Back to Journals » ClinicoEconomics and Outcomes Research » Volume 9
Cost-effectiveness of SQ® HDM SLIT-tablet in addition to pharmacotherapy for the treatment of house dust mite allergic rhinitis in Germany
Authors Green W, Kleine-Tebbe J, Klimek L, Hahn-Pedersen J, Nørgaard Andreasen J, Taylor M
Received 27 June 2016
Accepted for publication 20 October 2016
Published 16 February 2017 Volume 2017:9 Pages 77—84
DOI https://doi.org/10.2147/CEOR.S115931
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Samer Hamidi
William Green,1 Jörg Kleine-Tebbe,2 Ludger Klimek,3 Julie Hahn-Pedersen,4 Jakob Nørgaard Andreasen,4 Matthew Taylor1
1York Health Economics Consortium, University of York, York, UK; 2Allergy and Asthma Center, Westend, Berlin, 3Center for Rhinology and Allergology, Wiesbaden, Germany; 4ALK-Abelló, Hørsholm, Denmark
Background: Allergic rhinitis is a global health problem that burdens society due to associated health care costs and its impact on health. Standardized quality (SQ®) house dust mite (HDM) sublingual immunotherapy (SLIT)-tablet is a sublingually administered allergy immunotherapy tablet for patients with persistent moderate to severe HDM allergic rhinitis despite use of allergy pharmacotherapy.
Objective: To assess the cost-effectiveness of SQ HDM SLIT-tablet in Germany for patients suffering from HDM allergic rhinitis.
Methods: A pharmacoeconomic analysis, based on data collected in a double-blinded, phase III randomized placebo-controlled trial (n=992), was undertaken to compare SQ HDM SLIT-tablet in addition to allergy pharmacotherapy to placebo plus allergy pharmacotherapy. Quality-adjusted life year (QALY) scores and health care resource use data recorded in the trial were applied to each treatment group and extrapolated over a nine-year time horizon. A series of scenarios were used to investigate the impact of changes on long-term patient health for both treatment groups, which was measured by annual changes in QALY scores. Deterministic and probabilistic sensitivity analyses were also performed.
Results: In the base case analysis, compared with allergy pharmacotherapy, SQ HDM SLIT-tablet led to a QALY gain of 0.31 at an incremental cost of €2,276 over the nine-year time horizon, equating to an incremental cost-effectiveness ratio of €7,519. The treatment was cost-effective for all scenarios analyzed; however, results were sensitive to changes in individual parameter values during the deterministic sensitivity analysis.
Conclusion: SQ HDM SLIT-tablet in addition to pharmacotherapy is cost-effective compared with allergy pharmacotherapy plus placebo for the treatment of persistent moderate to severe HDM allergic rhinitis that is not well controlled by allergy pharmacotherapy.
Keywords: SQ HDM SLIT-tablet, allergic rhinitis, house dust mite, cost-utility analysis
Introduction
Allergic rhinitis is a chronic disease and a global health problem that can have a significantly detrimental effect on quality of life and is often associated with substantial health care costs. In 2004, a European study found the prevalence of allergic rhinitis to be between 18% and 26%, with the incidence expected to rise.1,2 Assessment of the disease through both general health and disease-specific questionnaires showed a substantial impairment of quality of life.3 Patients found that their daily activities, particularly those related to their professional, personal, and social life, were either moderately or severely impaired. A European survey found that a quarter of patients had to take time off work due to allergic rhinitis, and almost 40% of children with allergic rhinitis reported that they occasionally missed school due to their disease.4
Allergic rhinitis is associated not only with significant costs from a societal perspective, because of reduced working capacity and absence from work, but also from a health care perspective, with the main cost drivers being medication, hospitalization, and rehabilitation. A German cost of illness study found that the average annual cost of seasonal allergic rhinitis was €1,543 per adult, with direct costs accounting for only 42% of those costs.5
Sensitization to house dust mite (HDM) allergens is a common cause of respiratory allergies, being associated with both asthma and rhinitis. A study of 726 patients by Bauchau and Durham found that 49% of patients with a clinical diagnosis of allergic rhinitis were sensitive to HDM allergens.1 There is also evidence that when compared with other allergic sensitizations, patients with HDM allergy have a more severe condition due to the impact on quality of life and a greater number of comorbidities.6
There are three main therapeutic options for this condition: allergen avoidance, allergy pharmacotherapy, and allergy immunotherapy (AIT).7 One recently developed AIT, targeted specifically at HDM allergens, is the SQ® HDM sublingual AIT (SLIT)-tablet (Acarizax®; ALK-Abelló, Hørsholm, Denmark). This is a therapy option for patients suffering from HDM allergic rhinitis taking allergy pharmacotherapy but not obtaining satisfactory disease control. The therapy is administered sublingually as a tablet, rather than subcutaneously. SQ HDM SLIT-tablet is a 1:1 mixture of allergen extract from the two major mite species, Dermatophagoides pteronyssinus and Dermatophagoides farinae.8
The objective of this analysis was to assess the cost-effectiveness of SQ HDM SLIT-tablet in Germany for patients suffering from persistent moderate to severe HDM allergic rhinitis, with or without allergic asthma, despite the use of allergy pharmacotherapy.
Methods
Design and population
A pharmacoeconomic analysis was undertaken based on data collected from a multi-national, double-blinded, randomized controlled phase III trial, in which SQ HDM SLIT-tablet was compared with placebo.9 This was the MT-06 trial (NCT01454544), a one-year evaluation that aimed to assess the efficacy of SQ HDM SLIT-tablet in addition to pharmacotherapy in improving rhinitis symptoms and reducing the use of allergy pharmacotherapy in patients with or without asthma who also have a history of poor disease control. A total of 992 patients with a mean (standard deviation) age of 32.3 (10.9) years were recruited from 100 trial sites across 12 European countries. The trial was designed and conducted in accordance with the principles of the Declaration of Helsinki and conducted in compliance with the principles of the International Conference on Harmonization of Technical Requirement for Registration of Pharmaceuticals for Human Use (ICH) Good Clinical Practice. Subjects signed the informed consent form when entering the trial.
For the purpose of this evaluation, two treatment options were included: SQ HDM SLIT-tablet plus allergy pharmacotherapy (henceforth SQ HDM SLIT-tablet) and placebo plus allergy pharmacotherapy (henceforth pharmacotherapy) in line with the setup of the trial. Various types of allergy pharmacotherapies are available, namely oral and topical antihistamines and nasal corticosteroids, and both treatment groups had free access to these drugs, provided by ALK-Abelló, during the trial. Access to the data for this analysis/research was provided by ALK-Abello who funded the original trial.
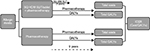
While the efficacy of SQ HDM SLIT-tablet is assessed for one year in the MT-06 trial, in clinical practice patients are treated for three years. There is clear evidence that AIT can lead to significant improvements during three years of continuous treatment,10 and similar effects are expected for SQ HDM SLIT-tablet. The exact level of effect after discontinuation of AIT, as observed for other AITs such as Grazax® (ALK-Abello, Horsholm, Denmark), is still to be elucidated. However, there is evidence that AIT can have a longer-term impact on patient outcomes, up to 12 years as reported in a consensus report by Burks et al, and this may be explained by the disease modification resulting in induction of immunological tolerance, which has been demonstrated for some forms of AIT.10–13 To investigate the potential long-term effect of treatment, a nine-year time period has been adopted for the analysis supported by expert advice. In the analysis, patients in the SQ HDM SLIT-tablet group remain on treatment for three years before switching to allergy pharmacotherapy only. In the comparator group, it is assumed patients remain on pharmacotherapy for the full time horizon. This assumption has been made because the patients included in the analysis are those with persistent moderate to severe forms of the disease and, therefore, require pharmacotherapy for ongoing control of disease symptoms with continuous treatment, an established option for patients with persistent allergic rhinitis.14
Quality of life
In order to quantify the magnitude of the benefits of treatment, patient quality of life was incorporated into the analysis using utility scores. The inclusion of utility score ensures that the impact of allergic rhinitis on patient health is captured appropriately. In the MT-06 trial, utility (ie, quality of life) was measured using the EQ-5D (EuroQol-five dimensions questionnaire) health survey, which is a standardized instrument widely used to quantify general health outcomes.15 Following one year of treatment, utility values of 0.926 and 0.915 were recorded for patients in the SQ HDM SLIT-tablet and pharmacotherapy groups, respectively. However, regression analysis was used to correct for skewed data that occurred because many patients were in perfect health. A two-stage approach was adopted for the regression analysis. This approach was adopted because it was shown to be a less biased regression method for analyzing utility data, and the analysis that was performed was very similar to that reported by Poole et al.15 In short, during the first stage, a binomial model was used to estimate the proportion of EQ-5D observations in which the patient was in perfect health (61.4%). Within this model, the EQ-5D index was modeled as a binary variable, indicating perfect health or imperfect health, with the inclusion of five predictor variables (asthma status, age, rhinitis daily symptom score, rhinitis daily medication, and smoking status). During stage two, a generalized, mixed linear model was applied to the imperfect health observations (38.6%), to estimate the EQ-5D index scores for SQ HDM SLIT-tablet and pharmacotherapy patients. Using this approach, utility values of 0.919 and 0.898 for SQ HDM SLIT-tablet and pharmacotherapy patients, respectively, were estimated (p<0.05), and these values were assigned to each treatment group and combined with the duration of time spent in that state of health in order to generate quality-adjusted life year (QALY) scores.
The long-term impact of each treatment option on patient quality of life was also examined using utility scores. To account for the impact of AIT during the treatment period, the analysis assumed that patients taking SQ HDM SLIT-tablet will have a 5% increase in utility during each year of treatment (ie, a 5% improvement in quality of life), while patients on pharmacotherapy were assumed to have a stable quality of life during this period. The stability of patients receiving pharmacotherapy was based on the conservative assumption that the improvement recorded for the placebo group at the end of the trial would remain constant during the first three years of treatment.
While it was expected that SQ HDM SLIT-tablet would have a disease-modifying effect on patients, as a result of the potential preventive and curative effect of AIT, due to a lack of data it has been conservatively assumed that there would be a decrease in utility of 10% during the years six to nine. This decline is to account for the potential loss of effect when the treatment has been stopped. During this period, it is also conservatively assumed that pharmacotherapy patients have a smaller decrease in utility of 5% per year. The rate of decline is assumed to be lower for pharmacotherapy patients, because during the treatment period (ie, the first three years of treatment), the utility gain was lower for these patients. The impact of changes to treatment effectiveness, such as longer-term improvements with SQ HDM SLIT-tablet, was examined via a series of scenarios that are discussed in more detail in the following section.
Health care resource use
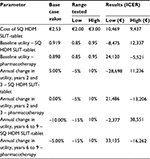
Costs for the analysis were estimated by taking health care resource use values from the MT-06 trial and multiplying by the unit price of that resource for the German market (Table 1). In the MT-06 trial, data were collected on four resources: doctors’ visits, desloratadine (5 mg) intake, budesonide (64 μg) intake, and azelastine (0.05%) intake. The cost of SQ HDM SLIT-tablet was also included within the treatment group. The annual costs generated were applied equally across all years in the model. Also included in the analysis was one extra doctor’s visit for all patients on SQ HDM SLIT-tablet. The treatment was suitable for home treatment, following an initial dose that was given under the supervision of a doctor.
  | Table 1 Health care resource use from phase III randomized controlled trial Notes: Five health care resources were included in the analysis. Unit prices for the resources (2015 prices) were combined with the resource use (mean annual values) to estimate the total annual cost. The resource use was recorded within the phase III randomized controlled trial (MT-06) by patients who used electronic diaries to record day-to-day usage. aSource: correspondence with ALK-Abello, Horsholm, Denmark; bsource: https://www.gkv-spitzenverband.de; csource: http://www.kbv.de/html/. Abbreviations: HDM, house dust mite; SLIT, sublingual allergy immunotherapy; SQ, standardized quality. |
To capture the indirect costs of allergic rhinitis on society, sick days were also incorporated into the analysis. Petersen et al previously found that AIT reduced the mean number of sick days per annum, from 6.75 to 4, in patients with HDM allergic rhinitis or allergic asthma.16 As data specific to SQ HDM SLIT-tablet are not available, it was assumed that this treatment would have a similar impact to that identified by Petersen et al. Therefore, these data were applied in the analysis and combined with a cost of €93.69 per sick day in Germany as estimated by Klussman et al.17 This equated to total annual indirect costs of €375 and €632 for SQ HDM SLIT-tablet and pharmacotherapy groups, respectively.
Pharmacoeconomic analysis
The analysis estimated the total costs and QALYs for one hypothetical adult patient receiving SQ HDM SLIT-tablet in addition to pharmacotherapy and one hypothetical adult patient receiving pharmacotherapy plus placebo over a nine-year period. In order to measure the cost-effectiveness of SQ HDM SLIT-tablet, it was necessary to compare the costs and QALYs generated by this treatment to those generated by pharmacotherapy. This comparison was facilitated via the calculation of the incremental cost-effectiveness ratio (ICER). This required the inclusion of a willingness-to-pay threshold, with a value of €40,000 per QALY adopted here. Rationing decisions in Germany are the responsibility of Gemeinsamer Bundesausschuss (G-BA) and are made on a case-by-case basis.18 Therefore, no defined threshold was used for German cost-effectiveness analyses. The value adopted here was based on a value of approximately £30,000, which is included in the range adopted by the National Institute for Health and Care Excellence in England.19
Because a nine-year time horizon was adopted, health outcomes and costs that occur in the future were given less value than those occurring in the present. This is due to the preference of humans to receive benefits in the present and incur costs later in the future. Therefore, cost and QALY values were discounted, using an annual discount rate of 3%, in line with German guidelines.20 The overall setup of the analysis is presented graphically in Figure 1.
Scenario analysis
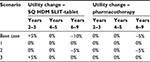
To investigate the impact of altering effectiveness assumptions, ie, utility values, a series of scenarios were conducted as part of the analysis. In these scenarios, an annual rate of utility change (ie, change in quality of life) was applied to the model. This rate of change was used to test the conservative assumptions regarding patient quality of life over time, which were adopted in the model base case.
In total, three separate rates were applied to the model: the annual rate of change while on treatment (years 2 and 3), the annual rate of change posttreatment (years 4 and 5), and the annual rate of change in all remaining years (years 6 to 9). For each of these rates, separate values were applied to the two treatment groups. The utility change for each scenario is summarized in Table 2.
For scenario one, it was assumed that patient health (ie, quality of life) remained stable for the full nine-year time horizon (ie, no change in utility scores) for both treatment groups. Scenario two was included to investigate the impact of a decline in health following treatment. Within this scenario, all patients (ie, both SQ HDM SLIT-tablet and pharmacotherapy patients) were assumed to have stable utility for the three years of treatment and two additional years, followed by a 5% decline in utility during the years six to nine. In scenario three, the impact of improved health on SQ HDM SLIT-tablet patients, without a subsequent decline, was investigated. Therefore, SQ HDM SLIT-tablet patients had a 5% improvement in utility for years two and three (based on the same assumptions mentioned in scenario one), followed by stability, while pharmacotherapy patients were assumed to be stable for all years.
Sensitivity analysis
In order to account for first-order uncertainty around the data used for input parameter values, one-way deterministic sensitivity analyses (DSA) were undertaken. This involved altering the value used for individual parameters, within realistic ranges, to see the impact on the model results, as measured by the ICER. In order to further investigate the uncertainty associated with the model, probabilistic sensitivity analysis (PSA) was also undertaken by varying all input parameters simultaneously across predefined ranges and distributions. Inputs with greater uncertainty, eg, those with wide confidence intervals, varied more than those with less uncertainty. During the PSA, 10,000 iterations of the model were run with a different point estimate drawn randomly for each input parameter during each iteration and the result was presented as the probability of cost-effectiveness for SQ HDM SLIT-tablet (ie, the proportion of results in which the ICER fell below the €40,000 threshold). The distributions applied for the PSA are summarized in Table 3.
Results
The results of the pharmacoeconomic analysis, which is based on the findings from the phase III randomized controlled trial of a sublingual tablet treatment for HDM allergic rhinitis, indicate that over the nine-year time horizon, SQ HDM SLIT-tablet patients generated 6.96 QALYs at a cost of €3,598, as compared with 6.65 QALYs at a cost of €1,301 for pharmacotherapy patients. Therefore, the addition of SQ HDM SLIT-tablet to pharmacotherapy as a treatment option for HDM led to a QALY gain of 0.31 years, with an incremental cost of €2,276. This equates to an ICER of €7,519. When indirect costs (ie, sick days) were also included, overall costs increased to €6,516 and €6,993 for SQ HDM SLIT-tablet and pharmacotherapy patients, respectively. Therefore, SQ HDM SLIT-tablet generated more QALYs at a lower cost, making it a dominant treatment option compared with pharmacotherapy. The ICER was €14,391 during the total period in which patients took the SQ HDM SLIT-tablet (3 years)and this indicates that the drug is also cost-effective over the treatment period.
The impact of changes in patient health over the nine-year time horizon was also investigated using analyses of different scenarios. In these analyses, SQ HDM SLIT-tablet remained cost-effective (as measured by the ICER) in all scenarios, including scenario two, in which a decreased effectiveness of treatment was assumed. The results of the base case and scenario analysis (ICER range of €3,359 to €14,551) are summarized in Table 4. The inclusion of indirect costs increased the magnitude of the results in all the three scenarios.
The results of the DSA indicate that the overall results of the analysis were sensitive to changes in the value of individual parameters. This is because for all parameters, with the exception of the unit price of the SQ HDM SLIT-tablet, the changes caused the ICER to increase above the €40,000 threshold adopted in this analysis, thereby indicating that the SQ HDM SLIT-tablet was no longer cost-effective. These results are summarized in Table 5. The results of the DSA were again unaffected by the inclusion of indirect costs. The results of the PSA indicate that SQ HDM SLIT-tablet has a probability of cost-effectiveness of 61.4%. When indirect costs were also included, the probability of cost-effectiveness increased to 68.4%.
Discussion
Allergic rhinitis is a condition with significant unmet need that can place a large burden on both patients and the health care system. The base case results of this analysis indicate that SQ HDM SLIT-tablet in addition to pharmacotherapy is cost-effective compared with allergy pharmacotherapy only in German patients with persistent moderate to severe HDM allergic rhinitis despite the use of symptom-relieving medication. The scenario analysis that was undertaken also indicates that the base case analysis may underestimate the true benefits of SQ HDM SLIT-tablet. This is because the treatment remained cost-effective in all scenarios assessed, with the incremental cost-effectiveness ratio reducing substantially within scenario three, a scenario that is plausible based on the potential for AIT to be both preventative and curative, as discussed previously.10–13
The model’s results remained robust during the changes implemented as part of the scenario analysis that was undertaken. However, the sensitivity analysis, which was also used to assess the robustness of the results, indicates that the model is sensitive to relatively small changes in individual input parameter values. As minor alterations in input parameters can have a large impact on the results of the model, there is a degree of uncertainty regarding the reliability of the results. This uncertainty may largely be explained by the lack of relevant data to quantify patient outcomes post-trial. Due to this deficiency, assumptions were made to predict patient outcomes (ie, utility) post-trial, and this is a limitation of the analysis. However, the authors of this paper believe the assumptions were relatively conservative and, therefore, the scenarios adopted may underestimate the benefits of SQ HDM SLIT-tablet. In reality, it is probable that the rate of decline in patient health will be substantially less for patients taking SQ HDM SLIT-tablet in addition to allergy pharmacotherapy as compared with those taking allergy pharmacotherapy only and, in fact, health may continue to improve.
Health care resource use in the analysis is based solely on data collected from the phase III clinical trial. As resource use within the trial was protocol-driven, it may not accurately reflect real-life practice. In particular, patients received more overall supervision and better education than patients in an everyday clinical setting, which reduced the number of additional visits to the doctor. Furthermore, medical resources that may be required by rhinitis patients, such as visits to a specialist, were not captured within the analysis as they were not recorded as part of the trial. Treatment compliance was also not considered within the analysis. This is because it would be necessary to model compliance for all therapies given to patients in both treatment groups, and a number of pharmacotherapies were available. It was therefore decided that there were insufficient long-term data to model how compliant patients were to all therapies. If patients receiving SQ HDM SLIT-tablet are not fully compliant with the daily treatment regimen, then this may reduce the efficacy of the treatment. However, previous evidence has shown that following 24 weeks of treatment with SQ HDM SLIT-tablet, the effect remained up to one year after therapy had been stopped altogether, indicating that missing of doses by patients is unlikely to have a major impact on efficacy.21 It should be noted that international treatment guidelines for AIT state that disease modification can only be expected after three years of treatment and that patients should stop taking SQ HDM SLIT-tablet if there is no observed improvement after one year.22
In the trial, there was an improvement in the placebo group, partially explained by the factors discussed previously (ie, supervision and education) and also as a result of the Hawthorne effect, which occurs when patients modify their behavior when they become aware that they are being monitored.23 Similarly, patient outcomes may have improved due to regression to the mean, whereby patient health was lower than normal at trial initiation, which means that over the course of the trial there was a greater probability that their health would improve regardless of the treatment. These changes might have caused an improvement in patient outcomes that may not be found in clinical practice.
Allergic rhinitis is a progressive condition where the patients will commonly experience regular changes in their overall health, and variations are often subtle. These will be driven by changes in a patient’s condition and symptom exacerbations. These variations would be better captured using a more complex modeling approach, such as a Markov model, which facilitates the use of health states to predict changes in patient outcomes. However, given the data that are currently available, developing a Markov model that accurately estimates changes in patient health (eg, disease severity) is a challenging proposition.
In the analysis, SQ HDM SLIT-tablet was compared with allergy pharmacotherapy based on the setup of MT-06 model. In Germany, subcutaneous AIT is considered the standard of care for the patient subgroup assessed in this analysis, and a range of AIT options are available. For emerging AIT treatments, such as SQ HDM SLIT-tablet, the efficacy of each individual product must be established using an appropriately designed clinical trial.24 Because a range of AIT treatments are available, it was not feasible to include them in the MT-06 trial. Nevertheless, in the future it may be worthwhile to compare SQ HDM SLIT-tablet with other AIT options if suitable data can be accessed to facilitate the comparison.
Conclusion
The results presented here suggest that SQ HDM SLIT-tablet in addition to pharmacotherapy is cost-effective compared with allergy pharmacotherapy alone in German patients with persistent moderate to severe HDM allergic rhinitis. The sensitivity analysis highlights a degree of uncertainty regarding these results, which can largely be explained by the lack of clarity in long-term patient outcomes when AIT treatment is stopped. Therefore, conservative assumptions have been adopted for the long-term effectiveness of the treatment, and these may underestimate the true benefits of SQ HDM SLIT-tablet.
Disclosure
SQ HDM SLIT-tablet, the drug considered in this study, is owned by ALK-Abelló. J Hahn-Pedersen and J Nørgaard Andreasen were both employed by ALK-Abelló at the time of study preparation. W Green and M Taylor are employed by York Health Economics Consortium (YHEC), who was sponsored by ALK-Abelló to undertake the cost-utility analysis of SQ HDM SLIT-tablet that this manuscript is based on. A separate consultancy fee was also provided to YHEC for the preparation of the study. L Klimek was an investigator in the MT-06 trial and has also received honoraria from ALK-Abelló for presentation of lectures and other consultancy services. J Kleine-Tebbe was an investigator in the MT-06 trial and has also received honoraria from ALK-Abelló for attendance at an advisory board and the presentation of lectures.
References
Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24(5):758–764. | ||
Canonica GW, Poulsen PB, Vestenbaek U. Cost-effectiveness of GRAZAX® for prevention of grass pollen induced rhinoconjunctivitis in Southern Europe. Respir Med. 2007;101(9):1885–1894. | ||
Bousquet J, Neukirch F, Bousquet PJ, et al. Severity and impairment of allergic rhinitis in patients consulting in primary care. J Allergy Clin Immunol. 2006;117(1):158–162. | ||
Valovirta E, Myrseth SE, Palkonen S. The voice of the patients: allergic rhinitis is not a trivial disease. Curr Opin Allergy Clin Immunol. 2008;8(1):1–9. | ||
Schramm B, Ehlken B, Smala A, Quednau K, Berger K, Nowak D. Cost of illness of atopic asthma and seasonal allergic rhinitis in Germany: 1-yr retrospective study. Eur Respir J. 2003;21(1):116–122. | ||
Frati F, Scurati S, Dell-Albani I, Puccinelli P, Incorvaia C, Passalacqua G. Evaluation of house dust mite allergy in real life: patients’ characteristics and satisfaction with treatment. Eur Ann Allergy Clin Immunol. 2014;46(1):17–21. | ||
Petersen KD, Gyrd-Hansen D, Dahl R. Health-economic analyses of subcutaneous specific immunotherapy for grass pollen and mite allergy. Allergol Immunopathol (Madr). 2005;33(6):296–302. | ||
Centre NHS. House dust mite allergen immunotherapy tablet (Mitizax) for house dust mite allergy-induced rhinitis and conjunctivitis – third line. 2013. Availabel from: http://www.hsric.nihr.ac.uk/topics/house-dust-mite-allergen-immunotherapy-tablet-mitizax-for-house-dust-mite-allergy-induced-rhinitis-and-conjunctivitis-third-line/. Accessed November 17, 2016. | ||
Demoly P, Emminger W, Rehm D, Backer V, Tommerup L, Kleine-Tebbe J. Effective treatment of house dust mite-induced allergic rhinitis with 2 doses of the SQ HDM SLIT-tablet: results from a randomized double-blind, placebo-controlled phase III trial. J Allergy Clin Immunol. 2016;137(2):444–451. | ||
Durham SR, Emminger W, Kapp A, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129(3):717–725e5. | ||
Burks A, Calderon M, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131(5):1288–1295. | ||
Bousquet J, Demoly P, Michel FB. Specific immunotherapy in rhinitis and asthma. Ann Allergy Asthma Immunol. 2001;87(1 Suppl 1):38–42. | ||
Marogna M, Spadolini I, Massolo A, Canonica G, Passalacqua G. Long-lasting effects of sublingual immunotherapy according to its duration: a 15-year prospective study. J Allergy Clin Immunol. 2010;126(5):969–975. | ||
Laekeman G, Simoens S, Buffels J, et al. Continuous versus on-demand pharmacotherapy of allergic rhinitis: evidence and practice. Respir Med. 2010;104(5):615–625. | ||
Poole CD, Bannister CA, Andreasen JN, et al. Estimation of health-related utility (EQ-5D index) in subjects with seasonal allergic rhinoconjunctivitis to evaluate health gain associated with sublingual grass allergen immunotherapy. Health Qual Life Outcomes. 2014;12:99. | ||
Petersen KD, Kronborg C, Larsen JN, Dahl R, Gyrd-Hansen D. Patient related outcomes in a real life prospective follow up study: Allergen immunotherapy increase quality of life and reduce sick days. World Allergy Organ J. 2013;6(1):15. | ||
Klussmann JP, Schädlich PK, Chen X, Rémy V. Annual cost of hospitalization, inpatient rehabilitation, and sick leave for head and neck cancers in Germany. Clinicoecon Outcomes Res. 2013;5:203–213. | ||
Ahlert M, Breyer F, Schwettmann L. How you ask is what you get: framing effects in willingness-to-pay for a QALY. Soc Sci Med. 2016;150:40–48. | ||
NICE. Guide to the Methods of Technology Appraisal 2013. Available from: https://www.nice.org.uk/process/pmg9/chapter/foreword. Accessed November 17, 2016. | ||
IQWiG. General Methods for the Assessment of the Relation of Benefits to Costs 2009. Available from: https://www.iqwig.de/download/General_Methods_for_the_Assessment_of_the_Relation_of_Benefits_to_Costs.pdf. Accessed June 24, 2016. | ||
Zieglmayer P, Nolte H, Nelson HS, et al. Evaluation of SQ-house dust mite sublingual immunotherapy tablet one-year after completion of a 24-week treatment period. J Allergy Clin Immunol. 2016;137(Suppl 2):AB62. | ||
Heads of Medicines Agencies. ACARIZAX – Summary of Product Characteristics. Available from: http://mri.medagencies.org/Human/Product/Details/45380. Accessed June 24, 2016. | ||
McCarney R, Warner J, IIiffee S, van Haselen R, Griffin M, Fisher P. The Hawthorne effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. | ||
Bachert C, Noergaard Andreasen J. Cost-effectiveness of immunotherapy in the treatment of seasonal allergic rhinitis: identifying product-specific parameters of relevance for health care decision-makers and clinicians. Int Arch Allergy Immunol. 2015;168(3):213–217. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.