Back to Journals » International Journal of Nanomedicine » Volume 14
Controlled-releasing hydrogen sulfide donor based on dual-modal iron oxide nanoparticles protects myocardial tissue from ischemia–reperfusion injury
Authors Wang W, Liu H, Lu YT, Wang X, Zhang B, Cong S , Zhao Y, Ji M , Tao H, Wei L
Received 3 September 2018
Accepted for publication 30 November 2018
Published 30 January 2019 Volume 2019:14 Pages 875—888
DOI https://doi.org/10.2147/IJN.S186225
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Wenshuo Wang,1,* Huan Liu,1,* Yuntao Lu,1,* Xiaole Wang,2,* Bohan Zhang,3 Shuo Cong,1 Yun Zhao,1 Minbiao Ji,3 Hongyue Tao,4 Lai Wei1,5
1Department of Cardiac Surgery, Zhongshan Hospital, Fudan University, Shanghai 200030, China; 2Department of Radiology, Second People’s Hospital of Nantong City, Nantong 226002, Jiangsu, China; 3State Key Laboratory of Surface Physics and Department of Physics, Fudan University, Shanghai 200433, China; 4Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai 200040, China; 5Department of Cardiac Surgery, Shanghai Public Health Clincal Center, Shanghai 201508, China
*These authors contributed equally to this work
Background: Hydrogen sulfide (H2S) has shown promising therapeutic benefits in reversing a variety of pathophysiological processes in cardiovascular system, including myocardial ischemia–reperfusion (IR) injury. However, the achievement of controlled and sustained release of H2S has been a technical bottleneck that limits the clinical application of the gas molecule.
Methods: The current study describes the development of mesoporous iron oxide nanoparticles (MIONs) which were loaded with diallyl trisulfide (DATS), a H2S donor compound, and calibrated by stimulated Raman scattering/transient absorption.
Results: The synthesized MIONs were characterized with excellent mesoporosity and a narrow size distribution, which enabled them to slow down the release of H2S to a suitable rate and prolong the plateau period. The controlled-release feature of DATS-MIONs resulted in little adverse effect both in vitro and in vivo, and their protective effect on the heart tissue that underwent IR injury was observed in the mouse model of myocardial ischemia. The rapid biodegradation of DATS-MIONs was induced by Kupffer cells, which were specialized macrophages located in the liver and caused limited hepatic metabolic burden.
Conclusion: The sustained-release pattern and excellent biocompatibility make DATS-MIONs a promising H2S donor for research and medical purposes.
Keywords: steady release, porous structure, biocompatibility, biodegeneration
Introduction
Myocardial ischemia–reperfusion (IR) injury is one of the major contributing factors to morbidity and mortality in patients with cardiovascular diseases, and significantly limits the clinical efficacy of various treatment strategies. Hydrogen sulfide (H2S) has been shown to offer protective effects against ischemia to a wide variety of tissues and organs, particularly the heart.1,2 For instance, Elrod et al demonstrated that enhanced production of endogenous H2S could significantly reduce the severity of myocardial infarction in a murine IR model.3 Because H2S is a gas molecule that shows considerable cytotoxicity at high concentrations, its controlled generation and site-specific delivery are particularly crucial yet challenging. The commonly used H2S donor sodium hydrosulfide hydrate (NaHS) suffers the obvious drawback of being unstable and producing H2S in an overly rapid, uncontrolled manner.4 It is therefore critical to develop new donor compounds and delivery systems that can achieve slow and sustained release of H2S.
Recently, diallyl trisulfide (DATS), which generates H2S in a slow and sustained manner by reacting with reduced endogenous glutathione, has exhibited remarkable therapeutic benefits in many cardiovascular diseases.5 Nevertheless, the poor solubility of DATS necessitates a suitable delivery system to increase its bioavailability and to allow the fine-tuning of its release profile. In a previous study, we reported the construction and use of mesoporous silica nanoparticles (MSNs) for loading and slow release of DATS.6 When administered in vivo, DATS-MSNs were found to significantly alleviate myocardial IR injury in a rat model.7 On the other hand, we recently constructed a mesoporous iron oxide framework with excellent effect on facilitating the regeneration of infarcted cardiac tissues.8 Based on our obtained results, we recognized that iron oxide could potentially be used as the material of choice to construct nanovehicle for therapeutic payloads due to its many favorable biochemical properties such as malleability and biodegradability. Herein, we report the synthesis, characterization, and evaluation of mesoporous iron oxide nanoparticles (MIONs) as a sustained-release and low-toxicity delivery system for H2S donors.
Methods
Preparation of DATS-MIONs
All chemicals and reagents were purchased from Sigma-Aldrich (St Louis, MO, USA), unless otherwise specified.
MIONs were synthesized by first preparing polystyrene nanoparticles and then coating them with iron oxide (Figure S1). To generate polystyrene nanoparticles, 18 mL of styrene, 2 mL of methacrylic acid, and 140 mL of deionized water were mixed together, dispersed thoroughly by sonication, and then added to a three-neck round-bottom flask. The suspension was heated to 75°C and stirred for 30 minutes. Next, 0.2 g of potassium peroxodisulfate was dissolved in 20 mL of deionized water, and the resultant solution was gradually added to the above flask with a dropper. The reaction was allowed to proceed at 75°C for another 10–24 hours to obtain the polystyrene nanoparticles.
In another three-neck round-bottom flask, 1 g of the prepared polystyrene nanoparticles was thoroughly dispersed in 170 mL of deionized water by sonication and mixed with 30 mL of ethylene glycol. Then, 0.446 g of ferrous chloride, 0.111 g of potassium nitrate, and 2 g of methenamine were sequentially added to the abovementioned polystyrene nanoparticle suspension. The flask was then filled with nitrogen and heated to 80°C. The reaction was allowed to proceed at 80°C for 3 hours. The resultant mixture was cooled to room temperature and centrifuged. The pelleted coated nanoparticles were collected, rinsed thoroughly with deionized water to remove all residual reagents, and then gradually heated to 500°C using a ceramic crucible at a rate of <10°C/min. The thermal reaction was allowed to proceed at 500°C for 3 hours to facilitate iron oxidation, followed by cooling to room temperature to obtain MIONs. The generated MIONs were imaged on Transmission Electron Microscope H-9500 (Hitachi Ltd., Tokyo, Japan). For the characterization of size distribution, MIONs were sonicated in deionized water for 1 minute and measured on a Zetasizer Nano particle size analyzer (Malvern Instruments, Malvern, UK) following the manufacturer’s instructions.
DATS was loaded into MIONs based on a previously described protocol with minor modifications.7 In brief, 1 mg of MIONs and 1 mg of DATS were sequentially mixed in 5 mL of distilled water, followed by incubation with continuous sonication at 0°C for 8 hours. The suspension was then centrifuged to pellet the generated DATS-MIONs. Free, residual DATS was removed from the surface of MIONs by washing with distilled water. The cleaned DATS-MIONs were freeze-dried under vacuum and weighed.
H2S release of DATS-MIONs
The release experiment was based on our previous protocol with minor modifications.9 DATS-MIONs were added at final concentrations of 10, 20, and 30 mg/mL into a glass chamber (World Precision Instruments, Sarasota, FL, USA) containing adherent endothelial cells in high-glucose DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 2 mM reduced glutathione (Thermo Fisher Scientific) (Figure S1). The resultant culture suspension was maintained at 37°C under hypoxic environment. H2S formation was monitored on an ISO-H2S-2 sensor (World Precision Instruments) attached to an Apollo 1100 Free Radical Analyzer (World Precision Instruments). The concentration of released H2S correlated proportionally to the current level on the electrode. A standard curve was generated based on serial dilution of NaHS solutions and was used to convert the current readings to concentration values.
In vitro cytotoxicity assay
As the endothelium of blood vessels is the first barrier for an intravenous drug, it is important to evaluate the toxicity of MIONs to endothelial cells. One hundred microliters of endothelial cell medium (ECM; ScienCell, Carlsbad, CA, USA) containing (103) human umbilical vein endothelial cells (HUVECs) was added to each well of a 96-well microelectrode plate (ACEA, San Diego, CA, USA) and incubated for 24 hours at 37°C. After the careful removal of the medium, 100 μL of pre-chilled solution containing 2 mM reduced glutathione (Thermo Fisher Scientific) in DMEM (Thermo Fisher Scientific) supplemented with 10% FBS (Thermo Fisher Scientific) was added to each well, followed by treatment with different concentrations of DATS-MIONs in the range of 0.12 μg/mL to 2 mg/mL at 37°C for 60 minutes. A control was included in which no DATS-MIONs were added. Following the treatment, the supernatant was replaced with an equal volume of ECM and then monitored on an xCELLigence RTCA DP station (ACEA).
To determine the optimal dose of DATS-MIONs for IR injury treatment, a similar experiment was performed using embryonic cardiomyocyte H9C2 cells based on the above procedures with minor modifications. Specifically, after treatment with different concentrations of DATS-MIONs, the cells were cultured under hypoxic conditions (95% N2/5% CO2) at 37°C for 60 minutes. After the replacement of the supernatant with ECM, the cells were subjected to reoxygenation. All the procedures were monitored on an xCELLigence RTCA DP station.
Animal myocardial IR model
The mice myocardial IR model was generated based on a previously described protocol.10 The Zhongshan Animal Use Committee approved the animal experiments, and all experiments were conducted in accordance with the committee’s guidelines approved by Fudan University Animal Care. Briefly, 12 mice were anesthetized with intraperitoneal injection of 250 μg/kg medetomidine hydrochloride (Merck, Darmstadt, Germany) and 50 mg/kg ketamine hydrochloride (Merck). Then, endotracheal intubation was performed to maintain normal respiratory function. Temporary ligation of the left anterior descending coronary artery was performed at around 3 mm distal to its origin between the conus artery and the left atrium with a 6-0 prolene suture. After the confirmation of ischemia in the anterior ventricular wall and the apex as indicated by a color change, the mice were maintained under ischemic conditions for 30 minutes and then subjected to reperfusion for 24 hours.
In vivo distribution and biodegradation of DATS-MIONs
Six normal mice (not subjected to surgical intervention) and MIONs conjugated with Cy7 were used. The mice were injected with the nanoparticles at a dose of 100 mg/kg through the tail vein. Whole-body fluorescence was monitored daily by in vivo bioluminescence imaging on IVIS Spectrum In Vivo Imaging System (PerkinElmer, Waltham, MA, USA). For macrophage depletion, the mice were intravenously injected with 0.2 mL of Clophosome (FormuMax Scientific Inc., Sunnyvale, CA, USA) through the tail vein 24 hours prior to the administration of DATS-MIONs. Six macrophage-depleted mice were then subjected to similar injection and monitoring procedures.
Cellular biodegradation of DATS-MIONs
Mice were anesthetized by an intraperitoneal injection of 1% pentobarbital sodium. The mice were then disinfected, and abdominal furs were removed by applying 8% sodium sulfide. A laparotomy procedure was then performed, followed by the puncture of the murine hepatic portal vein with a venous indwelling needle (24G). Caution was exercised to ensure that the needle was secured in place and its tip was not extended beyond the portal bifurcation. Then, the liver was perfused with 10 mL of 10% heparin solution and then pre-warmed, calcium/magnesium-free D-Hank’s solution at a rate of 100 mL/h. When the liver was swelled to approximately twice its original volume, the inferior vena was cut open while the perfusion was maintained at the same rate as above. Once no blood was visibly flowing from the inferior vena, all perihepatic ligaments were dissected to allow the removal and transfer of the liver together with the portal vein to a clean, sterile petri dish.
A total volume of 10 mL of 0.1% collagenase IV in calcium/magnesium-supplemented Hank’s solution was pre-warmed to 38°C and slowly infused into the liver through its portal vein. The perfused liver was allowed to stand for 10 minutes to ensure thorough digestion. After removing the liver capsule and the surrounding connective tissues, the liver was thoroughly rinsed and cleaned with a brush. The digested liver content was slowly aerated and filtrated through a 200-mesh filter into a 50-mL comical tube. The tube was centrifuged at 4°C, 100× g for 5 minutes, and the supernatant was transferred to another clean, sterile conical tube, followed by a second centrifugation at 4°C, 400× g for 10 minutes. The supernatant was then discarded, and the pelleted cells were thoroughly resuspended in 10 mL of PBS. The resultant cell suspension was then carefully layered on top of a 25%/50% Percoll gradient solution and centrifuged at 4°C, 900× g for 20 minutes. Approximately 15 mL of liquid was withdrawn from around the 50% interphase by piercing the tube with a needle. The liquid was mixed with 15 mL of PBS and centrifuged at 4°C, 800× g for 10 minutes. The cell pellet was resuspended in RPMI-1640 medium and grown in a culture flask at 37°C under 5% CO2 for 40 minutes. Nonadherent cells were discarded by decanting the supernatant and rinsing the flask twice with PBS. The adherent cells were then cultured in 8 mL of DMEM (Thermo Fisher Scientific) with 10% FBS (Thermo Fisher Scientific) and 1% penicillin/streptomycin (Thermo Fisher Scientific). Cells were preseeded into 35 mm dishes and cultured for at least 24 hours to make them completely adherent. When cells accounted for 70% of the volume of a dish, DMEM was replaced by 1 mL of DMEM containing 0.1 mg DATS-MIONs, and the resulting sample was incubated for different amounts of time. Before imaging, DATS-MIONs were removed from the medium by washing the cells three times with PBS (Thermo Fisher Scientific).
The design of our dual-modal stimulated Raman scattering (SRS)/transient absorption (TA) microscope is illustrated in Figure S2. In our setup, pulsed femtosecond laser beams from a commercial optical parametric oscillator (OPO) laser (Insight DS+; Newport, Irvine, CA, USA) were used as the laser source. The fundamental 1,040 nm laser was used as the Stokes beam for SRS and the pump beam for TA, while the tunable OPO output (690–1,300 nm) served as the pump beam for SRS and the probe beam for TA. The intensity of the 1,040 nm beam was modulated at 20 MHz using an electro-optical modulator. The two laser beams were combined through a dichromic mirror, spatially and temporally overlapped, delivered into the laser-scanning microscope (FV1200; Olympus, Tokyo, Japan), and focused onto the samples. The stimulated Raman loss and TA signal generated were optically filtered, detected by photodiode, and demodulated with a lock-in amplifier (HF2LI; Zurich Instruments) to feed the analog input of the microscope to form images.
In vivo toxicity assessment of DATS-MIONs
Twenty-seven normal mice (not subjected to surgical intervention) and regular DATS-MIONs (no fluorescence labeling) were used. The mice were injected with the nanoparticles at a dose of 100 mg/kg through the tail vein. Blood was withdrawn from tail vein from each animal on day 2 and day 14 after the injection and subjected to routine blood analysis. Briefly, the mice were anesthetized by injecting 50 mg/kg of pentobarbital sodium (ShangPharm, Shanghai, China) via the tail vein. Upon confirmation of anesthesia, 2.5×105 U/kg of heparin sodium (ShangPharm) was injected into each animal, which was then sacrificed by exsanguination in 1 minute. Blood was collected and subsequently analyzed on a Coulter LH 750 hematology analyzer (Beckman Coulter, Brea, CA, USA) and also on Hitachi 7180 chemistry analyzer (Hitachi Ltd.) following each manufacturer’s instructions.
To evaluate the toxicity effect of DATS-MIONs on liver, spleen, and kidney, mice were euthanized every week from week 1 to week 5. Vital organs such as liver, spleen, and kidney were harvested and fixed in 4% paraformaldehyde for 24 hours at room temperature. The fixed organ specimens were embedded in paraffin, cut into 7 μm-thick slices, and then mounted on slides. The sections were then stained with H&E and imaged under a light microscope at ×40 magnification. Six high-power fields were randomly selected in each photograph.
TUNEL assay
TUNEL assay was performed by using the In Situ Cell Death Detection Kit, TMR red (Roche, Basel, Switzerland), according to the manufacturer’s instructions. Briefly, 50 μL of rTdT Incubation Buffer was added to the fixed cells on slides, which were then allowed to stand in darkness under a humidified atmosphere for 60 minutes at 37°C. The slides were subsequently rinsed three times with 2X saline-sodium citrate buffer for 5 minutes each time, stained with DAPI, and visualized under a BX51 fluorescence microscope (Olympus). TUNEL-positive nuclei and total nuclei were indicated by red and blue fluorescence, respectively. The ratio of the number of TUNEL-positive nuclei to the total number of nuclei was calculated.
Echocardiography
The mice models mentioned in the above part were anesthetized by inhaled isoflurane (1%, Baxter) in normal air. Each animal was fastened to a heating pad and subjected to a chemical hair remover (Reckitt Benckiser, Slough, UK) to shave the chest. Pre-warmed ultrasound gel (Hankang Medical Equipment CO., LTD., Foshan, China) was applied to the thoracic wall to facilitate the visibility of the heart chambers. Echocardiography (M-mode and two-dimensional) was performed using a Vevo 2100 high-resolution ultrasound machine.
Statistical analysis
Data are expressed as mean ± standard error of mean. Statistical analysis was performed using SPSS (version 20.0; IBM Corp., Armonk, NY, USA) using either ANOVA or Student–Newman–Keuls multiple comparison test. P<0.05 was considered statistically significant.
Results
Characterization of DATS-MIONs
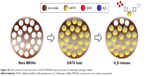
We first evaluated the physical characteristics of the iron oxide nanoparticles that we prepared by visualizing under a transmission electron microscope. As shown in Figure 1A, the nanoparticles existed in a monodisperse form with a mesoporous structure suitable for drug absorption. The nanoparticles had a narrow size distribution around 230±35 nm (Figure 1B). Following the encapsulation of DATS into MIONs, we found the average weight of the loaded nanoparticles was roughly 2.2 times that of their drug-free counterparts (Figure 1C). Therefore, the drug loading efficiency of our MIONs was calculated to be around 68.75%. We then investigated the H2S release profile of DATS-MIONs on a H2S-selective electrode (Figure 1D). H2S formation was observed as soon as the loaded nanoparticles were added to the solution. Based on the current generated on the electrode, the concentration of H2S released from DATS-MIONs was found to gradually increase over the first hour until reaching a plateau. The H2S level remained stable for at least 5 more hours and was proportional to the concentration of nanoparticles in the solution. The results indicated a steady and sustained H2S release profile for DATS-MIONs, suggesting that they could potentially simulate the biological effect of endogenous H2S. We next conducted biosafety study by incubating DATS-MIONs with HUVECs at varying concentrations. Monitoring of cell proliferation on a real-time cell analyzer indicated that the cellular toxicity of our nanoparticles was similar to that of commonly used composite biomaterials, with an LC50 of 0.36 μg/mL (Figure 1E). Combined, the above experimental data provided preliminary evidence for the feasibility of using DATS-MIONs as a slow-releasing H2S donor.
Distribution and biodegradation of DATS-MIONs
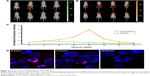
To investigate the in vivo distribution and metabolism of DATS-MIONs, bald mice were injected via the tail vein with 100 mg/kg of the nanoparticles loaded with Cy7-conjugated DATS, and visualized by bioluminescence imaging. As illustrated in Figure 2A, DATS-MIONs were primarily localized in the murine liver, and the hepatic fluorescence intensity reached its peak value around 1 day after the injection. The fluorescence level then steadily declined over the next 13 days, implying the gradual degradation and clearance of the nanoparticles. In comparison, the peak hepatic fluorescence intensity level in mice treated with Clophosome, a macrophage-depleting agent, did not occur until on day 8 and was 7.9 times of that in the untreated mice (Figure 2B and C). In vitro phagocytosis test showed that DATS-MIONs were endocytosed by Kupffer cells and largely degenerated within 12 hours (Figure 2D). Therefore, we concluded that DATS-MIONs were mainly metabolized by liver macrophages.
Administration of DATS-MIONs shows no detrimental effect on homeostasis
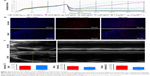
Following the intravenous administration of DATS-MIONs, we also performed routine blood test on the mice on day 2 and day 14. Compared with the results on day 0 (preinjection), we did not observe any appreciable changes in the level of alanine aminotransferase, aspartate aminotransferase, ALP, blood urea nitrogen, or creatinine over the course of treatment (Figure 3), suggesting that the nanoparticles did not negatively affect liver or kidney function. Similarly, no abnormal alterations were detected in any of the erythrocyte-related hematological parameters, such as the red blood cell count, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, blood hemoglobin level, red blood cell distribution width, and hematocrit (Figure 3). Finally, the infusion of DATS-MIONs did not lead to perturbation of white blood cell count or lymphocyte percentage in the murine subjects, which demonstrated that the nanoparticles led to no inflammatory response.
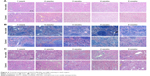
To further assess the toxicity of DATS-MIONs in vital organs, we repeated the abovementioned experiments and sacrificed the mice every week from week 1 to week 5. Murine livers, spleens, and kidneys were harvested and examined histologically. Hepatic lobules and the surrounding portal zone showed healthy and normal morphology in all euthanized animals regardless of the time of sacrifice (Figure 4A). The spleen tissues of all treated mice also exhibited normal histological characteristics such as the clear separation of the red pulp from the white pulp, and no significant reduction in the average size of splenic corpuscles or in the density of lymphocytes was observed (Figure 4B). Similarly, the injection of DATS-MIONs did not cause any appreciable damage to the renal glomeruli, tubules, or interstitium (Figure 4C).
DATS-MIONs significantly attenuate IR-induced heart injury in mice
Having demonstrated the biosafety of DATS-MIONs, we then investigated their effect on myocardial protection. We first determined the optimal dose of the nanoparticles in the embryonic cardiomyocyte cell line H9C2. The cells were treated with varying concentrations of DATS-MIONs 1 hour prior to the induction of IR and monitored on a real-time cell analyzer. We observed significant protective effect when the nanoparticles were added to a final concentration of 0.12 or 0.24 μg/mL (Figure 5A). Conversely, DATS-MIONs were found to reduce cell viability and inhibit cell proliferation above 0.48 μg/mL, possibly due to H2S overdose. Based on this value and the average blood volume of mice (5.85 mL/100 g), the suitable dose of DATS-MIONs was calculated to be 0.71 μg/100 g. We then pretreated DATS-MIONs with reduced glutathione for 1 hour and intravenously injected the drug-loaded nanoparticles via the tail vein into mice with IR-induced myocardial injury. TUNEL staining of the harvested myocardial tissues indicated that the injection of the drug-loaded nanoparticles led to a substantial decrease in the number of apoptotic cells compared to the control animals that did not receive treatment after the induction of IR (Figure 5B). In addition, the effect of DATS-MIONs on heart functions was tested on mice with myocardial infarction using echocardiogram. Compared to the control that did not receive any implant, mice with myocardial infarction that were injected with DATS-MIONs showed a significant improvement in several aspects of cardiac functions, including heart rate (fold-change=0.87, P<0.05), ejection fraction (fold-change=1.31, P<0.05), and fraction shortening (fold-change=1.55, P<0.05) (Figure 5C and D). Therefore, these findings provided preliminary evidence that DATS-MIONs could alleviate IR-induced injury in cardiac tissues.
Discussion
In the current study, we investigated the cardioprotective effect of DATS-MIONs against IR-induced myocardial injury in a murine model. Our nanoparticles showed a narrow size distribution, had a DATS loading efficiency of 68.75%, and generated H2S in a steady and sustained manner as evidenced by the in vitro release experiment. When administrated intravenously into healthy mice, DATS-MIONs demonstrated excellent biodegradability and were primarily metabolized by liver macrophages, Kupffer cells. In addition, the drug-loaded nanoparticles exhibited no appreciable toxicity based on the fact that no significant changes in common blood parameters or histological alterations in major organs were observed. Most importantly, the injection of DATS-MIONs in mice with IR-induced injury in cardiac tissues led to significant attenuation of cell apoptosis. Taken together, our study offered tentative evidence for the cardioprotective effect of DATS-MIONs against IR. Combined with their low toxicity, these nanoparticles show considerable promise as a sustained-release delivery system for therapeutic H2S donor compounds.
Several research groups have reported the development of H2S delivery systems that also showed a controlled and sustained release profile. Ali et al demonstrated the sustained formation of H2S from NaHS by dissolving the donor in a mixed solution of benzyl benzoate and benzyl alcohol containing polylactide or polylactide-co-glycolide.14 Their formulations produced H2S at a stable rate for at least 72 hours as opposed to the burst release of H2S normally observed in simple NaHS solutions, though no toxicological study was conducted. Morpholin-4-ium-methoxyphenyl-morpholino-phosphinodithioate (GYY4137) has been shown to be a slow and sustained H2S donor capable of alleviating IR-induced injury in cell- and animal-based models.11 Treatment of rats suffering from myocardial IR injury with an intraperitoneal administration of GYY4137 at a dose of 50 mg/kg significantly reduced the size of myocardial infarction, ameliorated heart function, and alleviated apoptotic activity in the cardiac tissues.12 Patil et al developed a sustained-release delivery system for GYY4137 based on a formulation that involved polylactide-co-glycolide polymer.13 The amount of H2S released from the formulation plateaued at around 30 minutes and remained stable for the remainder of the 72-hour observation period.14 Nevertheless, the formulation showed moderate cytotoxicity and its biological effects were not investigated. On the other hand, the H2S release profile of our DATS-MIONs was shown to be comparable to the aforementioned combinations of H2S donor and delivery system. In addition, our formulation showed relatively mild cytotoxicity below 0.48 μg/mL in cell-based experiments, and particularly did not seem to cause any observable injury or functional impairment of major organs when tested in the mouse model.
Iron oxide has been used as a key component in the construction of tissue scaffolds and drug carriers. Iron oxide offers excellent malleability and can be easily synthesized with tailored porosity. Equally importantly, iron oxide is biodegradable and shows little toxicity at low concentrations, making it well suited for biomedical applications.15 These advantages underlay our previous development of a mesoporous iron oxide framework that demonstrated a clear beneficial effect on the regeneration of heart tissues,8 and also serves as the basis for our choice of nanoparticle materials in the current study. It is worth mentioning that iron oxide possesses several other useful properties that could be exploited to further enhance its utility for drug delivery. The paramagnetic properties of iron oxide nanoparticles have led to the development of various magnetic drug-targeting strategies, in which an external magnetic field is used to direct the drug-loaded nanovehicle toward the pathological sites, thereby mitigating the potential side effect of the therapeutic agents.16 This has been employed, for example, by Alexiou et al to minimize the systemic toxicity of mitoxantrone, an antineoplastic agent, in a rabbit model of squamous cell carcinoma.17 On the other hand, surface functionalization of iron oxide nanoparticles has been explored to create opportunities for target-specific drug delivery, increased drug loading capacity, and improved biodegradable properties. For instance, coating of iron oxide nanoparticles with cyanoethyltrimethoxysilane was found to greatly facilitate their labeling and targeting of cells, possibly due to the introduction of abundant cyano groups.18 The findings obtained in these studies provided useful insights into how our DATS-MIONs could be further improved to achieve even better therapeutic effects.
In summary, we have reported the development of iron oxide-coated nanoparticles with excellent uniformity and mesoporosity that can be used as a delivery vehicle to achieve controlled and sustained release of H2S in biological systems. Our DATS-MIONs exhibited low organ toxicity and could attenuate IR-induced myocardial injury in a mouse model. We are currently investigating different surface modification strategies to further enhance the therapeutic properties of DATS-MIONs for treating IR and other cardiovascular diseases.
Acknowledgment
This work was supported by Shanghai Sailing Program (grant number 17YF1402100) and Natural Science Foundation of China (grant number 81801844).
Disclosure
The authors report no conflicts of interest in this work.
References
Dongó E, Hornyák I, Benkő Z, Kiss L. The cardioprotective potential of hydrogen sulfide in myocardial ischemia/reperfusion injury (review). Acta Physiol Hung. 2011;98(4):369–381. | ||
Nicholson CK, Calvert JW. Hydrogen sulfide and ischemia-reperfusion injury. Pharmacol Res. 2010;62(4):289–297. | ||
Elrod JW, Calvert JW, Morrison J, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104(39):15560–15565. | ||
Caliendo G, Cirino G, Santagada V, Wallace JL. Synthesis and biological effects of hydrogen sulfide (H2S): development of H2S-releasing drugs as pharmaceuticals. J Med Chem. 2010;53(17):6275–6286. | ||
Yu L, Li S, Tang X, et al. Diallyl trisulfide ameliorates myocardial ischemia-reperfusion injury by reducing oxidative stress and endoplasmic reticulum stress-mediated apoptosis in type 1 diabetic rats: role of SIRT1 activation. Apoptosis. 2017;22(7):942–954. | ||
Guo C, Sun X, Kong B. Mesoporous silica nanoparticles for glutathione-triggered long-range and stable releasing hydrogen sulfide. J Mater Chem B. 2015;3(21):4451–4457. | ||
Sun X, Wang W, Dai J, et al. A long-term and slow-releasing hydrogen sulfide donor protects against myocardial ischemia/reperfusion injury. Scientific Reports. 2017;7:3541. | ||
Wang W, Tao H, Zhao Y, et al. Implantable and biodegradable macroporous iron oxide frameworks for efficient regeneration and repair of infracted heart. Theranostics. 2017;7(7):1966–1975. | ||
Wang W, Sun X, Zhang H, et al. Controlled release hydrogen sulfide delivery system based on mesoporous silica nanoparticles protects graft endothelium from ischemia-reperfusion injury. Int J Nanomedicine. 2016;11:3255–3263. | ||
Yao LL, Huang XW, Wang YG, Cao YX, Zhang CC, Zhu YC. Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3beta-dependent opening of mPTP. Am J Physiol Heart Circ Physiol. 2010;298(5):H1310–H1319. | ||
Li L, Whiteman M, Guan YY, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117(18):2351–2360. | ||
Meng G, Wang J, Xiao Y, et al. GYY4137 protects against myocardial ischemia and reperfusion injury by attenuating oxidative stress and apoptosis in rats. J Biomed Res. 2015;29(3):203–213. | ||
Patil A, Singh S, Opere C, Dash A. Sustained-release delivery system of a slow hydrogen sulfide donor, gyy 4137, for potential application in glaucoma. AAPS PharmSciTech. 2017;18(6):2291–2302. | ||
Ali H, Opere C, Singh S. In vitro-controlled release delivery system for hydrogen sulfide donor. AAPS PharmSciTech. 2014;15(4):910–919. | ||
Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26(18):3995–4021. | ||
Frei EH. Magnetism and medicine. J Appl Phys. 1969;40(3):955–957. | ||
Alexiou C, Arnold W, Klein RJ, et al. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 2000;60(23):6641–6648. | ||
Forge D, Laurent S, Gossuin Y, Roch A, vander Elst L, Muller RN. An original route to stabilize and functionalize magnetite nanoparticles for theranosis applications. J Magn Magn Mater. 2011;323(5):410–415. |
Supplementary materials
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.