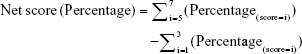Back to Journals » Patient Preference and Adherence » Volume 12
Current and future options for opioid use disorder: a survey assessing real-world opinion of service users on novel therapies including depot formulations of buprenorphine
Authors Gilman M , Li L, Hudson K , Lumley T, Myers G, Corte C, Littlewood R
Received 18 July 2018
Accepted for publication 28 August 2018
Published 11 October 2018 Volume 2018:12 Pages 2123—2129
DOI https://doi.org/10.2147/PPA.S180641
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Mark Gilman,1 Li Li,2 Kerrie Hudson,3 Tara Lumley,2 Georgia Myers,2 Camilla Corte,2 Richard Littlewood2
1Discovering Health, Manchester, UK; 2Applied Strategic, London, UK; 3The Well Communities CIC, Lancaster, UK
Purpose: Integrated treatment for opioid use disorder (OUD) includes opioid agonist therapy (OAT) such as methadone and buprenorphine with well-evidenced benefits. Treatment with typical existing oral medications is associated with burdens and limits to successful outcomes (frequent dosing, attendance for collection/consumption, difficulty in achieving optimal dosing, misuse, diversion, accidental exposure, and stigma from the treatment process). Novel medications include injected depot formulations with less frequent administration, providing consistent drug levels after dosing. This survey assesses the opinion of those with OUD treatment services lived experience to inform future medication choices.
Patients and methods: A survey of people with experience of OUD pharmacotherapy – the treatment system – was completed. Participants reviewed statements describing elements of OUD care using 7-point Likert scales to indicate their level of agreement or disagreement. Data were assessed using descriptive analysis.
Results: In total, 35 people (16 in treatment; 19 with previous history of treatment) completed the survey. Average drug-use duration, 20 years, commonly included injected opioids. The majority agreed treatment was effective, but not tailored to their individual needs and limited normal day-to-day activities. Opinions on novel depot medications included the following: agreement on its potential to make life easier, reduce stigma, free-up time for preferred activities. Participants did not report concerns over the effectiveness and safety of depot medications, nor about reduced contact with treatment services that could be associated with less frequent dosing.
Conclusion: This survey provides a useful initial record of the opinions of people experienced in OUD treatment services on novel depot medications, which may result in important benefits. Care providers and policy makers should continue to work with those with lived experience to understand the specific opportunity provided by such innovation.
Keywords: opioid agonist therapy, lived experience, innovation
Introduction
Opioid use disorder (OUD) is an important individual and public health problem worldwide,1 treatment of which is effective and well evidenced. Integrated treatment programs combine pharmacotherapy (commonly methadone, buprenorphine, and other choices) and psychosocial support.2,3 Engagement with high-quality treatment for OUD is associated with benefits such as reduced all-cause and overdose mortality rate,4 transmission risk for blood borne virus (BBV),5 and crime rates.6 Current treatments do not meet the needs of all people with OUD; engagement with treatment may be 50% on average in Europe.7 Conventional approaches to OUD care delivery demand daily oral administration in most cases, which may explain, in part, variable adherence to suggested treatment regimens and engagement with treatment services in general. It has been observed that it is considerably more difficult to collect prescribed medication for OUD when compared with illicit drugs. Medication is also associated with risks including misuse, diversion, and accidental exposure, including to children.8 Achieving consistent, therapeutic dosing levels may be difficult. Adherence to agreed treatment regimens is also often challenging; some are involved in a repeated cycle of episodes of entering and leaving treatment.9 Others may consider opioid agonist therapy (OAT) as a supplementary source rather than a substitute for problem drugs. Important factors facilitating treatment success include greater flexibility, less pressure to reduce treatment dose, and reduced time of supervised dose.10 Medication options including weekly or monthly buprenorphine depot formulations are approved in the USA11 and under review by regulatory agencies in Europe.12 These medications provide sustained release of active medication over a weekly/monthly duration, which implies a reduced need for daily attendance at clinics or pharmacies, improved adherence, and sustained effective dosing levels.13–15 Take home medications by definition are not required; diversion and misuse are very unlikely.13,16,17
To date, there is limited insight from the broad experience of people treated for OUD on how to approach such innovative options. Understanding the opinion of people engaged with drug treatment services (DTS), currently or in the past, informs treatment decisions.10,18,19 This work aims to pilot a survey of people with lived experience of treatment for OUD to understand the real-world perspective on future medication choices. The objective is to assess experience from current and former users of the treatment system, to provide evidence to inform choices about the future of OUD therapy and new products, which change the burden of treatment.
Materials and methods
A survey of treatment experience was designed by experts familiar with OUD care and patient experience assessment. The survey was developed with input from treatment service providers, people in treatment for OUD, and those abstinent in the long term but with a previous history of problem drug use. The study was a survey of a group of people self-identifying as having a history (current or former) of engagement with DTS in England.
The study was performed at a national service user involvement conference,20 a meeting for people engaged in OUD care, attended by current and former users of treatment services. Participants completed a self-directed, interactive digital survey online. Experienced facilitators were trained and assisted in navigating the online tool to assist when participants were unfamiliar with use of the technology. Data were collected in one session. All attendees at the meeting were invited to participate.
Eligibility criteria included experience of using DTS for OUD. Those interested in participating in the survey indicated this to a survey facilitator in a private setting. Participants were selected based on their self-reported treatment experience, defined by a short, pre-survey qualification interview performed using an online tool. Only those with a reported history of OUD and engagement with DTS completed the survey. No other exclusionary criteria were applied. Participants were not paid.
The survey consisted of 20 questions; areas of questioning included basic demographics, history of substance use, and personal history of treatment for drug-related problems. The main focus of the survey was the participant’s opinion on current approaches to OUD care and future treatment. Future treatment options, as an injected product providing consistent levels of medication requiring weekly to monthly injections, were described in materials presented with the survey. Potential bias from participant selection was addressed by using a standard system to define eligibility criteria. The survey was completed in a nonclinical setting, led by facilitators who were not healthcare professionals. Participants were asked to indicate the level of agreement or disagreement with a series of statements describing current and future OUD treatment. Standard 7-point Likert scales (disagree strongly, disagree moderately, disagree slightly, neutral, agree slightly, agree moderately, agree strongly) were presented on a tablet computer with each statement.21
Data were collected from all participants by selecting a position on a graphical presentation of a visual scale. A net response score for all participants was calculated as the difference between fraction of positive responses (score ≥5) and fraction of negative responses (score ≤3) for each statement presented. The net score ranges from −100% (lowest) to 100% (highest); a score <0 suggests an overall negative view while a score >0 indicates a positive opinion. The absolute value reflects the degree of overall agreement or disagreement.
|
Ethics committee review and approval was not sought in accordance with guidance from a Medical Research Council and Health Research Authority tool for assessment of projects.22 All participants recorded their consent to participate in the survey prior to beginning. The study population for the survey was defined based on an assessment of the number of potential participants available and a frequency of likely participation, estimated by people familiar with the event attendees. The target study sample size was 30 participants, in line with guidance.23 Data were analyzed using simple descriptive statistics. The sample included people with current and former treatment experience; P-values for the comparison of scores between current and former users were calculated using nonparametric Mann–Whitney test.24
Results
In total, 35 people (16 in treatment; 19 with previous history of treatment) completed the survey. 40 people showed initial interest in the survey; 5 people did not meet eligibility criteria. The description of the population is summarized in Table 1. Average problem drug-use history was 20 years and the majority of participants injected opioids.
  | Table 1 Demographics for survey participants |
The majority of participants were male (66%); 31% were female and one person was transexual. Of the 35 participants, 19 had previous experiences of treatment programs but reported they were abstinent from opioids in the long term, the remainder (16) were currently engaged with a drug treatment program. The majority of participants in treatment gained their income from welfare payments in part or entirely, lived in rented accommodation, and were treated with buprenorphine or methadone that required collection on a daily (50% of those in treatment) basis. The majority (81%) of participants engaged with treatment services received OAT prescriptions (31% methadone, 25% buprenorphine, 13% buprenorphine/naloxone, 13% other); 19% did not receive medication. “Other” therapy options included Lofexidine; often participants chose not to disclose the name of therapy where “other” was indicated.
The most common goal in engaging with treatment was to achieve abstinence and not use any illicit drugs (69%); 11% wanted to seek long-term stable maintenance therapy of methadone or buprenorphine and avoid illicit drug use; 11% considered therapy as a “supplement” for illicit drug use; 9% indicated other reasons.
All participants reported their views on experience with the treatment service. Results are summarized in Figure 1. The majority (net score, +44%) agreed that treatment allowed them to maintain a normal life; a small majority reported that steps to gain access to treatment programs were acceptable to them (net score +3%). The majority (net score +32%) considered that steps to collect medication limited normal activities of living; the majority (net score +18%) agreed that reducing daily activities to get medications would be attractive. The group did not consider that treatment services offered choice tailored to their specific needs (net score −24%) or that representation of their views was sufficient.
Results of the assessment of opinion of participants on a depot form of medication are summarized in Figure 2. The majority considered depot medication would make life easier (net score +40%), reduce stigma of treatment (net score +49%), and release time for preferred activities (net score +63%). Overall, participants did not indicate agreement with statements describing potential limitations such as the lack of contact with pharmacy services (net score −47%), loss of control over therapy (net score −44%), and lack of possibility to use on top (net score −29%).
Analysis of the Likert scale scores (1–7) showed that people who are currently in treatment programs are more likely to agree with the statement “I would have less control over my therapy with a depot medication” when compared with those with a previous treatment experience (P<0.01). No significant difference between the two groups was found in responses to all the other statements in the survey (P>0.05).
Discussion
This survey of people with lived experience of OUD treatment describes their opinions of treatment, including novel depot forms of medication for OUD with less frequent dosing. The participants have 400 years of combined experience in the treatment system. The results of this work provide evidence of the potential benefits of such novel depot forms of medication, linking potential characteristics of medications, assumed benefits, and potential disadvantages or concerns with the feelings of potential recipients. Benefits, which may reduce the burden of treatment, are positively held by participants; indirect benefits including reducing stigma, improving quality of life, and increasing time available to complete other activities of living are identified. Concerns about effectiveness of novel medications or the loss of contact with pharmacy services are not reported by the majority. It is noted that participants did not uniformly report the same opinions; however, in many cases, the responses indicated agreements with potential positive interpretation of the depot forms of medication. In all cases, a smaller sized subgroup did state opposing views, indicating potential concerns about less frequent treatment approaches.
This survey is based on the opinion of 35 people with many years of experience of OUD treatment. Although this is the largest survey of its kind to date, it is limited, as with any qualitative assessment, by the small population size. Age and gender of the study population followed a similar profile to that reported in national statistics for the general population receiving OUD treatment in England.25 The nature of recruitment approach implies a certain type of population – those able or wishing to attend a congress for people with experience of OUD treatment. This setting for the study was detached from the typical formal clinical setting – this may avoid bias of selecting participants who are overly familiar with such research projects. It is important to engage with people in this way to build a full picture of opinion. Other approaches to recruit study populations should be considered. Social desirability tendencies should be considered when interpreting results presented, due to the sensitive and self-reported nature of data generated. The survey was anonymous and carried out in a private setting to minimize social desirability pressures. The sample included people with current and former OUD treatment experience; comparison between current and former users showed that patients with current treatment experience are more likely to report concern over loss of control in therapy when depot medication becomes available compared with those with former treatment experience. Further studies should investigate the different opinions between the two groups. This study was performed in England only; opinions in other countries should also be assessed.
Results suggest that the potential benefits of depot medications – especially reducing the treatment burden – may resonate with people in treatment. The results indicate that there are a range of opinions, especially in relation to concerns about novel therapy options. This is consistent with results from similar recent studies.17 It is important to understand the drivers of different opinion and the characteristics of groups with differing opinion, and to approach the introduction of the novel medications with implied needs in mind. This will allow the optimal choice of medication, whether novel or otherwise, to be selected and to avoid problems associated with future change. This study expands on previous qualitative and anecdotal evidence;17 structured and quantitative data were generated through use of Likert scales to build on existing narrative reports and allow firmer conclusions to be drawn, particularly where patient opinion differs among subgroups. Findings should be considered in the approach to future treatment and medication options; it will be important to expand on this pilot data and assess views on therapy options for OUD in a wider lived experience population.
Conclusion
Important progress has been made in managing OUD but there are still significant unmet needs; innovation can be a key part of future success. The introduction of novel depot medication choices with less frequent administration can be associated with important benefits. It is pertinent to understand the range of opinions of different groups and to use these insights to help work with individuals who wish to try depot medications. Further work with people with lived experience of OUD care is likely to improve understanding and is recommended.
Acknowledgment
The authors are grateful to all participants.
Disclosure
The authors report no conflicts of interest in this work.
References
Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. | ||
Schuckit MA. Treatment of Opioid-Use Disorders. N Engl J Med. 2016;375(4):357–368. | ||
Clinical Guidelines on Drug Misuse and Dependence Update 2017 Independent Expert Working Group. Drug Misuse and Dependence: UK Guidelines on Clinical Management. London: Department of Health; 2017. | ||
Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. | ||
Gowing L, Mf F, Bornemann R, Le S, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection (Review8). 2011. | ||
Bukten A, Skurtveit S, Gossop M, et al. Engagement with opioid maintenance treatment and reductions in crime: a longitudinal national cohort study. Addiction. 2012;107(2):393–399. | ||
Wright N, Reimer J, Somaini L, et al. Are we ready to treat hepatitis C virus in individuals with opioid use disorder. Eur J Gastroenterol Hepatol. 2017;29(11):1206–1214. | ||
Stöver H. Barriers to opioid substitution treatment access, entry and retention: a survey of opioid users, patients in treatment, and treating and non-treating physicians. Eur Addict Res. 2011;17(1):44–54. | ||
Fischer G, Nava F SH. Outcomes of opioid-dependence treatment across Europe: identifying opportunities for improvement. Editorial Board. Heroin Addict Relat Clin Probl. 2012;14:39–50. | ||
Brandt L, Unger A, Moser L, Fischer G, Jagsch R. Opioid maintenance treatment – a call for a Joint European Quality Care Approach. Eur Addict Res. 2016;22(1):36–51. | ||
FDA approves first once-monthly buprenorphine injection, a medication-assisted treatment option for opioid use disorder. Available from: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm587312.htm. Accessed September 26, 2018. | ||
Camurus. Transforming treatments by long-acting medications Forward-looking statements. 2017. Available from: https://www.camurus.com/wp-content/uploads/2017/12/Camurus-Company-Presentation-CARNEGIE-NYC-171205.pdf. Accessed September 26, 2018. | ||
Rosenthal RN, Goradia VV. Advances in the delivery of buprenorphine for opioid dependence. Drug Des Devel Ther. 2017;11:2493–2505. | ||
Indivior. SUBLOCADE-FDA Approves SUBLOCADETM (Buprenorphine Extended-Release), the First and Only Once-Monthly Injectable Buprenorphine Formulation to Treat Moderate to Severe Opioid Use Disorder. 2018. Available from: http://www.indivior.com/investor-news/fda-approves-sublocade-buprenorphine-extended-release-first-monthly-injectable-buprenorphine-formulation-treat-moderate-severe-opioid-use-disorder/. Accessed September 26, 2018. | ||
Camurus. Camurus Announces European Medicines Agency Validation of CAM2038 Marketing Authorization Application for Treatment of Opioid Dependence. 2017. Available from: http://mb.cision.com/Main/13456/2356920/729320.pdf. Accessed September 26, 2018. | ||
Lofwall MR, Walsh SL, Nunes EV, et al. Weekly and monthly subcutaneous buprenorphine depot formulations vs daily sublingual buprenorphine with naloxone for treatment of opioid use disorder: a randomized clinical trial. JAMA Intern Med. 2018;178(6):764–773. | ||
Neale J, Tompkins CNE, Mcdonald R, Strang J. Implants and depot injections for treating opioid dependence: qualitative study of people who use or have used heroin. Drug Alcohol Depend. 2018;189:1–7. | ||
Fischer J, Jenkins N, Bloor M, Neale J, Berney L. Drug User Involvement in Treatment Decisions. York: Joseph Rowntree Foundation; 2007. | ||
Metz VE, Brandt L, Unger A, Fischer G. Substance abuse/dependence treatment: a European perspective. Subst Abus. 2014;35(3):309–320. | ||
Drink and Drug News. Get connected. 2018. Available from: https://drinkanddrugsnews.com/wp-content/uploads/2018/03/DDN-March2018.pdf. Accessed September 25, 2018. | ||
Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;22(140):55. | ||
NHS Health Research Authority. Do I need NHS REC approval? 2018 Published. Available from: http://www.hra-decisiontools.org.uk/ethics/. Accessed September 25, 2018. | ||
Johanson GA, Brooks GP. Initial scale development: sample size for pilot studies. Educ Psychol Meas. 2010;70(3):394–400. | ||
Sullivan GM, Artino AR. Analyzing and interpreting data from likert-type scales. J Grad Med Educ. 2013;5(4):541–542. | ||
Public Health England. Adult substance misuse statistics from the National Drug Treatment Monitoring System (NDTMS). 2016;2017:1–74. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/658056/Adult-statistics-from-the-national-drug-treatment-monitoring-system-2016-2017.pdf. Accessed September 26, 2018. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.