Back to Journals » Clinical Ophthalmology » Volume 14
Corneal Endothelial Cell Changes After Phacoemulsification Combined with Excisional Goniotomy with the Kahook Dual Blade or iStent: A Prospective Fellow-Eye Comparison
Authors Dorairaj S , Balasubramani GK
Received 23 May 2020
Accepted for publication 6 July 2020
Published 24 November 2020 Volume 2020:14 Pages 4047—4053
DOI https://doi.org/10.2147/OPTH.S263072
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Syril Dorairaj,1 Goundappa K Balasubramani2
1Department of Ophthalmology, Mayo Clinic, Jacksonville, FL, USA; 2Department of Epidemiology, University of Pittsburgh, Pittsburgh, PA, USA
Correspondence: Syril Dorairaj
Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224, USA
Tel +1 201-704-7896
Email [email protected]
Purpose: To characterize changes in endothelial cell density and morphology following phacoemulsification combined with either excisional goniotomy with the Kahook Dual Blade (KDB-phaco) or first-generation iStent trabecular microbypass implantation (iStent-phaco).
Setting: A single clinical practice.
Methods: Twenty-one adult subjects from one center with visually significant cataract and mild-moderate open-angle glaucoma underwent KDB-phaco or iStent-phaco in one eye and the alternate procedure in the fellow eye as part of a prospective, multicenter, randomized trial. Specular microscopy and pachymetry were performed before and 6– 29 months after surgery. Parameters analyzed included change from baseline of endothelial cell density (ECD), the coefficient of variation (CV), the percent of hexagonal cells (%HEX), and central corneal thickness (CCT).
Results: Baseline ECD, CV, %HEX, and CCT were similar between groups. A mean (standard deviation) of 18.2 (5.8) months postoperatively (range 12.5– 28.7 months), the change from baseline in ECD was − 90 ± 226 cells/mm2 (− 3.4%) in KDB-phaco eyes (p=0.083) and − 239 ± 247 cells/mm2 (− 9.0%) in iStent-phaco eyes (p< 0.001); the change in iStent-phaco eyes was significantly greater than in KDB-phaco eyes (p=0.013). The magnitude of Endothelial Cell Loss (ECL) was not correlated with length of postoperative follow-up time in either group. No significant differences in change from baseline in CV, %HEX, or CCT were noted with the exception of a decrease in %HEX in iStent-phaco eyes (p=0.017). No eyes manifested corneal edema, decompensation, or other cornea-related complications.
Conclusion: Both KDB-phaco and iStent-phaco are associated with postoperative ECL, with iStent-phaco producing significantly greater ECL than KDB-phaco. The clinical significance of these findings is unclear, and future studies are warranted to more robustly characterize long-term effects of glaucoma surgical procedures—with and without permanent implants—on ECD and corneal health and to develop evidence-based guidelines for the pre- and post-operative evaluation of corneal health in eyes undergoing glaucoma surgery.
Keywords: glaucoma, excisional goniotomy, Kahook Dual Blade, iStent trabecular microbypass stent, endothelial cell loss
Introduction
The emergence of minimally invasive glaucoma surgeries (MIGS)—procedures generally designed for greater safety compared to traditional procedures such as trabeculectomy or tube-shunt implantation, although in some cases with lesser efficacy—has expanded the indications for glaucoma surgery. Once reserved for cases recalcitrant to medical and laser therapy, glaucoma surgery is now routinely offered earlier in the treatment cascade—particularly in eyes undergoing elective cataract surgery—to reduce intraocular pressure (IOP) and/or to ease the IOP-lowering medication burden.1,2 The rapid expansion of MIGS procedures in a relatively short time period precludes the availability of long-term efficacy and safety data for these treatments. The importance of long-term outcome data has been highlighted by the late corneal endothelial cell loss (ECL) seen with eyes undergoing CyPass supraciliary micro-stent implantation (Alcon Labs, Fort Worth, TX) in combination with cataract surgery versus cataract surgery alone.3 These ECL findings only manifested in postoperative years 4 and 5 and were severe enough to warrant removal of the product from the global marketplace.3,4
The CyPass experience has brought greater scrutiny to the effects of glaucoma and its therapy on corneal endothelial cell (CEC) health. CECs line the posterior, innermost aspect of the cornea and are responsible for maintaining corneal nutrition and hydration homeostasis for optimal optical clarity.5 Relative to the slow rate of ECL associated with age—approximately 0.6% per year6—the presence of glaucoma accelerates this rate.7,8 Topical glaucoma medical therapy has not been associated with changes in endothelial cell density (ECD) or morphology.9,10 Surgical interventions for glaucoma, however, have been linked to ECL. Cataract surgery by phacoemulsification in eyes with glaucoma,3,11 as in eyes without glaucoma,12–15 produces acute ECL that diminishes over time. Unaugmented trabeculectomy produces only modest ECL,16,17 while mitomycin c-augmented trabeculectomy17–19 and tube-shunt procedures produce ECL approximating the effect of phacoemulsification.20–23
The effect of MIGS procedures on ECL is less well characterized. Implanting 2 original iStent trabecular microbypass devices (Glaukos, San Clemente, CA) as a standalone procedure had no significant effect on ECD at 6 months.24 The second-generation iStent Inject device produced comparable ECL when combined with phacoemulsification as phacoemulsification alone at 24 months.11 The Hydrus Schlemm’s canal implant produced insignificantly higher ECL combined with phacoemulsification than phacoemulsification alone (17.2% versus 11.7%);25 the device’s Instructions for Use contain a warning that central ECL ≥30% was more common in Hydrus-phacoemulsification eyes than in phacoemulsification-only eyes.26 The XEN gel stent for subconjunctival filtration produced similar ECL rates when performed as a standalone procedure or combined with phacoemulsification.27 Minimally invasive procedures that do not require permanent implantation of a device—such as Trabectome trabecular ablation (MicroSurgical Technology, Redmond, WA)—have no significant effects on ECD.28,29 It should be noted that these cited studies, as well as the current study, are of relatively short duration (2 years or less); the CyPass experience suggests that late ECL can occur 4 or 5 years postoperatively in eyes with insignificant ECL at 2 years, underscoring the need for long-term safety assessment of new procedures.
To date, there have been no reports of the effects of excisional goniotomy using the Kahook Dual Blade (KDB, New World Medical, Rancho Cucamonga, CA) on ECD. In this report, we describe the relative effects of KDB-phaco and iStent-phaco on ECD and endothelial morphology in an analysis of paired fellow eyes with mild to moderate OAG.
Methods
This was a post hoc follow-up study of subjects who previously participated in a multicenter clinical trial comparing the efficacy and safety of KDB-phaco versus iStent-phaco (www.clinicaltrials.org registration: NCT02784249).30 In that trial, subjects were randomly assigned to undergo phacoemulsification combined with either excisional goniotomy with the KDB (KDB-phaco) or iStent implantation (iStent-phaco) in the study eye. If the fellow eye was eligible and enrolled, it underwent the alternative procedure, typically 2–3 weeks after the first eye. An investigator in the clinical trial (SD) routinely obtained specular microscopy to assess ECD preoperatively in all eyes undergoing cataract surgery. Following the report of late ECL associated with the CyPass device, the investigator obtained postoperative specular microscopy on all subjects at their next scheduled visit; in some cases, this occurred after completion of the clinical trial. The current fellow-eye analysis includes data from all subjects with both eyes enrolled in the clinical trial at the investigator’s site. The data collection protocol was reviewed and approved by the Mayo Clinic ethics committee, the study was conducted in accordance with the tenets of the Declaration of Helsinki, and all subjects provided written informed consent to participate. Reasonable requests for data sharing submitted to Dr. Syril Dorairaj will be considered.
The eligibility criteria for the clinical trial (and thus for inclusion in this analysis) have been described previously.30 Briefly, eligible subjects were aged 18–90 years, diagnosed with mild to moderate OAG (including eyes with pigmentary and pseudoexfoliation components), with medically treated IOP between 14 and 28 mmHg, and a visually significant cataract planned for elective extraction. Subjects were excluded for use of oral medications that could affect IOP, prior glaucoma surgery, recent (≤3 months) glaucoma laser therapy, occludable angles, or documented history of IOP elevation to corticosteroid exposure. Phacoemulsification and intraocular lens implantation were performed in standard fashion. Both the excisional goniotomy and the iStent implantation were performed according to manufacturers’ recommendations by a surgeon with extensive angle surgery experience (SD).31,32 The corneal endothelium was analyzed by a noncontact-type specular microscope (Konan CellChek XL Specular Microscope; Konan Medical Inc., Hyogo, Japan). All examinations were carried out in a dimly lit room by experienced ophthalmic technicians. Patients were asked to look at the fixation light built into the device, and one image was automatically taken at the center of the cornea to count central corneal ECD. No eye drops were used before obtaining the ECD and measurements were made during office hours (9:00 AM to 2 PM). Data provided by automated analysis of the specular microscopy imaging included endothelial cell density (ECD), coefficient of variation (CV), the proportion of hexagonal cells (%HEX), and central corneal thickness (CCT).
Descriptive statistics were reported as means and their standard deviations for continuous data and numbers and percentages for categorical data. Baseline means of ECD, CV, %HEX, and CCT, as well as changes in ECD, CV, %HEX, and CCT from preoperatively to postoperatively, were compared between treatment groups using paired t-tests (for changes within eyes). Between-group changes in ECD, CV, %HEX, and CCT were also compared using paired t-tests (for changes between paired fellow eyes). Correlation of ECD with time from surgery was evaluated using Pearson’s r2. The level of significance was p<0.05. No formal power and sample size calculations were undertaken; the sample size was limited to the number of participants in the clinical trial who had both eyes enrolled at the investigator’s study site.
Results
Overall, 42 eyes of 21 subjects were included in this analysis. Each subject underwent KDB-phaco in one eye and iStent-phaco in the fellow eye determined by randomization of the procedure in the first eye and the alternate procedure in the fellow eye. Demographic data are given in Table 1 and were balanced between groups owing to the fellow-eye nature of the study. All eyes in this analysis had moderate glaucoma based on International Classification of Disease 10 criteria and were using a mean of 1.5 (0.1) medications per eye.
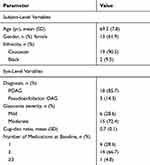 |
Table 1 Subject Demographic Data |
Preoperative and postoperative ECD, CV, %HEX, and CCT values for each group are given in Table 2. Mean preoperative values for ECD, CV, %HEX, and CCT were statistically similar between groups. Postoperative scans were performed on average 18.2 (5.8) months after surgery (range 12.5–28.7 months). The only significant changes from baseline within groups were reductions in ECD (p<0.001) and %HEX (p=0.017) in the iStent-phaco group. Change in ECD from pre- to postoperatively was significantly greater ECL in iStent-phaco eyes compared to KDB-phaco eyes (−9.0% versus −3.4%, p=0.013). The distribution of ECL in each group is given in Figure 1. No statistically significant between-group differences were seen in changes of CV, %HEX, or CCT. There was no significant correlation between magnitude of ECL and time from surgery in eyes undergoing KDB-phaco (r2=0.0009, p=0.898) or iStent-phaco (r2=0.0003, p=0.937).
 |
Table 2 ECD, CV, %HEX, and CCT Data by Group (n=42 Eyes of 21 Subjects) |
 |
Figure 1 Distribution of ECL by treatment group. |
No eyes developed focal or diffuse corneal edema, corneal decompensation, or other cornea-related side effects attributable to ECL.
Discussion
In this sample of patients randomized to undergo phacoemulsification combined with either KDB excisional goniotomy or iStent trabecular microbypass implantation in one eye, and who ultimately underwent the alternate procedure in the fellow eye, greater ECL was seen in eyes undergoing iStent-phaco. Neither procedure had statistically significant effects on CCT or CEC morphology, and ECL was not found to be greater at longer follow-up times.
This is the first report of which we are aware to characterize the effects of excisional goniotomy on ECD, although a prior histological analysis reported the presence of nodular excrescences on Descemet’s membrane in one eye undergoing KDB-phaco, the clinical significance of which is unknown.33 Studies of related procedures have been reported. A study of ab interno canalectomy (a precursor to excisional goniotomy) found no evidence of CEC damage on histological examination of eye bank eyes.34 Microhook trabeculotomy using a specialized blade designed to incise TM was associated with 6% ECL 9.5 months postoperatively in Japanese eyes.35 Incisional goniotomy with the Trabectome has been reported to produce no changes in ECD through 629 or 3628 months of follow-up.
Effects of first-generation iStent implantation on ECD were not reported in the device’s pivotal trial.36 In a series of 10 Japanese eyes with OAG undergoing standalone implantation of 2 first-generation iStents, no ECL was observed through 6 months of follow-up.24 In a prospective, uncontrolled case series of 20 eyes undergoing combined iStent-phaco, mean ECD decreased from 2290 to 1987 cells/mm2 (13.2% decrease) at 12 months.37 The second-generation iStent Inject’s pivotal trial evaluated ECD and found a 13.1% reduction at 24 months postoperatively in the iStent-phaco group compared to a 12.3% reduction in eyes undergoing phacoemulsification only; most of this ECL (12.5% and 11.6%, respectively) occurred within the first 3 months and ongoing ECL was minimal thereafter.11 Further, the proportion of eyes with ECL >30% at 24 months was similar between groups (10.4% versus 9.5%, respectively).
Because eyes in this study underwent both a glaucoma procedure and phacoemulsification, it is not possible to determine the direct effects of the glaucoma procedures alone on ECD over time. The magnitude of ECL seen in the iStent eyes in the current study is consistent with ECL reported after phacoemulsification alone in eyes with OAG,3,11 while the ECL rate in the excisional goniotomy eyes was somewhat lower than would be expected based on the literature. One possible explanation is that cataracts were less dense in the excisional goniotomy eyes, requiring less ultrasound power and causing less ECL. However, eyes were assigned to treatment by randomization, which in theory should balance potential confounding factors such as this. The relatively small sample size, however, could in theory lead to imbalances between groups. Given that phacoemulsification was standardized in all eyes; a more obvious factor to which the difference in ECL may be attributed is the difference in glaucoma procedures. While excisional goniotomy and iStent implantation are both trabecular meshwork-based procedures developed to enhance aqueous humor flow from the anterior chamber into Schlemm’s canal, one accomplishes this goal without the permanent implantation of a device while the other requires a permanent device implantation. It is logical to consider that the presence of a foreign body in the anterior chamber, in close proximity to the cornea, may affect ECD over time. In the 5-year COMPASS XT study of the CyPass supraciliary micro-stent, the device’s position within the eye was identified as a factor associated with ECD; specifically, eyes with devices that protruded farther into the anterior chamber and were thus closer to the cornea had greater ECL than eyes with devices properly positioned with little protrusion.3 Similarly, while trabeculectomy is associated with an acute reduction in ECD that stabilizes postoperatively,18,38,39 Ex-Press mini-shunt39 or tube-shunt implantation21,38,40 is associated with ongoing ECL. Evidence for a mechanical effect of the implant on ECD includes focal ECL in the area of the cornea closest to the tube tip after Ahmed implantation,20 and a case of focal corneal endothelial injury has been reported in an eye with a dislocated XEN gel stent.41 When positioned correctly, the iStent should be adequately spaced from the corneal endothelium, precluding direct mechanical trauma, although ECL was seen in eyes with correctly positioned CyPass devices so proper device position may not completely prevent ECL.
While this is a small series, the study is strengthened by the randomized nature of treatment assignment, which minimizes selection bias that can occur in nonrandomized or retrospective studies. Also, the use of fellow eyes as controls increases the effective power of the small sample by virtue of minimizing between-subject variability between treatment groups. Limitations of the study include its external validity, being the product of a single surgeon, and the fact that pre- and postoperative imaging was performed only once per eye. Also, the limited follow-up time (mean 18.2 months) precludes detection of late effects of these procedures on ECD and corneal health as was seen in the COMPASS XT supraciliary micro-stent trial,3 although the variability of the time between pre-and postoperative assessments permitted an analysis of the possible relationship between ECL and postoperative time (none was seen in either group).
In summary, both KDB-phaco and iStent-phaco are associated with postoperative ECL, with iStent-phaco producing significantly greater ECL than KDB-phaco. The clinical significance of these findings is unclear, and guidelines for serial evaluation of corneal health following glaucoma surgical procedures are lacking. Future studies are warranted to more robustly characterize long-term effects of glaucoma surgical procedures—with and without permanent implants—on ECD and corneal health and to develop evidence-based guidelines for the pre- and postoperative evaluation of corneal health in eyes undergoing glaucoma surgery.
Acknowledgments
New World Medical supported the clinical trial from which the data in this paper are drawn, and also provided support to Tony Realini, MD, MPH, for assistance in preparing the manuscript for publication.
Disclosure
Dr Goundappa K Balasubramani reports personal fees from New World Medicals Inc., during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206.
2. Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi:10.1371/journal.pone.0183142
3. Lass JH, Benetz BA, He J, et al. Corneal endothelial cell loss and morphometric changes 5 years after phacoemulsification with or without CyPass micro-stent. Am J Ophthalmol. 2019;208:211–218. doi:10.1016/j.ajo.2019.07.016
4. US Food and Drug Administration. UPDATE: potential eye damage from alcon CyPass micro-stent used to treat open-angle glaucoma: FDA safety communication. 2018. Available from: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm624283.htm.
5. Edelhauser HF, Geroski DH, Stern ME. Glucose metabolism in the cornea and lens in elasmobranchs, teleosts and mammals: response to thiol-oxidation. Fed Proc. 1980;39(14):3213–3221.
6. Edelhauser HF. The balance between corneal transparency and edema: the proctor lecture. Invest Ophthalmol Vis Sci. 2006;47(5):1754–1767.
7. Cho SW, Kim JM, Choi CY, Park KH. Changes in corneal endothelial cell density in patients with normal-tension glaucoma. Jpn J Ophthalmol. 2009;53(6):569–573. doi:10.1007/s10384-009-0740-1
8. Gagnon MM, Boisjoly HM, Brunette I, Charest M, Amyot M. Corneal endothelial cell density in glaucoma. Cornea. 1997;16(3):314–318. doi:10.1097/00003226-199705000-00010
9. Ranno S, Fogagnolo P, Rossetti L, Orzalesi N, Nucci P. Changes in corneal parameters at confocal microscopy in treated glaucoma patients. Clin Ophthalmol. 2011;5:1037–1042.
10. Baratz KH, Nau CB, Winter EJ, et al. Effects of glaucoma medications on corneal endothelium, keratocytes, and subbasal nerves among participants in the ocular hypertension treatment study. Cornea. 2006;25(9):1046–1052. doi:10.1097/01.ico.0000230499.07273.c5
11. Samuelson TW, Sarkisian SR, Lubeck DM, et al. Prospective, Randomized, controlled pivotal trial of an Ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811–821. doi:10.1016/j.ophtha.2019.03.006
12. Wilczynski M, Drobniewski I, Synder A, Omulecki W. Evaluation of early corneal endothelial cell loss in bimanual microincision cataract surgery (MICS) in comparison with standard phacoemulsification. Eur J Ophthalmol. 2006;16(6):798–803. doi:10.1177/112067210601600603
13. Wilczynski M, Supady E, Loba P, Synder A, Palenga-Pydyn D, Omulecki W. Comparison of early corneal endothelial cell loss after coaxial phacoemulsification through 1.8 mm microincision and bimanual phacoemulsification through 1.7 mm microincision. J Cataract Refract Surg. 2009;35(9):1570–1574. doi:10.1016/j.jcrs.2009.05.014
14. Dick B, Kohnen T, Jacobi KW. [Endothelial cell loss after phacoemulsification and 3.5 vs. 5 mm corneal tunnel incision]. Ophthalmologe. 1995;92(4):476–483.
15. Dick HB, Kohnen T, Jacobi FK, Jacobi KW. Long-term endothelial cell loss following phacoemulsification through a temporal clear corneal incision. J Cataract Refract Surg. 1996;22(1):63–71.
16. Smith DL, Skuta GL, Lindenmuth KA, Musch DC, Bergstrom TJ. The effect of glaucoma filtering surgery on corneal endothelial cell density. Ophthalmic Surg. 1991;22(5):251–255.
17. Sihota R, Sharma T, Agarwal HC. Intraoperative mitomycin C and the corneal endothelium. Acta Ophthalmol Scand. 1998;76(1):80–82. doi:10.1034/j.1600-0420.1998.760115.x
18. Storr-Paulsen T, Norregaard JC, Ahmed S, Storr-Paulsen A. Corneal endothelial cell loss after mitomycin C-augmented trabeculectomy. J Glaucoma. 2008;17(8):654–657. doi:10.1097/IJG.0b013e3181659e56
19. Zarei R, Zarei M, Fakhraie G, et al. Effect of mitomycin-C augmented trabeculectomy on corneal endothelial cells. J Ophthalmic Vis Res. 2015;10(3):257–262.
20. Kim CS, Yim JH, Lee EK, Lee NH. Changes in corneal endothelial cell density and morphology after Ahmed glaucoma valve implantation during the first year of follow up. Clin Exp Ophthalmol. 2008;36(2):142–147.
21. Kim KN, Lee SB, Lee YH, Lee JJ, Lim HB, Kim CS. Changes in corneal endothelial cell density and the cumulative risk of corneal decompensation after Ahmed glaucoma valve implantation. Br J Ophthalmol. 2016;100(7):933–938. doi:10.1136/bjophthalmol-2015-306894
22. Iwasaki K, Arimura S, Takihara Y, Takamura Y, Inatani M. Prospective cohort study of corneal endothelial cell loss after Baerveldt glaucoma implantation. PLoS One. 2018;13(7):e0201342. doi:10.1371/journal.pone.0201342
23. Tan AN, Webers CA, Berendschot TT, et al. Corneal endothelial cell loss after Baerveldt glaucoma drainage device implantation in the anterior chamber. Acta Ophthalmol. 2017;95(1):91–96. doi:10.1111/aos.13161
24. Shiba D, Hosoda S, Yaguchi S, Ozeki N, Yuki K, Tsubota K. Safety and efficacy of two trabecular micro-bypass stents as the sole procedure in Japanese patients with medically uncontrolled primary open-angle glaucoma: a pilot case series. J Ophthalmol. 2017;2017:9605461.
25. Fea AM, Consolandi G, Pignata G, et al. A comparison of endothelial cell loss in combined cataract and MIGS (Hydrus) procedure to phacoemulsification alone: 6-month results. J Ophthalmol. 2015;2015:769289.
26. Ivantis, Inc. Hydrus microstent instructions for use. Irvine, CA; 2018.
27. Gillmann K, Bravetti GE, Rao HL, Mermoud A, Mansouri K. Impact of combined XEN gel stent implantation on corneal endothelial cell density: 2-year results. J Glaucoma. 2019.
28. Kasahara M, Shoji N, Matsumura K. The influence of trabectome surgery on corneal endothelial cells. J Glaucoma. 2019;28(2):150–153. doi:10.1097/IJG.0000000000001128
29. Maeda M, Watanabe M, Ichikawa K. Evaluation of trabectome in open-angle glaucoma. J Glaucoma. 2013;22(3):205–208.
30. Falkenberry S, Singh IP, Crane CJ, et al. Excisional goniotomy versus trabecular micro-bypass stent implantation: a prospective randomized clinical trial in eyes with mild to moderate open-angle glaucoma. J Cataract Refract Surg. 2020;46:1165–1171. doi:10.1097/j.jcrs.0000000000000229
31. New World Medical, Inc. Kahook Dual Blade. Instructions for Use. Rancho Cucamonga, CA; 2018.
32. Glaukos Corporation. iStent trabecular micro-bypass stent. Directions for use/package insert. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf8/p080030c.pdf.
33. Swaminathan SS, Monsalve P, Zhou XY, et al. Histologic analysis of trabecular meshwork obtained from Kahook Dual Blade goniotomy. Am J Ophthalmol. 2018;192:198–205. doi:10.1016/j.ajo.2018.05.028
34. Ferrari E, Bandello F, Ortolani F, Petrelli L, Marchini M, Ponzin D. Ab-interno trabeculo-canalectomy: surgical approach and histological examination. Eur J Ophthalmol. 2002;12(5):401–405. doi:10.1177/112067210201200510
35. Tanito M, Ikeda Y, Fujihara E. Effectiveness and safety of combined cataract surgery and microhook ab interno trabeculotomy in Japanese eyes with glaucoma: report of an initial case series. Jpn J Ophthalmol. 2017;61(6):457–464.
36. Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. doi:10.1016/j.ophtha.2010.07.007
37. Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, et al. Mid-term evaluation of the new Glaukos iStent with phacoemulsification in coexistent open-angle glaucoma or ocular hypertension and cataract. Br J Ophthalmol. 2013;97(10):1250–1255. doi:10.1136/bjophthalmol-2012-302394
38. Kim MS, Kim KN, Kim CS. Changes in corneal endothelial cell after Ahmed glaucoma valve implantation and trabeculectomy: 1-year follow-up. Korean J Ophthalmol. 2016;30(6):416–425. doi:10.3341/kjo.2016.30.6.416
39. Arimura S, Takihara Y, Miyake S, et al. Randomized clinical trial for early postoperative complications of Ex-PRESS Implantation versus trabeculectomy: complications postoperatively of Ex-PRESS versus trabeculectomy study (CPETS). Sci Rep. 2016;6:26080.
40. Lee EK, Yun YJ, Lee JE, Yim JH, Kim CS. Changes in corneal endothelial cells after Ahmed glaucoma valve implantation: 2-year follow-up. Am J Ophthalmol. 2009;148(3):361–367. doi:10.1016/j.ajo.2009.04.016
41. Gillmann K, Bravetti GE, Mermoud A, Mansouri K. Anterior chamber XEN gel stent movements: the impact on corneal endothelial cell density. J Glaucoma. 2019;28(6):e93–e95.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
