Back to Journals » Clinical Ophthalmology » Volume 15
Cost–Benefit Analysis of Single versus Repeated Use of Single-Use Devices in Cataract Surgery
Authors Naoum P , Palioura S, Naoum V , Nomikos N , Bachtalia K, Zisis K, Athanasakis K, Kyriopoulos J
Received 18 November 2020
Accepted for publication 18 February 2021
Published 12 April 2021 Volume 2021:15 Pages 1491—1501
DOI https://doi.org/10.2147/OPTH.S292849
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Panagiota Naoum,1 Sotiria Palioura,2 Vasiliki Naoum,1 Nikos Nomikos,1 Konstantina Bachtalia,1 Konstantinos Zisis,1 Kostas Athanasakis,1,3 John Kyriopoulos1
1Institute for Health Economics, Athens, Greece; 2Athens Vision Eye Institute, Athens, Greece; 3Department of Public Health Policy, School of Public Health, University of West Attica, Athens, Greece
Correspondence: Panagiota Naoum
Institute for Health Economics, Aldou Manoutiou 10, Athens, 11521, Greece
Tel +30 210 6455562
Email [email protected]
Purpose: To estimate the net cost effect associated with the real-world practice of repeated use of designated single-use medical devices (SUDs) versus their proper single use in cataract surgery in Greece.
Design: A cost-benefit analysis model was constructed in the form of a decision tree.
Methods: A digital expert panel was assembled in order to estimate the probabilities of intraoperative and postoperative complications associated with single and repeated use of SUDs. Unit costs for the management of each complication were obtained from the official Greek bulletins. A Monte Carlo-type sensitivity analysis was performed to assess the robustness of the results.
Results: Based on the probabilities of complications attained from the expert panel, repeated use of SUDs is associated with a higher chance of complications compared to single use, which results in higher cost of complication management. Under the healthcare sector perspective, the total expected cost per cataract surgery is 1,403.98€ (1,244.20€ the initial cost of cataract surgery plus 159.78€ the cost of adverse events) in the case of single use, while for repeated use the total cost is 1,486.29€ (1,146.86€ + 339.43€, respectively) and, thus, repeated use of SUDs in cataract surgery results in 82.31€ higher expected cost per patient compared to their single use. Moreover, the societal perspective analysis indicated even higher additional costs in the case of SUD reuse (108.24€).
Conclusion: Repeated use of SUDs in cataract surgery is not appropriate, it jeopardizes patient safety and carries a legal liability for the reuser. The present study, which is the first to attach a monetary value to the common yet questionable practice of SUD reuse, shows that it is not cost beneficial. Therefore, it is expected that the results will have implications in policy formulations to improve the delivery of cataract healthcare.
Keywords: cataract, cataract surgery, single-use devices, SUDs
Introduction
Cataract surgery is a common ophthalmic procedure with remarkably high success rates. While it significantly improves quality of life, it inevitably induces a significant financial burden for patients, their families, the society and the health care system. The current standard of care is the use of single-use devices (SUDs) in cataract surgery. SUDs share specific properties that allow mass production on one hand but are not resistant to aggressive physical or chemical retreatments, high temperatures and sterilization processes on the other.1 Even though SUDs are by definition intended for single use, an increasing number of such medical devices are being reprocessed and reused.
Reports by the European Commission,2 the Medicine and Healthcare Products Regulatory Agency,3 and the EUCOMED Industry Guide1 emphasize that reuse of SUDs affects the safety and performance of SUDs, thus exposing patients and users to unnecessary risks such as cross-infection, endotoxin reaction, chemical burns, and sensitization. Reuse is also associated with significant legal implications for the user, who is held fully responsible for the safety and effectiveness of reprocessed and reused SUDs.1–4
Despite the physical and legal risks, reuse of SUDs has been common practice in cataract surgery.5–10 Commonly reused SUDs include the phaco cassette with tip and sleeve, incisional knives, cannulas, cystotomes and left-over ophthalmic viscosurgical devices (OVDs).5–10 Phaco tip reuse not only decreases the efficiency of phacoemulsification,10 but has also been associated with dissemination of metallic foreign bodies from micro-breakages of the tip,8,9 as well as wound burns due to ineffective use of ultrasound energy and prolonged surgical time.10 Any cataract SUD reuse has also been linked to the development of the potentially blinding toxic anterior segment syndrome (TASS).5–7
Advocates of SUD reuse in cataract surgery are driven by the assumption that reuse would lower the primary cost of the procedure. Given that reuse is associated with a higher risk of intraoperative and postoperative complications, the secondary costs of such complications need to be accounted for before reaching the conclusion that reuse is cost-effective. The practice of SUD reuse in cataract surgery is common practice both in the public and in the private sectors in Greece, though there are no exact estimates. Patients are rarely made aware of the reuse. To address this issue, we performed a cost benefit analysis of single versus repeated use of cataract surgery SUDs in the healthcare setting of Greece in 2019.
Materials and Methods
The cost benefit method of analysis compares interventions and their consequences by analyzing costs and benefits in terms of monetary outcomes.11 This specific method was selected because it allows policy makers to attain a better estimate of the real-world impact of interventions. The two comparator interventions in the analysis were (a) single and (b) repeated use of SUDs in cataract surgery.
The base case scenario of the analysis was conducted under the healthcare sector perspective, which includes formal medical costs borne by third-party payers (ie insurance agencies) or paid by patients as out-of-pocket expenses. Additionally, an alternative scenario analysis was conducted to examine the impact of SUD reuse under the societal perspective.
Regardless of SUD reuse, the cost estimation for each complication included the baseline cost of cataract surgery, accompanied by (wherever applicable) the cost of medication for its management, the cost of any additional required hospitalizations, as well as the cost of any postoperative physician visits. In order to account for the cost of SUDs, acquirement of a new set of SUDs was incorporated in the single-use scenario, while sterilization of the SUDs set was incorporated in the reuse scenario. For the alternative scenario, lost productivity and cost of leisure time were taken into account, as well as patient transportation costs for physician visits.
A decision tree was constructed for the cost-benefit model. This method provides a visualization of the objective along with all the items of the model and makes quantification of the decision problem easier.12 The analysis was performed using the Microsoft Excel 2016 software (Microsoft Corporation, Seattle, WA). The model was designed to incorporate the management cost of the main intraoperative and postoperative complications of cataract surgery. The vast majority of complications associated with SUD reuse occur intraoperatively or in the immediate postoperative period. Thus, a 1-year time frame was selected for the health economics analysis of this study. Thus, no discount rates were deemed necessary for the model.
The SUDs selected for the analysis were the ones that are most likely to be reused by ophthalmic surgeons in their practice, namely: incisional knives, left-over OVDs, phaco cassettes with tips and sleeves, cystotomes, and hydrodissection and anterior chamber cannulas. In order to simulate the real-world clinical practice of SUD reuse in Greece, where facilities that adopt the reuse route tend to reuse the whole set of the aforementioned SUDs, our analysis has focused on the economic impact of SUD reuse as a group rather than examining each one of them separately. For the purpose of this study, SUDs were considered to be re-sterilized by autoclave. In order to capture the minimum economic effect of SUD reuse, we have considered a single pattern of reuse.
Clinical Data
In Greece, there are no standard therapeutic protocols with respect to the detailed management of the complications used in the model. In order to capture the overall management of each complication in the cost model as accurately as possible, the research team consulted an ophthalmic surgeon expert in the field.
The complications were selected after an extensive review of the international literature and consultation with the cataract surgery expert, in order to include the ones for which the probability of occurrence is most likely affected by the repeated use of SUDs. Given the 1-year horizon of the model, complications that might occur after that time period were not included in the model. The complications that were selected for the model and comprised the main branches of the decision tree are presented in Table 1. Some of the primary complications develop into sub-branches, since they may be accompanied by specific concomitant secondary complications, which affect the management of the respective primary complication and, thus, the accompanying cost (Table 1). It should be noted that the occurrence of the concomitant secondary complications is related to cataract surgery alone and is not necessarily associated with single or repeated use of the selected SUDs. For example, the occurrence of a posterior capsule rent may be related to the reuse of the phaco cassette, tip and sleeve, but whether the nucleus drops or not is not related to the SUD reuse. We should note that the rate of endophthalmitis reported by the expert panel reflects the general practice in Greece of intracameral antibiotic use at the end of cataract surgery, in adherence to the European Society for Cataract and Refractive Surgery (ESCRS) guidelines (recommendations).13
 |
Table 1 List of Primary and Possible Concomitant Secondary Complications Included in the Model |
Digital Expert Panel
In Greece, no epidemiological data are available on the incidence of complications associated with cataract surgery. Except for toxic anterior segment syndrome (TASS),14 an extensive literature review failed to identify any published incidence rates of complications in the case of reuse. Therefore, in order to obtain the necessary clinical data for the cost model, a digital expert panel was assembled. The method of expert elicitation in economic evaluation is considered appropriate when the value in question is unknown.15 A variety of published studies utilize this method in the population of health economics models.16–18 The panel consisted of 12 senior ophthalmic surgeons, experts in cataract surgery. For this purpose, all experts were asked to answer a structured questionnaire that consisted of two groups of questions. The goal of the first set of questions was to estimate the incidence of the selected primary complications separately for the single and the repeated use of SUDs in cataract surgery, while the second set of questions aimed at estimating the proportion of the concomitant secondary complications that are associated with cataract surgery itself. Upon completion of data collection via the expert panel, incidence rates of each complication were estimated for SUD single use and reuse, respectively. These incidence rates were then used to estimate the relative risk (RR) of reuse vs single use as per the formula: RR= incidence rate reuse/incidence rate single use. It should be noted that the relative risk indicates how different the incidence of each complication is when SUDs are reused compared to their single use and cannot directly be compared across complications.
Cost Data
The unit costs and outcomes for the resources utilized in the model were obtained from the respective official Greek bulletins. In particular, inpatient and outpatient medication costs were sourced from the Official Medication Price Bulletin published in 2019.19 Hospitalization cost estimations were based on the official Greek DRG (Diagnosis Related Groups) system, adjusted for wages.20,21
As far as outpatient visits after cataract surgery are concerned, there is no corresponding official decree determining the cost of the visit for the third-party payer. Therefore, the cost of a doctor’s office visit, in the context of complication management, was calculated on the basis of the fee paid for afternoon clinic visits in public hospitals.22 In addition, the cost of spectacles was sourced from the relevant reimbursement fee paid by the Hellenic National Organization for Health Services Provision (EOPYY).23 Finally, costs of lost productivity and leisure time were estimated by utilizing the mean daily wage24 and the minimum daily wage25 in Greece, respectively. Cost unit data and outcome analysis refer to 2019 prices in euros (€). Patient transportation cost for physician visits was assumed to be 5.00€.
This cost benefit model was based on the mean of the probabilities derived from the expert panel. As there is uncertainty regarding the validity of these values, a sensitivity analysis using a Monte Carlo-type model was undertaken for the base case scenario (healthcare sector perspective). In this model, simultaneous changes in the values for the probability of each complication are examined through a series of simulations.26 The sensitivity analysis was performed in Excel 2016 software, using the statistical tools included in the software’s add-ons. A total of 10,000 simulations were performed, generating random values based on the Poisson distribution for both single and repeated use of SUDs.
Results
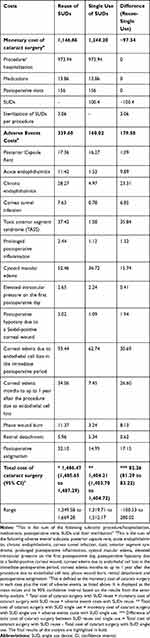
The monetary cost of cataract surgery was estimated based on the wage-adjusted Greek DRG system, the cost of intraoperative and postoperative medications and the cost of postoperative office visits. In the case of single use, the additional cost of SUDs, which was calculated to be 100.4€, was added to the cost of cataract surgery. On the other hand, in the case of repeated use, only the sterilization cost of SUDs was included in the cost estimate. A conservative approach was adopted, and, thus, we assumed that the SUDs were sterilized as a batch, resulting in a cost of sterilization per procedure equal to 3.06€.27 Therefore, when SUDs are reused, the total monetary cost of cataract surgery is estimated at 1,146.86€, while when SUDs are not reused, the total cost is estimated at 1,244.20€ (Table 2). It should be noted that the costs presented in Table 2 are unadjusted for the relative risk of each complication.
 |
Table 2 Model Inputs |
Based on the probabilities of complications attained from the expert panel, repeated use of SUDs is associated with a higher chance of complications compared to single use (Table 2). It is noteworthy that the relative risk (RR) is particularly high for TASS (RR: 23.727) followed by cornea tunnel infection (RR: 9.459) and acute endophthalmitis (RR: 7.204). Similar to the cost estimate of cataract surgery, costs of adverse events were also estimated based on the wage-adjusted Greek DRG system, the cost of relevant medications and the cost of any additional office visits (Table 2). The highest cost is observed for the management of chronic endophthalmitis, followed by cornea tunnel infection and TASS.
Table 3 shows the expected additional cost of each complication per cataract surgery per patient. When SUDs are reused, the cost of each complication is higher due to its higher probability of occurrence compared to when SUDs are not reused. The greatest additional costs are seen in the cases of endophthalmitis, cornea tunnel infection, TASS, cystoid macular edema, corneal edema and phaco wound burn. The total additional cost per cataract surgery under a single-use policy was estimated at 159.78€, and under a reuse policy at 339.43€.
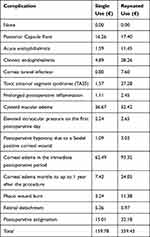 |
Table 3 Expected Additional Cost of Each Complication per Cataract Surgery per Patient, Healthcare Sector Perspective |
Therefore, the total cost per cataract surgery is 1,403.98€ (1,244.20€, the monetary cost of cataract surgery, plus 159.78€ for the cost of adverse events) in the case of single use, while for repeated use the total cost is 1,486.29€ (1,146.86€ + 339.43€, respectively). Thus, based on the results of the base case analysis, single use of SUDs results in a lower expected cost per cataract surgery per patient compared to reuse of SUDs. This is justified by the increased likelihood of complications in the case of reuse, which, in turn, leads to a higher expected additional cost. Consequently, single use of SUDs results in 82.31€ lower expected cost per patient compared to the repeated use of SUDs in cataract surgery. In the alternative scenario (societal perspective), total cost per cataract surgery in the case of single use was 1,506.20€ (1,327.33€, the cost of cataract surgery, + 178.87€ for the cost of adverse events) while in the case of repeated use it was 1,614.44€ (1,229.99€ + 384.45€ respectively), indicating a higher cost up to 108.24€. The expected additional cost per complication under the societal perspective is presented in Table 4.
 |
Table 4 Expected Additional Cost of Each Complication per Cataract Surgery per Patient, Societal Perspective |
Sensitivity Analysis
In each of the 10,000 Monte Carlo simulations, simultaneous changes in the probability variables (obtained from the expert panel) were performed and the difference between the total expected cost under a reuse versus a single use policy was calculated. According to the results of the sensitivity analysis, the mean difference amounts to 82.26€ (95% CI: 81.29–83.22) with a range from −100.53€ to 280.02€. (Table 5 and Figure 1). In 95% of the simulations, the total expected cost of SUD reuse is higher than the total expected cost of SUD single use by at least 2.65€. In the remaining 5% of the simulations, the difference takes values from −100.53€ up to 2.61€. In particular, when the probabilities of complications have very low values, the additional cost of adverse events per patient is low too. Therefore, in the remaining 5% of the 10,000 simulations, the difference between single and repeated use is lower than 2.65€ and, in a proportion of these, the total expected cost of SUD single use exceeds the total expected cost of SUD reuse.
Discussion
The present study is the first to report a cost-benefit analysis on the single and repeated use of SUDs in cataract surgery. Based on our findings, it is evident that SUD reuse in cataract surgery is not cost-beneficial. At first glance, it may seem that reuse of SUDs is economically favorable since reprocessing has a substantially lower cost compared to the purchase of new SUDs. However, when all accompanying costs are inserted in the cost equation, the result is the opposite.
The incidence rates of cataract surgery complications for SUD single use, which derived from the expert panel, are in line with relevant published studies. In particular, the published incidence rate of endophthalmitis with the use of intracameral antibiotics,28,29 which is routinely done in Greece, is 0.012% and the incidence rate derived from the expert panel was 0.02%. For postoperative retinal detachment, the reported incidence rate is 0.30% at 4 years30 and the panel’s estimate was 0.26%. Finally, the published incidence rate of postoperative cystoid macular edema in the case of SUD single use ranges from 0.1% to 2.35%31,32 and the panel’s estimate was 1.22%. Regarding SUD reuse, published literature on the incidence rates of complications is scarce. Notably, only one study from India has been identified to investigate the impact of SUD reuse on the incidence of TASS.14 It estimated an incidence rate of 0.22%, while in our study it was 0.48%. However, these incidence rates are not comparable, since this published study does not discuss the exact pattern and type of reuse in terms of medical devices and disposables. To the extent of our knowledge, no other studies have been published on the incidence rate of cataract surgery complications in the case of SUD reuse.
Based on the expert panel estimates, the likelihood of complications is higher when SUDs are reused compared to their single use, which leads to higher complication management costs. Consequently, the additional cost required to manage the complication, or, in other words, the negative benefit, outweighs the initial benefit provided by not purchasing new SUDs. In particular, when SUDs are reused there are savings of 97.34€ per cataract surgery, but also an additional cost of 179.65€ from managing the resultant complications. Therefore, instead of net savings, an additional burden of 82.31€, in fact, arises, under the healthcare sector perspective. Additionally, the societal perspective analysis, in which lost productivity and cost of leisure time were taken into consideration, indicated a higher cost of 108.24€ per cataract surgery in the case of the SUD reuse policy. The relative risk of adverse events associated with reuse varies from 1.070 for posterior capsule rent to 23.727 for TASS (Table 2). For complications in the lower end of this spectrum, the benefit of single use may not be easily perceived by surgeons in their everyday practice. Therefore, it is of utmost importance to assess the impact of adverse events associated with SUD reuse on the healthcare system as a whole rather than on a single center and/or surgeon so as to capture these seemingly small differences in the risks and costs of complications.
Apart from the financial consequences and the patient safety issues associated with the single or the repeated use of SUDs, ethical concerns and medico-legal implications arise as well.33,34 Reuse of an SUD should not be performed without patient awareness of such practice. In other words, SUD reuse should be included in the informed consent the patient signs prior to surgery.33 From a legal standpoint, in Europe, the United States, Canada and Australia, liability for the safety and performance of a reused SUD is assumed by the reuser (ie the hospital, clinic and/or health care provider) who chooses to use such a device in patient care.35,36 If the practice of SUD reuse is to be adopted, attention should be paid at the reprocessing methods used, which should include more than just the standard cleaning and sterilization that is commonly done. In fact, the World Health Organization advises that SUD reprocessing be performed by licensed reprocessors following certified procedures.37 The alternative to SUD reprocessing that could save cost and reduce the environmental waste associated with single use would be designated reusable devices with manufacturer standards as to the cleaning and resterilization of such devices. This would ensure the safety of the procedure, it would make cataract surgery more accessible in low per capita healthcare settings and it would reduce the carbon footprint associated with SUD waste.38
Our study bears some limitations that should be acknowledged. Cost calculations are estimated from the third party payer and societal perspectives, while the cost borne by the patient in private settings is not included in the analysis. Similarly, indirect costs related to informal care (ie family/friends who provide unpaid care to the patient) are also not incorporated in the analysis. Moreover, the present study does not incorporate the impact of cataract surgery and its complications on patient quality of life. In addition, our study assumes that SUDs are reused only once, which is a conservative estimate. In everyday practice, sterilization and reuse of SUDs might take place more than once, which may dramatically increase the incidence of adverse events and eventually the expected total cost of cataract surgery. Finally, this study does not take into account potential equipment failure due to SUD reuse, which would extend the duration of the procedure and limit the total daily case load of the operating room. Overall, the present analysis most likely underestimates the actual cost that arises from the repeated use of SUDs.
Conclusions
In conclusion, our study is the first to attach a monetary value to the common yet questionable practice of SUD reuse. Repeated use of SUDs in cataract surgery is not appropriate, it jeopardizes patient and staff safety and it carries a legal liability for the reuser. This study also shows that it is not cost beneficial. We, thus, expect that our results will have an impact on policy formulations and influence hospital budget spending for the sector of cataract surgery.
Abbreviations
OVD, ophthalmic viscosurgical device; SUD, single-use device; TASS, toxic anterior segment syndrome.
Ethics Statement
Ethical approval was not required for the present study. The participants were asked to provide only cumulative estimates based on their clinical practice and, thus, the study did not involve access to any patient data or protected health information (as defined by HIPAA) by the research team. Furthermore, the invitation letters explained to participants that their responses would be fully anonymized.
Disclosure
The present study has been financially supported by Alcon Laboratories Single Member S.A.C.I. Mrs Panagiota Naoum, Mrs Vasiliki Naoum, Mr Nikos Nomikos and Dr Konstantina Bachtalia, report grants from Alcon Laboratories Single Member S.A.C.I., during the conduct of the study. Dr Sotiria Palioura reports personal fees from Alcon, during the conduct of the study; personal fees from Alcon Laboratories, outside the submitted work; and is a consultant for Alcon Laboratories Single Member S.A.C.I. The authors report no other potential conflicts of interest for this work.
References
1. Eucomed Industry. Guide for Manufacturers to Implement the Requirement Introduced by Directive 2007/47/EC for Information on Single-Use Medical Devices. Eucomed; 2009.
2. European Commission. Report on the Issue of the Reprocessing of Medical Devices in the European Union, in Accordance with Article 12a of Directive 93/42/EEC. Brussels: European Commission; 2010.
3. MHRA. Single-Use Medical Devices: Implications and Consequences of Reuse. MHRA; 2013.
4. de Sousa Martins B, Queiroz e Melo J, Logarinho Monteiro J, Rente G, Teixeira Bastos P. Reprocessing of single-use medical devices: clinical and financial results. Port J Public Heal. 2018;36(3):150–156. doi:10.1159/000496299
5. Mathys KC, Cohen KL, Bagnell CR. Identification of unknown intraocular material after cataract surgery: evaluation of a potential cause of toxic anterior segment syndrome. J Cataract Refract Surg. 2008;34(3):465–469. doi:10.1016/j.jcrs.2007.10.047
6. Cutler Peck CM, Brubaker J, Clouser S, Danford C, Edelhauser HE, Mamalis N. Toxic anterior segment syndrome: common causes. J Cataract Refract Surg. 2010;36(7):1073–1080. doi:10.1016/j.jcrs.2010.01.030
7. Bodnar Z, Clouser S, Mamalis N. Toxic anterior segment syndrome: update on the most common causes. J Cataract Refract Surg. 2012;38(11):1902–1910. doi:10.1016/j.jcrs.2012.06.053
8. Chaudhari M, Agarwala NS, Nayak BK. Determination of the nature and origin of the metallic foreign particles appearing on the iris after phacoemulsification. J Cataract Refract Surg. 2013;39(7):1008–1010. doi:10.1016/j.jcrs.2013.02.040
9. Angmo D, Khokhar SK, Ganguly A. Intraoperative fracture of phacoemulsification tip. Middle East Afr J Ophthalmol. 2014;21(1):86–88. doi:10.4103/0974-9233.124116
10. Demircan S, Gokce G, Atas M, Baskan B, Goktas E, Zararsiz G. The impact of reused phaco tip on outcomes of phacoemulsification surgery. Curr Eye Res. 2016;41(5):636–642. doi:10.3109/02713683.2015.1039654
11. Cost-Benefit Analysis. York Health Economics Consortium. 2016. Available from: https://www.yhec.co.uk/glossary/cost-benefit-analysis/.
12. Ryder HF, McDonough C, Tosteson ANA, Lurie JD. Decision analysis and cost-effectiveness analysis. Semin Spine Surg. 2009;21(4):216–222. doi:10.1053/j.semss.2009.08.003
13. Endophthalmitis Study Group. European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978–988. doi:10.1016/j.jcrs.2007.02.032.
14. Sengupta S, Chang DF, Gandhi R, Kenia H, Venkatesh R. Incidence and long-term outcomes of toxic anterior segment syndrome at Aravind Eye Hospital. J Cataract Refract Surg. 2011;37(9):1673–1678. doi:10.1016/j.jcrs.2011.03.053
15. O’Hagan A, Buck CE, Daneshkhah A, et al. Uncertain Judgements: Eliciting Experts’ Probabilities. Chichester, UK: John Wiley & Sons, Ltd; 2006. doi:10.1002/0470033312
16. Soares MO, Bojke L, Dumville J, Iglesias C, Cullum N, Claxton K. Methods to elicit experts’ beliefs over uncertain quantities: application to a cost effectiveness transition model of negative pressure wound therapy for severe pressure ulceration. Stat Med. 2011;30(19):2363–2380. doi:10.1002/sim.4288
17. Wilson ECF, Usher-Smith JA, Emery J, Corrie PG, Walter FM. Expert elicitation of multinomial probabilities for decision-analytic modeling: an application to rates of disease progression in undiagnosed and untreated melanoma. Value Health. 2018;21(6):669–676. doi:10.1016/j.jval.2017.10.009
18. Rossi SH, Blick C, Nathan P, Nicol D, Stewart GD, Wilson ECF. Expert elicitation to inform a cost-effectiveness analysis of screening for renal cancer. Value Health. 2019;22(9):981–987. doi:10.1016/j.jval.2019.03.018
19. Ministry of Health. Medication Price Bulletin. Available from: https://www.moh.gov.gr/articles/times-farmakwn/deltia-timwn/6053-epikairopoihsh-deltiwn-timwn-farmakwn-logw-dioikhtikwnmetabolwn-kai-allaghs-f-p-a-12-2-2019.
20. Greek Diagnostic Related Groups System. [homepage on the internet]. Available from: http://kenicd.e-healthnet.gr/.
21. Hellenic Ministry of finance, Hellenic Ministry of labour, social insurance and welfare, Hellenic Ministry of health. ΦΕΚ 3096/Β/23.11.2012: Αριθμ. Υ4α/oικ.105494 [Government Gazette Issue 3096/Β/23.11.2012: Joint Ministerial Decision Υ4α/oικ.105494]; Athens: National Printing Office. Available from: http://www.et.gr/idocs-nph/search/pdfViewerForm.html?args=5C7QrtC22wEbA_BZxkczbHdtvSoClrL8Hcsihn8f1EIfP1Rf9veiteJInJ48_97uHrMts-zFzeyCiBSQOpYnTy36MacmUFCx2ppFvBej56Mmc8Qdb8ZfRJqZnsIAdk8Lv_e6czmhEembNmZCMxLMtUp6gROBB3AeQKo8RTWTCbwwgxBtjpIVgh1q-uwg-7Fa.
22. Ministry of Finance, Ministry of Health. ΦΕΚ 1958/B/12-8-2013, Απόφαση Αριθμ Oικ. 72944 [Government Gazette Issue 1958/B/12-8-2013, Joint Ministerial Decision 72944]. Athens: National Printing Office. Available from http://www.et.gr/idocs-nph/search/pdfViewerForm.html?args=5C7QrtC22wEaosRGzKxO6XdtvSoClrL8vnZOzRHI6VLuFUDqazHcNeJInJ48_97uHrMts-zFzeyCiBSQOpYnTy36MacmUFCx2ppFvBej56Mmc8Qdb8ZfRJqZnsIAdk8Lv_e6czmhEembNmZCMxLMtbrLuXTahBK3eaKz7OnbV4kUsGqhjeki8Zogqmlz3hjF.
23. Ministry of Finance, Ministry of Health. ΦΕΚ 4898/Β/1-11-2018, Απόφαση Αριθμ. ΕΑΛΕ/Γ.Π. 80157 [Government Gazette Issue 4898/Β/1-11-2018, Joint Ministerial Decision ΕΑΛΕ/Γ.Π. 80157]. Athens: National Printing Office. Available from https://eopyy.gov.gr/Files/%CE%A6%CE%95%CE%9A%204898%20%CF%84%20%CE%92%202018%20-%20%CE%9D%CE%95%CE%9F%CE%A3%20%CE%95.%CE%9A.%CE%A0.%CE%A5%2001%2011%202018.pdf.
24. Labour Institute GSEE. Greek Economy and Employment. Annual Report 2019.; 2019. Available from: https://www.inegsee.gr/ekdosi/etisia-ekthesi-2019-ine-gsee-i-elliniki-ikonomia-ke-i-apascholisi/.
25. Ministry of Labour, Social Insurance and Social Solidarity. ΦΕΚ 173/Β/30-1-2019, Υπoυργική Απόφαση Αριθμ. oικ. 4241/127/2019 [Government Gazette Issue 173/Β/30-1-2019, Ministerial Decision 4241/127/2019];. Athens: National Printing Office; Available from http://www.et.gr/idocs-nph/search/pdfViewerForm.html?args=5C7QrtC22wFqnM3eAbJzrXdtvSoClrL8LHF9k8yiZ3t5MXD0LzQTLf7MGgcO23N88knBzLCmTXKaO6fpVZ6Lx3UnKl3nP8NxdnJ5r9cmWyJWelDvWS_18kAEhATUkJb0x1LIdQ163nV9K–td6SIuf2F2zpv5lDUhp0ASkQZV75FWh666bqpExbOic1jRo33.
26. Harrison RL. Introduction to Monte Carlo Simulation. AIP Conf Proc. 2010;17–21. doi:10.1063/1.3295638
27. Landouzy M, Rossi M, Okiemy E, Velu MBI Comparative medical economical study about cataract surgery sets: single use versus reusable. Poster ID-146 presented at: 16th World Sterilization Congress & Annual conference of AFS; October 7-10; 2015; Lille, France. Available from: https://www.sf2s-sterilisation.fr/wp-content/uploads/2016/08/ID146_WFHSS_2015.pdf.
28. Creuzot-Garcher C, Benzenine E, Mariet AS, et al. Incidence of Acute Postoperative Endophthalmitis after Cataract Surgery: a Nationwide Study in France from 2005 to 2014. Ophthalmology. 2016;123(7):1414–1420. doi:10.1016/j.ophtha.2016.02.019
29. Yannuzzi NA, Si N, Relhan N, et al. Endophthalmitis after clear corneal cataract surgery: outcomes over two decades. Am J Ophthalmol. 2017;174:155–159. doi:10.1016/j.ajo.2016.11.006
30. Day AC, Donachie PHJ, Sparrow JM, Johnston RL. Royal College of Ophthalmologists’ National Ophthalmology Database. United Kingdom national ophthalmology database study of cataract surgery: report 3: pseudophakic retinal detachment. Ophthalmology. 2016;123(8):1711–1715. doi:10.1016/j.ophtha.2016.04.002
31. Henderson BA, Kim JY, Ament CS, Ferrufino-Ponce ZK, Grabowska A, Cremers SL. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg. 2007;33(9):1550–1558. doi:10.1016/j.jcrs.2007.05.013
32. Zur D, Loewenstein A. Postsurgical cystoid macular edema. Dev Ophthalmol. 2017;58:178–190. doi:10.1159/000455280
33. Hailey D, Jacobs PD, Ries NM, Polisena J. Reuse of single use medical devices in Canada: clinical and economic outcomes, legal and ethical issues, and current hospital practice. Int J Technol Assess Health Care. 2008;24(04):430–436. doi:10.1017/S0266462308080562
34. Collier R. The ethics of reusing single-use devices. CMAJ. 2011;183(11):1245. doi:10.1503/cmaj.109-3907
35. Eucomed. Eucomed White Paper on the Reuse of Single Use Devices. Eucomed; 2009.
36. Costa FS, Olivia Pereira M, Esteves C, Carvalho J Reprocessing of Single-Use Medical Devices in hospital environment: evolution and future perspectives. In:
37. World Health Organization. Decontamination and Reprocessing of Medical Devices for Health-Care Facilities. WHO; 2016.
38. Chang DF. Needless waste and the sustainability of cataract surgery. Ophthalmology. 2020;127(12):1600–1602. doi:10.1016/j.ophtha.2020.05.002
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.