s actions and has shown face, content, construct and concurrent validity in 28 volunteers.110
In comparison to VR simulators, fewer bench models for robotic surgery have been validated. Three basic skills have been validated by Ramos et al111 in a group of novices and experts using global evaluative assessment of robotic skills. 3D printed kidney and tumor models have been used for robotic-assisted partial nephrectomy (RAPN). SIMPLE-PN demonstrated face, content, construct and concurrent validity in two studies of small size (8 participants).112,113 However, Monda et al114 involved 24 participants in validating their model for RAPN measuring similar metrics of warm ischemia, preserved renal parenchyma and surgical margins. They established face, content and construct validity. Recently two new models for UVA simulation have emerged applying 3D-printed technology and silicone using the da Vinci system.115,116 The larger sample group (20) of Johnson et al115 consisted of experts, intermediates and novices and demonstrated face, content and construct validity for their low-cost ($2.50), high-fidelity model.
Various animal models have been used for robotic training however fewer than other disciplines. A porcine kidney with a Styrofoam ball to replicate a kidney tumor was used for a RAPN simulator. The authors showed face, content and construct validity among 46 participants.117 Pig bowel was introduced in an abdominal trainer to simulate spatial constraints and using the da Vinci SI robotic system validated the model for face, content and construct.118 Again using porcine material, a female genitourinary tract was employed to teach steps of RARP. Seminal vesicles and dorsal venous complex were mimicked by the fallopian tubes and the introitus was used as the prostate. The model showed face, content and construct validity.120 The proventriculus and the proximal part of a chicken’s esophagus stand to simulate UVA during RARP. Posterior fascial reconstruction was also performed between the tissues on the posterior surface of the esophagus and the serosa of the proventriculus. Face and content validity were demonstrated.119
Basic skills and RARP have been taught on cadavers and some studies showed validity121,122 although robust evidence is lacking. The European Association of Urology has a validated robotic training curriculum that integrates cadaveric training.125
Remaining on the topic of prostate cancer, tissue diagnosis is essential. The Biopsym is a virtual reality simulator for transrectal ultrasound-guided (TRUS) biopsy consisting of a haptic device connected to a 3D ultrasound image library.126 The haptic stylus mimics the movement of ultrasound probe while corresponding 2D image slices are projected in real-time. This model has demonstrated face, content and construct validity.127,128 Another VR simulator uses an ultrasound probe inside a mock pelvis, synchronized with a 3D ultrasound image by a 3D magnetic tracking system. This was shown to have face, content and construct validity.129
With the growing popularity of transperineal biopsies, two simulators have been developed: an augmented reality simulator by the University of Florida130 and a virtual reality haptics-enabled simulator by the University of Chester.131 These simulators are not commercially available and no validation studies have been published to date.
Low dose-rate brachytherapy seed implantation and high dose-rate brachytherapy source positioning is technically very similar to transperineal prostate biopsy. A brachytherapy training program using a bench-top simulator (made from a prostate phantom and dummy seeds) showed promise at improving residents’ skill acquisition by training residents to perform high-quality implants.132 A virtual reality haptic simulator for seed implantation was developed using multiple haptic devices to represent an ultrasound probe and needle.133 Advanced computerized simulation is also used in the treatment planning for high dose-rate brachytherapy to optimize radiation dose delivery and minimize source positioning errors.134 These simulators are not yet validated in the literature.
Sehrawat et al135 developed a computer-based simulation tool to train urology residents. Simulated prostate cancer cases were created, and residents had to plan the layout of probes as well as depth insertion to achieve the perfect treatment outcome. Trainees were allowed unlimited attempts to solve six cases within 50 mins. It was found that with just 50 mins of planning practice, novice resident performance in planning increased significantly from 2.2% to 31.1%.135
External beam radiation therapy for prostate cancer involves meticulous planning to maximize therapeutic benefit and minimize toxicity to surrounding organs. A prostate fossa contouring simulator was developed to teach and improve contouring accuracy during treatment planning. After sufficient practice with this simulator, novice medical students were able to contour the prostate fossa therapy with near “excellent agreement” with plans by expert radiation oncologists.136
Non-Technical Skills
Recently there has been more focus on non-technical skills (NTS) training in surgery. NTS is an emerging field of research. So far there is no standardized training and the subject is yet to be implemented in surgical training across specialties.
A systematic review by Anderson et al137 showed that 14.4% of surgical patients will experience adverse effects of various severity, out of these 5.2% are potentially preventable. The same paper indicated more incidents were caused by errors in NTS than faults in the operating technique.137 Similarly, a report to the National Patient Safety Agency in the UK found that almost half of the incidents had a failure in NTS.138 Recently, however, a meta-analysis139 failed to find a statistically significant improvement of patient’s outcomes after NTS training of theatre staff. They did nonetheless recognize that their conclusion was drawn from a small number of heterogeneous studies.139 Three separate categories of NTS have been recognized: social, cognitive skills and personal resource factors.140 The metrics for assessments include NOTSS – Non-Technical Skills for Surgeons, NOTECHS – Non-technical Skills and OTAS – Observational Teamwork Assessment for Surgery.141
A high-fidelity simulated ward round has been used as part of NTS training and assessment in the UK Urology Simulation Bootcamp for newly appointed senior urology trainees. Forty-eight doctors participated and individually led a simulated ward round where distractions were introduced in an evolving urology-related scenario. Freeze-frames and whole-group structured debriefing and feedback were also offered. The mean NOTTS scores indicated that NTS performances could be improved.142
A prospective cohort study143 using a team-training scenario where residents performed a laparoscopic partial nephrectomy on a validated simulator showed that non-technical skills performance (based on NOTSS score) was significantly affected by the residents’ level of training therefore establishing construct and face validity. By contrast, another study which used a critical scenario during laparoscopic radical nephrectomy found that urology resident training correlated with technical performance but not with NTS. They also indicated face validity.144
In a prospective simulation study including 17 urology residents, significant improvement was noted on validated teamwork instruments between scenarios based on resident and expert evaluation. Face and content validity were also shown.145
Face, content and construct validity were demonstrated in a distributed simulation environment where participants performed a TURP in a portable, simulated operating room. NOTECHS scale was used for evaluation, debriefing and feedback was received. One of the advantages of this environment is that it can be set up in any open space.146
Brunckhorst et al147 looked at the relationship between technical and non-technical skills in a simulation-based ureteroscopy training environment. They concluded that a strong correlation does exist between the two, which was demonstrated to be irrespective of training received. It was also shown that all non-technical skill sets are important in technical performance advancing the notion of training and assessing both skills simultaneously.147
Discussion
Reflections
This non-systematic review provides a comprehensive update on simulators used in urology and their validity. As outlined, there are many different simulators from the very basic to the extremely sophisticated and expensive. Different technologies present with different advantages and limitations each with their own field of application. It is therefore essential to establish the learning objectives from the outset where a simulator can help, thus identifying the most appropriate method of achieving the learning goal.
Simulation should complement essential urological training and be gauged at the beginning of the learning curve in a safe and protective environment where mistakes are not catastrophic, then practice should progress with clinical and skill acquisition. Simulation is no substitute for clinical practice.
There is still ongoing expert debate as to whether simulation can be used for assessment purposes, especially for summative assessment. To our knowledge, there is no criteria to formally validate the training potential of individual simulators. Similarly, there is paucity of evidence supporting the use of simulation assessment tools for high-stakes assessments, which is a problem in the whole surgical literature. Perhaps the time has come when we should temper developing new simulators and further our research on the educational impact such as establishing the transfer of skill from simulation into practice.
Unfortunately, we still lack an agreed universally accepted curricula despite some efforts such as EUREP (European Urology Residency Program) or FLS (Fundamentals of Laparoscopic Surgery). We certainly need future research that should result in a multicentre international agreement. Even so, it is likely that only large centres might be able to offer such program and we might be heading towards a centralised simulation delivery.
We should not take lightly that the delivery process is resource-demanding from time spent in organising and design, selecting expert tutors, logistical equipment to the appropriate venue to time that might not be remunerated or reimbursed. There are also inequalities in what can be provided, for example large teaching centres will have money and resources to engage in simulation or even simulation centres/hubs whilst other organisations might struggle with this.
Surprisingly, despite a lot of attention in recent time, non-technical skills training failed to improve complication rates in the operating theatre according to the latest meta-analysis.139 Interestingly as well, surgeons and anaesthetists were the main actors failing to show improvements. However, it is of note to mention that most of the studies that looked at this were underpowered and heterogeneous. We still need well-designed and well-conducted prospective, randomised trials to better our understanding. Also, a standardized training curriculum is needed where the framework should combine the most useful and effective modalities tailored for individual specialties.
We believe simulation to be an important adjunct to a modern competency-based urological training, with residents receiving continuous exposure throughout their training and can also help in continuous professional development. In doing so trainers can also provide targeted learning and bridge the gap depending on training needs.
Limitations
We accept this is a non-systematic review and is by no means exhaustive of all simulators used in urology. A systematic review would have offered better focus and logical progressive sequence and perhaps even clarity. Nevertheless, this paper is providing a thorough, comprehensive and contemporary review on urology simulators and the evidence for their validity.
Future Directions
It is certain that urology is at the forefront of technology and so embracing the advancements and new developments only comes naturally. As simulation methods become increasingly realistic, virtual reality (VR) will continue to progress and augmented (AR) and mixed reality (MR) will have a more formalized and less futuristic role to play.
In the era of Snapchat and Pokémon Go it is almost inevitable not to find AR applied in surgery. For example, Gunner Goggles Series148 features a mobile app to be used in conjunction with the medical books to enhance learning by integrating AR using animations, visuals and 3D models to clarify complex anatomy, conditions or various concepts. HoloHuman149 is being advertised as the world’s most complete anatomy application for mixed reality and HoloLens from Microsoft. It boasts more than 13,000 separate anatomical structures and offers a life-like alternative to the traditional cadaveric dissections.
We postulate that AR/MR are the best examples of symbiosis between simulation and its direct application in real life. It is not surprising then to see different head-mounted displays (HMD) already been utilized in practice. There are currently three types: see-through HMDs, heads-up HMDs and non-see-through HMDs. A recent systematic review150 found that the primary use of HMD was for image guidance and AR and secondly for data display. Intra-operative education also featured either trainer or trainee directed. Overall, see-through HMDs were the most common type reported but in education and training, in particular, heads-up HMD led the way. There are of course limitations in the use of these devices and the same paper identified concerns about patient information security or privacy, heavy or cumbersome devices, battery life, lag time in AR and the dependence on wireless internet or Bluetooth connection.150 It is however promising technology for simulation. The use of the headset display allows for portability and removes the need for multiple screens. The higher degree of immersiveness offered by holograms allows for better spatial awareness and can be experienced by multiple users simultaneously.
Porpiglia et al151 compared outcomes between using AR vs standard procedure. Their hyper-accuracy 3D reconstruction software-based integration was used in 21 RAPN. They concluded that a higher number of patients were undergoing global ischemia in the control group (80%) as opposed to AR group (24%). The surgeons also adhered to the preoperative management plan of the renal pedicle in 90% of the AR cases vs 61% in the control group. Similarly, 3D elastic AR was found to correctly identify capsular involvement in RARP in 100% of cases when compared to 47% in the 2D MRI cognitive group.152
A systematic review of AR in urological interventions153 ascertained that overall the available literature regarding AR is largely limited to reports without control groups and mainly adopted by larger or academic centers. A major limitation of AR-enhanced surgery, particularly in operations involving soft tissues that suffer deformations, is the inaccuracy in registration that translates into poor navigation precision. This is where improvement in technology is awaited. Importantly also, more simulation using AR could help reduce the anxieties in accepting and finding more clinical applications.153
Telemedicine AR is an exciting avenue where one surgeon is in the operating room and the other anywhere in the world. The expert surgeon can watch and potentially correct the surgeon operating in real-time. Proximie, a London-based company has gained prominence with its use of AR technology and telemedicine to democratize global surgical training. Their AR technology enables the trainer/expert to observe an operation remotely and provide real-time guidance by pointing or drawing over the operative field, which is then overlaid and visualized by the operating surgeon.154
Artificial intelligence (AI) has the flexibility and capability to work and learn from large data. Deep-learning methods make minimal assumptions and give better predictions compared to traditional data especially when dealing with huge dimensional data. AI can help in predictions with early, accurate and individualized decisions. However, there have been instances where traditional statistics outperformed AI making it hard to justify direct application in clinical life. AI needs rigorous quality control, regulations and external validation. AI systems require continuous training by data from clinical studies and independent validation to maximize their potential.155 They can potentially be used for assessing and predicting training skill progression among surgical trainees.
With the ever-growing range of simulators and the exciting potential of new emerging technologies, we believe that there has been no better era to undergo urological training.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sealy W. Halsted is dead: time for change in graduate surgical education. Curr Surg. 1999;56(1–2):34–39. doi:10.1016/S0149-7944(99)00005-7
2. Marron CD, Byrnes CK, Kirk SJ. An EWTD-compliant shift rota decreases training opportunities. Bull R Coll Surg Engl. 2005;87(7):246–248. doi:10.1308/147363505X46880
3. Young M, Kailavasan M, Taylor J, et al. The success and evolution of a urological “boot camp” for newly appointed UK urology registrars: incorporating simulation, nontechnical skills and assessment. J Surg Educ. 2019;76(5):1425–1432. doi:10.1016/j.jsurg.2019.04.005
4. Somani B, Van Cleynenbreugel B, Gozen A, et al. Outcomes of European Basic Laparoscopic Urological Skills (EBLUS) Examinations: results from European School of Urology (ESU) and EAU Section of Uro-Technology (ESUT) over 6 years (2013–2018). Eur Urol Focus. 2019. doi:10.1016/j.euf.2019.01.007
5. Tiong HY, Zhu G, Ong TA, et al. Performance in fundamentals in laparoscopic surgery (FILSTM) reflects global rating scales in objective structured assessment of technical skills (OSATS) for porcine laparoscopic surgery. Int J Urol. 2017;24(Suppl. 1):S45–S46.
6. Campain N, Kailavasan M, Chalwe M, et al. An evaluation of the role of simulation training for teaching surgical skills in Sub-Saharan Africa. World J Surg. 2017;42(4):923–929. doi:10.1007/s00268-017-4261-7
7. Gaba D. The future vision of simulation in health care. Qual Saf Health Care. 2004;13(Suppl 1):S2–S10. doi:10.1136/qshc.2004.009878
8. McGaghie WC, Issenberg SB, Petrusa ER, Scalese R. A critical review of simulation-based medical education research: 2003–2009. Med Educ. 2010;44(1):50–63. doi:10.1111/j.1365-2923.2009.03547.x
9. Maruthappu M, Duclos A, Lipsitz S, Orgill D, Carty M. Surgical learning curves and operative efficiency: a cross-specialty observational study. BMJ Open. 2015;5(3):e006679. doi:10.1136/bmjopen-2014-006679
10. da Cruz J, Dos Reis S, Cunha Frati R, et al. Does warm-up training in a virtual reality simulator improve surgical performance? A prospective randomized analysis. J Surg Educ. 2016;73(6):974–978. doi:10.1016/j.jsurg.2016.04.020
11. Aydin A, Raison N, Khan MS, Dasgupta P, Ahmed K. Simulation-based training and assessment in urological surgery. Nat Rev Urol. 2016;13(9):503–519. doi:10.1038/nrurol.2016.147
12. Kailavasan M, Abdul-Rahman A, Hanchanale V. The validation of the clinical male pelvic trainer Mk 2-advanced models for scrotal examination simulation. J Surg Educ. 2017;74(3):423–430. doi:10.1016/j.jsurg.2016.10.008
13. Edwards S, Cass G, Lenguerrand E, Fox R, Crofts J. Realism and construct validity of novel pelvic models of common gynecologic conditions. Int J Gynaecol Obstet. 2014;124(3):270–273. doi:10.1016/j.ijgo.2013.09.016
14. Abdulmajed MI, Thomas M, Shergill IS. A new training model for adult circumcision. J Surg Educ. 2012;69(4):447–448. doi:10.1016/j.jsurg.2011.12.004
15. Parnham A, Campain N, Biyani CS, Muneer A, Venn S. Validation of a reusable model for simulation training of adult circumcision. Bull R Coll Surg Engl. 2015;97:383–385. doi:10.1308/rcsbull.2015.383
16. Pathak R, Alford S, Igel T. Mp23-07 vasectomy simulation module: didactic, audio-visual, and live-simulation experience. J Urol. 2015;193:e269. doi:10.1016/j.juro.2015.02.1250
17. Shergill IS, Shaikh T, Arya M, Junaid I. A training model for suprapubic catheter insertion: the UroEmerge suprapubic catheter model. Urology. 2008;72(1):196–197. doi:10.1016/j.urology.2008.03.021
18. Singal A, Halverson A, Rooney DM, Davis LM, Kielb SJ. A validated low-cost training model for suprapubic catheter insertion. Urology. 2015;85(1):23–26. doi:10.1016/j.urology.2014.08.024
19. Hossack T, Chris BB, Beer J, Thompson G. A cost-effective, easily reproducible, suprapubic catheter insertion simulation training model. Urology. 2013;82(4):955–958. doi:10.1016/j.urology.2013.06.013
20. Palvolgyi R, Lee A, Ramirez F, et al. VesEcho Training System: suprapubic catheterization under ultrasound guidance. Urol Pract. 2018;5(1):63–68. doi:10.1016/j.urpr.2017.01.003
21. Nonde J, Adam A, Laher AE. Validation of a low cost, disposable, and ultrasound-guided suprapubic catheter insertion trainer. Urology. 2018;115:45–50. doi:10.1016/j.urology.2018.02.013
22. Dai JC, Ahn JS, Cannon ST. Acute ischemic priapism management: an educational and simulation curriculum. MedEdPORTAL. 2018;14:1073. doi:10.15766/mep_2374-8265.10731
23. Ahmed K, Aydin A, Dasgupta P, Khan MS, McCabe JE. A novel cadaveric simulation program in urology. J Surg Educ. 2015;72(4):556–565. doi:10.1016/j.jsurg.2015.01.005
24. Cabello R, González C, Quicios C, et al. An experimental model for training in renal transplantation surgery with human cadavers preserved using W. Thiel’s embalming technique. J Surg Educ. 2015;72(2):192–197. doi:10.1016/j.jsurg.2014.10.002
25. Available from: https://limbsandthings.com/uk/specialties/urology/?filters=tasktrainers.packs.
26. Gao W, Ou T, Jia J, et al. Development and evaluation of a training model for paracentetic suprapubic cystostomy and catheterization. Clinics (Sao Paulo). 2019. doi:10.6061/clinics/2019/e435
27. Available from: https://www.baus.org.uk/professionals/events/2664/emergency_urology_cadaveric_courses_2019.
28. Yiasemidou M, Roberts D, Glassman D, Tomlinson J, Biyani S, Miskovic D. A multispecialty evaluation of Thiel cadavers for surgical training. World J Surg. 2017;41(5):1201–1207. doi:10.1007/s00268-016-3868-4
29. Aydin A, Ahmed K, McCabe JE, Khan MS, Dasgupta P, Sahai A. Validation of a dry-lab training model for cystoscopy and delivery of intravesical botulinum-toxin injections. J Endourol. 2015;29(S1):A80–A81.
30. Soria F, Morcillo E, Serrano A, et al. Development and validation of a novel skills training model for retrograde intrarenal surgery. J Endourol. 2015;29(11):1276–1281. doi:10.1089/end.2015.0421
31. Mishra S, Sharma R, Kumar A, Ganatra P, Sabnis RB, Desai MR. Comparative performance of high-fidelity training models for flexible ureteroscopy: are all models effective? Indian J Urol. 2011;27(4):451–456. doi:10.4103/0970-1591.91431
32. Matsumoto ED, Hamstra SJ, Radomski SB, Cusimano MD. A novel approach to endourological training: training at the surgical skills center. J Urol. 2001;166(4):1261–1266. doi:10.1016/S0022-5347(05)65749-7
33. Chou DS, Abdelshehid C, Clayman RV, McDougall EM. Comparison of results of virtual-reality simulator and training model for basic ureteroscopy training. J Endourol. 2006;20(4):266–271. doi:10.1089/end.2006.20.266
34. Khan SM, Ahmed K, Gavazzi A, et al. Development and implementation of centralized simulation training: evaluation of feasibility, acceptability and construct validity. BJU Int. 2013;111(3):518–523. doi:10.1111/j.1464-410X.2012.11204.x
35. Brewin J, Ahmed K, Khan MS, Jaye P, Dasgupta P. Face, content, and construct validation of the Bristol TURP trainer. J Surg Educ. 2014;71(4):500–505. doi:10.1016/j.jsurg.2014.01.013
36. de Vries AH, van Genugten HG, Hendrikx AJ, et al. The Simbla TURBT simulator in urological residency training: from needs analysis to validation. J Endourol. 2016;30(5):580–587. doi:10.1089/end.2015.0723
37. Aydin A, Ahmed K, Brewin J, Khan MS, Dasgupta P, Aho T. Face and content validation of the prostatic hyperplasia model and holmium laser surgery simulator. J Surg Educ. 2014;71(3):339–344. doi:10.1016/j.jsurg.2013.11.004
38. Villa L, Şener TE, Somani BK, et al. Initial content validation results of a new simulation model for flexible ureteroscopy: the key-box. J Endourol. 2017;31(1):72–77. doi:10.1089/end.2016.0677
39. Villa L, Sener TE, Cloutier J, et al. Preliminary results of intensive training on a simulation model for flexible ureteroscopy in medical students: the Kidney-Box (K-BOX) model.
40. Blankstein U, Lantz AG, D’A Honey RJ, Pace KT, Ordon M, Lee JY. Simulation-based flexible ureteroscopy training using a novel ureteroscopy part-task trainer. Can Urol Assoc J. 2015;9(9–10):331–335. doi:10.5489/cuaj.2811
41. Al-Jabir A, Aydin A, Abe T, et al. Validation of the advanced scope trainer for flexible ureterorenoscopy training. Urology. 2017;110:45–50. doi:10.1016/j.urology.2017.07.047
42. Ghazi A, Campbell T, Melnyk R, et al. Validation of a full-Immersion simulation platform for percutaneous nephrolithotomy using three-dimensional printing technology. J Endourol. 2017;31(12):1314–1320. doi:10.1089/end.2017.0366
43. Maldonado Alcaraz E, Moreno J, Montoya G, Torres-Mercado L, López V, Serrano-Brambila E. Use of a novel radiation-free fluoroscopy emulator (iPERC) to improve surgical skills in percutaneous nephrolithtotomy. Eur Urol Suppl. 2015;14(2):eV2. doi:10.1016/S1569-9056(15)61087-9
44. Dolmans VEMG, Schout BMA, de Beer NAM, Hendrikx AJM. Determination of construct validity of the URO mentor, a virtual reality simulator for endourological procedures. J Soc Simul Healthcare. 2006;1(3):192. doi:10.1097/01266021-200600130-00053
45. Gettman MT, Le CQ, Rangel LJ, Slezak JM, Bergstralh EJ, Krambeck AE. Analysis of a computer based simulator as an educational tool for cystoscopy: subjective and objective results. J Urol. 2008;179(1):267. doi:10.1016/j.juro.2007.08.146
46. Gettman MT, Le CQ, Rangel LJ, Slezak JM, Bergstralh EJ, Krambeck AE. Development of a standardized curriculum for teaching cystoscopic skills using a computer-based endourologic simulator. Simul Healthc. 2009;4(2):92–97. doi:10.1097/SIH.0b013e3181871c3e
47. Schout BM, Muijtjens AM, Hendrikx AJ, et al. Acquisition of flexible cystoscopy skills on a virtual reality simulator by experts and novices. BJU Int. 2010;105(2):234–239. doi:10.1111/j.1464-410X.2009.08733.x
48. Schout BM, Ananias HJ, Bemelmans BL, et al. Transfer of cysto-urethroscopy skills from a virtual-reality simulator to the operating room: a randomized controlled trial. BJU Int. 2010;106(2):226–231. doi:10.1111/j.1464-410X.2009.09049.x
49. Schout BM, Muijtjens AM, Hendrikx AJ, et al. Acquisition of flexible cystoscopy skills on a virtual reality simulator by experts and novices. BJU Int. 2010;105(2):234–239. doi:10.1111/j.1464-410X.2009.08733.x
50. Dolmans VE, Schout BM, de Beer NA, Bemelmans BL, Scherpbier AJ, Hendrikx AJ. The virtual reality endourologic simulator is realistic and useful for educational purposes. J Endourol. 2009;23(7):1175–1181. doi:10.1089/end.2008.0487
51. Aloosh M, Couture F, Fahmy N, Elhilali M, Andonian S Assessment of urology postgraduate trainees’ competencies in flexible ureteroscopic stone extraction.
52. Brunckhorst O, Aydin A, Abboudi H, et al. Simulation-based ureteroscopy training: a systematic review. J Surg Educ. 2015;72(1):135–143. doi:10.1016/j.jsurg.2014.07.003
53. Matsumoto ED, Pace KT, D’A Honey RJ. Virtual reality ureteroscopy simulator as a valid tool for assessing endourological skills. Int J Urol. 2006;13(7):896–901. doi:10.1111/iju.2006.13.issue-7
54. Ogan K, Jacomides L, Shulman MJ, Roehrborn CG, Cadeddu JA, Pearle MS. Virtual ureteroscopy predicts ureteroscopic proficiency of medical students on a cadaver. J Urol. 2004;172(2):667–671. doi:10.1097/01.ju.0000131631.60022.d9
55. Mishra S, Kurien A, Patel R, et al. Validation of virtual reality simulation for percutaneous renal access training. J Endourol. 2010;24(4):635–640. doi:10.1089/end.2009.0166
56. Mishra S, Kurien A, Ganpule A, Muthu V, Sabnis R, Desai M. Percutaneous renal access training: content validation comparison between a live porcine and a virtual reality (VR) simulation model. BJU Int. 2010;106(11):1753–1756. doi:10.1111/j.1464-410X.2010.09753.x
57. Noureldin YA, Fahmy N, Anidjar M, Andonian S. Is there a place for virtual reality simulators in assessment of competency in percutaneous renal access? World J Urol. 2016;34(5):733–739. doi:10.1007/s00345-015-1652-y
58. Schulz GB, Grimm T, Buchner A, et al. Validation of a high-end virtual reality simulator for training transurethral resection of bladder tumors. J Surg Educ. 2019;76(2):568–577. doi:10.1016/j.jsurg.2018.08.001
59. Tjiam IM, Berkers CH, Schout BM, et al. Evaluation of the educational value of a virtual reality TURP simulator according to a curriculum-based approach. Simul Healthc. 2014;9(5):288–294. doi:10.1097/SIH.0000000000000041
60. Bright E, Vine S, Wilson MR, Masters RS, McGrath JS. Face validity, construct validity and training benefits of a virtual reality TURP simulator. Int J Surg. 2012;10(3):163–166. doi:10.1016/j.ijsu.2012.02.012
61. Sweet R, Kowalewski T, Oppenheimer P, Weghorst S, Satava R. Face, content and construct validity of the University of Washington virtual reality transurethral prostate resection trainer. J Urol. 2004;172:1953–1957. doi:10.1097/01.ju.0000141298.06350.4c
62. Hudak SJ, Landt CL, Hernandez J, Soderdahl DW. External validation of a virtual reality transurethral resection of the prostate simulator. J Urol. 2010;184(5):2018–2022. doi:10.1016/j.juro.2010.06.141
63. Källström R, Hjertberg H, Kjölhede H, Svanvik J. Use of a virtual reality, real-time, simulation model for the training of urologists in transurethral resection of the prostate. Scand J Urol Nephrol. 2005;39(4):313–320. doi:10.1080/00365590510031246
64. Källström R, Hjertberg H, Svanvik J. Construct validity of a full procedure, virtual reality, real-time, simulation model for training in transurethral resection of the prostate. J Endourol. 2010;24(1):109–115. doi:10.1089/end.2009.0114
65. Tjiam IM, Berkers CH, Schout BM. Evaluation of the educational value of a virtual reality TURP simulator according to a curriculum-based approach. Simul Healthc. 2014;9(5):288–294. doi:10.1097/SIH.0000000000000041
66. Kuronen-Stewart C, Ahmed K, Aydin A, et al. Holmium laser enucleation of the prostate: simulation-based training curriculum and validation. Urology. 2015;86(3):639–646. doi:10.1016/j.urology.2015.06.008
67. Angulo JC, Arance I, García-Tello A, et al. Virtual reality simulator for training on photoselective vaporization of the prostate with 980 nm diode laser and learning curve of the technique. Actas Urol Esp. 2014;38(7):451–458. doi:10.1016/j.acuro.2014.02.013
68. Aydin A, Muir GH, Graziano ME, Khan MS, Dasgupta P, Ahmed K. Validation of the GreenLight™ simulator and development of a training curriculum for photoselective vaporisation of the prostate. BJU Int. 2015;115(6):994–1003. doi:10.1111/bju.12842
69. Matsuda K, Kinoshita H, Okamoto Y. Prostatic Hyperplasia Model and Prostate Surgery Simulator. World Intellectual Property Organization. 2013. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2013073210&_cid=P12-K7KCCJ-32997-1
70. Lovegrove CE, Abe T, Aydin A, et al. Simulation training in upper tract endourology: myth or reality? Minerva Urol Nefrol. 2017;69(6):579–588. doi:10.23736/S0393-2249.17.02873-9
71. Noureldin YA, Andonian S. Simulation for percutaneous renal access: where are we? J Endourol. 2017;31(Suppl 1):S10–S19. doi:10.1089/end.2016.0587
72. Yoshida T, Inoue T, Taguchi M, Matsuzaki T, Matsuda T. Development of a new experimental model for in vitro study of retrograde intrarenal surgery: the “T-box”. Int J Urol. 2018;25(10):898–900. doi:10.1111/iju.13753
73. Klein JT, Rassweiler J, Rassweiler-Seyfried MC. Validation of a novel cost effective easy to produce and durable in vitro model for kidney-puncture and percutaneous nephrolitholapaxy-simulation. J Endourol. 2018;32(9):871–876. doi:10.1089/end.2017.0834
74. Available from: https://www.virtamed.com/en/news/video-custom-made-virtamed-simulator-nxtheras-rezum-bph-treatment/.
75. Available from: https://www.virtamed.com/en/about-us/virtamed-story/custom-simulator-neotract-urosim-urolift/.
76. Huri E, Skolarikos A, Tatar İ, et al. Simulation of RIRS in soft cadavers: a novel training model by the Cadaveric Research On Endourology Training (CRET) Study Group. World J Urol. 2016;34(5):741–746. doi:10.1007/s00345-015-1676-3
77. Bowling CB, Greer WJ, Bryant SA, et al. Testing and validation of a low-cost cystoscopy teaching model: a randomized controlled trial. Obstet Gynecol. 2010;116(1):85–91. doi:10.1097/AOG.0b013e3181e45a52
78. Bele U, Kelc R. Upper and lower urinary tract endoscopy training on Thiel-embalmed cadavers. Urology. 2016l;93:27–32. doi:10.1016/j.urology.2016.01.040
79. Mains E, Tang B, Golabek T, et al. Ureterorenoscopy training on cadavers embalmed by Thiel’s method: simulation or a further step towards reality? Initial report. Cent European J Urol. 2017;70(1):81–87. doi:10.5173/ceju.2017.913
80. Rai B, Tang B, Healy S, et al. Face validity study of cadavers using Thiel method of embalming for endoscopic surgery in urology. Urology. 2014;84(4):S137–138.
81. Soria F, Morcillo E, Serrano A, et al. Development and validation of a novel skills training model for retrograde intrarenal surgery. J Endourol. 2015;29(11):1276–1281. doi:10.1089/end.2015.0421
82. Qiu Z, Yang Y, Zhang Y, Sun YC. Modified biological training model for percutaneous renal surgery with ultrasound and fluoroscopy guidance. Chin Med J (Engl). 2011;124(9):1286–1289.
83. Farhan B, Soltani T, Do R, Perez C, Choi H, Ghoniem G. Face, content, and construct validations of endoscopic needle injection simulator for transurethral bulking agent in treatment of stress urinary incontinence. J Surg Educ. 2018;75(6):1673–1678. doi:10.1016/j.jsurg.2018.04.011
84. Grimsby GM, Andrews PE, Castle EP, Wolter CE, Patel BM, Humphreys MR. Urologic surgical simulation: an endoscopic bladder model. Simul Healthc. 2011;6(6):352–355. doi:10.1097/SIH.0b013e3182211096
85. Fernandez A, Chen E, Moore J, et al. A phantom model as a teaching modality for laparoscopic partial nephrectomy. J Endourol. 2012;26(1):1–5. doi:10.1089/end.2011.0131
86. Lee JY, Mucksavage P, Canales C, McDougall EM, Lin S. High-fidelity simulation based team training in urology: a preliminary interdisciplinary study of technical and nontechnical skills in laparoscopic complications management. J Urol. 2012;187(4):1385–1391. doi:10.1016/j.juro.2011.11.106
87. Tunitsky E, Murphy A, Barber MD, Simmons M, Jelovsek JE. Development and validation of a ureteral anastomosis simulation model for surgical training. Female Pelvic Med Reconstr Surg. 2013;19(6):346–351. doi:10.1097/SPV.0b013e3182a331bf
88. Sabbagh R, Chatterjee S, Chawla A, Hoogenes J, Kapoor A, Matsumoto ED. Transfer of laparoscopic radical prostatectomy skills from bench model to animal model: a prospective, single-blind, randomized, controlled study. J Urol. 2012;187(5):1861–1866. doi:10.1016/j.juro.2011.12.050
89. Fernández-Tomé B, Díaz-Güemes I, Enciso Sanz S, et al. Validation of a new artificial model for simulated training of a laparoscopic vesicourethral anastomosis. Actas Urol Esp. 2019;23:
90. Molinas CR, Binda MM, Mailova K, Koninckx PR. The rabbit nephrectomy model for training in laparoscopic surgery. Hum Reprod. 2004;19(1):185–190. doi:10.1093/humrep/deh025
91. Teber D, Guven S, Yaycioglu O, et al. Single-knot running suture anastomosis (one-knot pyeloplasty) for laparoscopic dismembered pyeloplasty: training model on a porcine bladder and clinical results. Int Urol Nephrol. 2010;42(3):609–614. doi:10.1007/s11255-009-9668-0
92. Jiang C, Liu M, Chen J, et al. Construct validity of the chicken crop model in the simulation of laparoscopic pyeloplasty. J Endourol. 2013;27(8):1032–1036. doi:10.1089/end.2013.0085
93. Singh AG, Jai SJ, Ganpule AP, Vijayakumar M, Sabnis RB, Desai MR. Face, content, and construct validity of a novel chicken model for laparoscopic ureteric reimplantation. Indian J Urol. 2018;34(3):189–195. doi:10.4103/iju.IJU_46_18
94. Laguna MP, Arce-Alcazar A, Mochtar CA, Van Velthoven R, Peltier A. de la Rosette JJ. Construct validity of the chicken model in the simulation of laparoscopic radical prostatectomy suture. J Endourol. 2006;20(1):69–73. doi:10.1089/end.2006.20.69
95. Yang RM, Bellman GC. Laparoscopic urethrovesical anastomosis: a model to assess surgical competency. J Endourol. 2006;20(9):679–682. doi:10.1089/end.2006.20.679
96. Boon JR, Salas N, Avila D, Boone TB, Lipshultz LI, Link RE. Construct validity of the pig intestine model in the simulation of laparoscopic urethrovesical anastomosis: tools for objective evaluation. J Endourol. 2008;22(12):2713–2716. doi:10.1089/end.2008.0058
97. Available from: https://cleruro.files.wordpress.com/2019/05/program-web-compressed.pdf.
98. Rai BP, Stolzenburg JU, Healy S, Tang B, Jones P, Sweeney C. Preliminary validation of Thiel embalmed cadavers for laparoscopic radical nephrectomy. J Endourol. 2015;29(5):595–603. doi:10.1089/end.2014.0719
99. Moglia A, Ferrari V, Morelli L, Ferrari M, Mosca F, Cuschieri A. A systematic review of virtual reality simulators for robot-assisted surgery. Eur Urol. 2016;69(6):1065–1080. doi:10.1016/j.eururo.2015.09.021
100. Hertz AM, George EI, Vaccaro CM, Brand TC. Head-to-head comparison of three virtual-reality robotic surgery simulators. JSLS. 2018;22(1):
101. MacCraith E, Forde JC, Davis NF. Robotic simulation training for urological trainees: a comprehensive review on cost, merits and challenges. J Robot Surg. 2019;13(3):371–377. doi:10.1007/s11701-019-00934-1
102. Whitehurst SV, Lockrow EG, Lendvay TS, et al. Comparison of two simulation systems to support robotic-assisted surgical training: a pilot study (Swine model). J Minim Invasive Gynecol. 2015;22(3):483–488. doi:10.1016/j.jmig.2014.12.160
103. Whittaker G, Aydin A, Raison N, et al. Validation of the RobotiX mentor robotic surgery simulator. J Endourol. 2016;30(3):338–346. doi:10.1089/end.2015.0620
104. Harrison P, Raison N, Abe T, et al. The validation of a novel robot-assisted radical prostatectomy virtual reality module. J Surg Educ. 2018;75(3):758–766. doi:10.1016/j.jsurg.2017.09.005
105. Chowriappa A, Raza SJ, Fazili A, et al. Augmented-reality-based skills training for robot-assisted urethrovesical anastomosis: a multi-institutional randomised controlled trial. BJU Int. 2015;115(2):336–345. doi:10.1111/bju.12704
106. Kang SG, Cho S, Kang SH, et al. The Tube 3 module designed for practicing vesicourethral anastomosis in a virtual reality robotic simulator: determination of face, content, and construct validity. Urology. 2014;84(2):345–350. doi:10.1016/j.urology.2014.05.005
107. Kim JY, Kim SB, Pyun JH, et al. Concurrent and predictive validation of robotic simulator Tube 3 module. Korean J Urol. 2015;56(11):756–761. doi:10.4111/kju.2015.56.11.756
108. Shim JS, Noh TI, Kim JY, et al. Predictive validation of a robotic virtual reality simulator: the tube 3 module for practicing vesicourethral anastomosis in robot-assisted radical prostatectomy. Urology. 2018;122:32–36. doi:10.1016/j.urology.2018.08.013
109. Hung AJ, Shah SH, Dalag L, Shin D, Gill IS. Development and validation of a novel robotic procedure specific simulation platform: partial nephrectomy. J Urol. 2015;194(2):520–526. doi:10.1016/j.juro.2015.02.2949
110. Xu S, Perez M, Perrenot C, Hubert N, Hubert J. Face, content, construct, and concurrent validity of a novel robotic surgery patient-side simulator: the Xperience™ team trainer. Surg Endosc. 2016;30(8):3334–3344. doi:10.1007/s00464-015-4607-x
111. Ramos P, Montez J, Tripp A, Ng CK, Gill IS, Hung AJ. Face, content, construct and concurrent validity of dry laboratory exercises for robotic training using a global assessment tool. BJU Int. 2014;113(5):836–842. doi:10.1111/bju.12559
112. Ghazi A, Stone J, Candela B, Richards M, Joseph J. Simulated inanimate model for physical learning experience (simple) for robotic partial nephrectomy using a 3d printed kidney model. J Urol. 2015;193:e778. doi:10.1016/j.juro.2015.02.2285
113. Candela B, Stone J, Park J, et al. Concurrent validity of a simulated inanimate model for physical learning experience in partial nephrectomy (SIMPLE-PN). J Urol. 2016;195:e220. doi:10.1016/j.juro.2016.02.2787
114. Monda SM, Weese JR, Anderson BG, et al. Development and validity of a silicone renal tumor model for robotic partial nephrectomy training. Urology. 2018;114:114–120. doi:10.1016/j.urology.2018.01.030
115. Johnson BA, Timberlake M, Steinberg RL, Kosemund M, Mueller B, Gahan JC. Design and validation of a low-cost, high-fidelity model for urethrovesical anastomosis in radical prostatectomy. J Endourol. 2019;33(4):331–336. doi:10.1089/end.2018.0871
116. Shee K, Koo K, Wu X, Ghali FM, Halter RJ, Hyams ES. A novel ex vivo trainer for robotic vesicourethral anastomosis. J Robot Surg. 2019;2019(Epub):28.
117. Hung AJ, Ng CK, Patil MB, et al. Validation of a novel robotic-assisted partial nephrectomy surgical training model. BJU Int. 2012;110(6):870–874. doi:10.1111/j.1464-410X.2012.10953.x
118. von Rundstedt FC, Aghazadeh MA, Scovell J, et al. Validation of a simulation-training model for robotic intracorporeal bowel anastomosis using a step-by-step technique. Urology. 2018;120:125–130. doi:10.1016/j.urology.2018.07.035
119. Cacciamani G, De Marco V, Siracusano S, et al. A new training model for robot-assisted urethrovesical anastomosis and posterior muscle-fascial reconstruction: the Verona training technique. J Robot Surg. 2017;11(2):123–128. doi:10.1007/s11701-016-0626-4
120. Alemozaffar M, Narayanan R, Percy AA, et al. Validation of a novel, tissue-based simulator for robot-assisted radical prostatectomy. J Endourol. 2014;28(8):995–1000. doi:10.1089/end.2014.0041
121. Bertolo R, Garisto J, Dagenais J, Sagalovich D, Kaouk JH. Single session of robotic human cadaver training: the immediate impact on urology residents in a teaching hospital. J Laparoendosc Adv Surg Tech A. 2018;28(10):1157–1162. doi:10.1089/lap.2018.0109
122. Volpe A, Ahmed K, Dasgupta P, et al. Pilot validation study of the European Association of Urology Robotic Training Curriculum. Eur Urol. 2015;68(2):292–299. doi:10.1016/j.eururo.2014.10.025
123. Wiener S, Haddock P, Shichman S, Dorin R. Construction of a urologic robotic surgery training curriculum: how many simulator sessions are required for residents to achieve proficiency? J Endourol. 2015;29(11):1289–1293. doi:10.1089/end.2015.0392
124. Mills JT, Hougen HY, Bitner D, Krupski TL, Schenkman NS. Does robotic surgical simulator performance correlate with surgical skill? J Surg Educ. 2017;74(6):1052–1056. doi:10.1016/j.jsurg.2017.05.011
125. Novara G, Volpe A, Ahmed K, Dasgupta P, Van Der Poel H, Mottrie A. 193 validation of the European Association of Urology Robotic Training Curriculum: pilot study II. Eur Urol Suppl. 2015;14(2):e193. doi:10.1016/S1569-9056(15)60195-6
126. Sclaverano S, Chevreau G, Vadcard L, et al. Biopsym: a simulator for enhanced learning of ultra- sound-guided prostate biopsy. Stud Health Technol Inform. 2009;142:301–306.
127. Fiard G, Selmi S-Y, Promayon E, et al. Initial validation of a virtual-reality learning environment for prostate biopsies: realism matters!. J Endourol. 2014;28:453–458. doi:10.1089/end.2013.0454
128. Fiard G, Selmi S, Promayon E, Descotes J, Troccaz J. Simulation-based training for prostate biopsies: towards the validation of the Biopsym simulator. Minim Invasive Ther Allied Technol. 2019;1–7. doi:10.1080/13645706.2019.1653926
129. Chalasani V, Cool D, Sherebrin S, Fenster A, Chin J, Izawa J. Development and validation of a virtual reality transrectal ultrasound guided prostatic biopsy simulator. Can Urol Assoc J. 2011;19–26. doi:10.5489/cuaj.09159
130. Available from: https://simulation.health.ufl.edu/technology-development/augmented-reality-mixed-simulation/trus-pbx-sim/mixed-simulator-of-transrectal-transperineal-ultrasound-guided-prostate-biopsy-video/.
131. Ritsos PD, Edwards MR, Shergill IS, John NW A Haptics-enabled simulator for transperineal ultrasound-guided biopsy.
132. Thaker N, Kudchadker R, Swanson D, et al. Establishing high-quality prostate brachytherapy using a phantom simulator training program. Inter J Radiat Oncol Biol Phys. 2014;90(3):579–586. doi:10.1016/j.ijrobp.2014.06.036
133. Goksel O, Sapchuk K, Morris W, Salcudean S. Prostate brachytherapy training with simulated ultrasound and fluoroscopy images. IEEE Trans Biomed Eng. 2013;60(4):1002–1012. doi:10.1109/TBME.2012.2222642
134. Poder J, Carrara M, Howie A, Cutajar D, Bucci J, Rosenfeld A. Derivation of in vivo source tracking error thresholds for TRUS-based HDR prostate brachytherapy through simulation of source positioning errors. Brachytherapy. 2019;18(5):711–719. doi:10.1016/j.brachy.2019.05.001
135. Sehrawat A, Keelan R, Shimada K, Wilfong D, McCormick J, Rabin Y. Simulation-based cryosurgery training. Technol Cancer Res Treat. 2016;15(6):805–814. doi:10.1177/1533034615611509
136. Gunther JR, Liauw SL, Choi S, et al. A prostate fossa contouring instructional module: implementation and evaluation. J Am Coll Radiol. 2016;13(7):835–841.e1. doi:10.1016/j.jacr.2016.02.030
137. Anderson O, Davis R, Hanna GB, Vincent CA. Surgical adverse events: a systematic review. Am J Surg. 2013;206(2):253–262. doi:10.1016/j.amjsurg.2012.11.009
138. Panesar SS, Carson-Stevens A, Mann BS, Bhandari M, Madhok R. Mortality as an indicator of patient safety in orthopedics: lessons from qualitative analysis of a database of medical errors. BMC Musculoskelet Disord. 2012;13(1):93. doi:10.1186/1471-2474-13-93
139. Leuschner S, Leuschner M, Kropf S, Niederbichler AD. Non-technical skills training in the operating theatre: a meta-analysis of patient outcomes. Surgeon. 2019;17(4):233–243. doi:10.1016/j.surge.2018.07.001
140. Ounounou E, Aydin A, Brunckhorst O, Khan MS, Dasgupta P, Ahmed K. Nontechnical skills in surgery: a systematic review of current training modalities. J Surg Educ. 2019;76(1):14–24. doi:10.1016/j.jsurg.2018.05.017
141. Kwong JC, Lee JY, Goldenberg MG. Understanding and assessing nontechnical skills in robotic urological surgery: a systematic review and synthesis of the validity evidence. J Surg Educ. 2019;76(1):193–200. doi:10.1016/j.jsurg.2018.05.009
142. Somasundram K, Spence H, Colquhoun AJ, Mcilhenny C, Biyani CS, Jain S. Simulation in urology to train non-technical skills in ward rounds. BJU Int. 2018;122(4):705–712. doi:10.1111/bju.2018.122.issue-4
143. Abdelshehid CS, Quach S, Nelson C, et al. High-fidelity simulation-based team training in urology: evaluation of technical and nontechnical skills of urology residents during laparoscopic partial nephrectomy. J Surg Educ. 2013;70(5):588–595. doi:10.1016/j.jsurg.2013.04.009
144. Lee JY, Mucksavage P, Canales C, McDougall EM, Lin S. High-fidelity simulation based team training in urology: a preliminary interdisciplinary study of technical and nontechnical skills in laparoscopic complications management. J Urol. 2012;187(4):1385–1391. doi:10.1016/j.juro.2011.11.106
145. Gettman MT, Pereira CW, Lipsky K, et al. Use of high-fidelity operating room simulation to assess and teach communication, teamwork and laparoscopic skills: initial experience. J Urol. 2009;181(3):1289–1296. doi:10.1016/j.juro.2008.11.018
146. Brewin J, Tang J, Dasgupta P, et al. Full immersion simulation: validation of a distributed simulation environment for technical and non-technical skills training in urology. BJU Int. 2015;116(1):156–162. doi:10.1111/bju.2015.116.issue-1
147. Brunckhorst O, Shahid S, Aydin A, et al. The relationship between technical and nontechnical skills within a simulation-based ureteroscopy training environment. J Surg Educ. 2015;72(5):1039–1044. doi:10.1016/j.jsurg.2015.04.002
148. Available from: https://www.elsevier.com/search-results?query=gunner%20goggles&labels=books&page=1#top.
149. Available from: https://3d4medical.com/press/pearson-and-3d4medical-mixed-reality-and-hololens.
150. Rahman R, Wood ME, Qian L, Price CL, Johnson AA, Osgood GM. Head-mounted display use in surgery: a systematic review. Surg Innov. 2019;27:88–100. Epub 2019 Sept 12.
151. Porpiglia F, Fiori C, Checcucci E, Amparore D, Bertolo R. Hyperaccuracy three-dimensional reconstruction is able to maximize the efficacy of selective clamping during robot-assisted partial nephrectomy for complex renal masses. Eur Urol. 2018;74(5):651–660. doi:10.1016/j.eururo.2017.12.027
152. Porpiglia F, Checcucci E, Amparore D, et al. Three-dimensional elastic augmented-reality robot-assisted radical prostatectomy using hyperaccuracy three-dimensional reconstruction technology: a step further in the identification of capsular involvement. Eur Urol. 2019;76(4):505–514. doi:10.1016/j.eururo.2019.03.037
153. Bertolo R, Hung A, Porpiglia F, Bove P, Schleicher M, Dasgupta P. Systematic review of augmented reality in urological interventions: the evidences of an impact on surgical outcomes are yet to come. World J Urol. 2019; 1–10. Epub 2019 Mar 2.
154. Available from: https://www.proximie.com.
155. Chen J, Remulla D, Nguyen JH, et al. Current status of artificial intelligence applications in urology and their potential to influence clinical practice. BJU Int. 2019;2019(Epub):20.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
