Back to Journals » Research and Reports in Neonatology » Volume 7
Congenital chylothorax: current perspectives and trends
Authors Krishnamurthy MB, Malhotra A
Received 15 June 2017
Accepted for publication 17 October 2017
Published 11 December 2017 Volume 2017:7 Pages 53—63
DOI https://doi.org/10.2147/RRN.S128703
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Robert Schelonka
Mohan Bagur Krishnamurthy,1 Atul Malhotra1,2
1Monash Newborn, Monash Children’s Hospital, 2Department of Paediatrics, Monash University, Melbourne, VIC, Australia
Abstract: Congenital chylothorax (CC) is the most common cause of pleural effusion in the perinatal period. The etiology is unknown in the majority of the cases. However, in some cases, it can be associated with various syndromes and genetic conditions. CC is associated with a high mortality rate. Most of the clinical manifestations are secondary to pressure effects (pulmonary hypoplasia) and loss of protein and lymphatic fluid (hydrops, malnutrition). Conservative management in the neonatal period is effective in up to 80% of cases and includes pleural drainage, parenteral nutrition/enteral medium-chain triglyceride-based formulae, and medications such as octreotide. Surgical intervention (pleurodesis, thoracic duct ligation/embolization, pleuroperitoneal shunt) may be required in persistent cases. A universal consensus on management of CC is unavailable, and data on the safety of medication use for CC in neonates are sparse.
Keywords: pleural effusion, nonimmune hydrops fetalis, octreotide, pleurodesis
Introduction
Congenital chylothorax (CC) is a rare but important clinical condition during the perinatal period, the management of which can be challenging in severe cases. There are no universally accepted guidelines for the management of CC. Little progress has been made in the past decade toward the management of CC and has focused mainly on medications for the treatment of CC. This review aims to summarize the current literature regarding pathophysiology, clinical manifestations, and management options for CC.
Definition, prevalence, and associations
Chylothorax, which is defined as accumulation of lymph in the pleural cavity, is the most common form of pleural effusion encountered in the perinatal period. It is a rare occurrence, estimated to affect 1 in 10,000 births, with a mortality rate ranging between 20% and 60%.1–4 If chylothorax is associated with hydrops fetalis, mortality can be as high as 98%.1 The most serious consequences of fetal chylothorax are pulmonary hypoplasia, congestive heart failure, and hydrops.1,2,5
CC can be an isolated manifestation or may be associated with genetic conditions such as trisomy 21 (which is associated with 4.9% of isolated pleural effusions), monosomy X,1,2 Turner,4,6,7 and Noonan syndromes4,6,7 (Table 1). Congenital lymphangiectasia can occur as part of these conditions. Associated anomalies and malformations can be present in up to 80% of cases which are often difficult or resistant to treatment.8 Lymphatic developmental anomalies associated with chylothorax may be limited to the lungs or involve other organ systems.9 Other syndromes associated with chylothorax are X-linked myotubular myopathy,10 missense mutation in integrin α9β1,11,12 and Gorham–Stout syndrome.13
  | Table 1 Causes of CC Abbreviation: CC, congenital chylothorax. |
Chylothorax can also occur secondary to rupture or laceration of the thoracic duct, from sudden hyperextension of the neck, or stretching of the chest wall during child birth,14,15 after surgeries involving structures in the neck and thorax16–18 such as vascular rings,19 and diaphragmatic hernia.20 In children, the reported incidence of chylothorax after cardiothoracic surgery is between 0.85% and 6.6%.16,21 These are termed secondary chylothorax and not the focus of this review.
Pathophysiology
The human lymphatic system has three main functions: transportation of lipids and lipid-soluble vitamins to the systemic circulation, collection and return of excess fluid and extravasated proteins from the interstitial spaces to the circulation, and return of lymphocytes to the circulation.14,15
Chyle (from the Greek word “juice”22) is a milky body fluid which is non-inflammatory, alkaline, and bacteriostatic composed mainly of fat (emulsified/free fatty acids, cholesterol), electrolytes, proteins, glucose, and abundant lymphocytes. It is formed in the small intestine during digestion of fatty foods, and taken up by lymph vessels specifically known as lacteals. The protein content of chyle is usually >3 g/L, and the electrolyte composition is similar to that of serum.23 The absolute cell count is >1000 cells/L and the lymphocyte count ranges from 400 to 6800/mm3, with most being T lymphocytes.15,24 The thoracic duct transports between 1.5 and 2.5 L of chyle daily (maximum 4 L/day in a healthy adult). Flow through the thoracic duct can vary widely from as low as 14 mL/hour in the fasting state to more than 100 mL/hour in postprandial state. Thoracic duct flow is related to the response of the duct wall smooth muscle to splanchnic and vagal stimulation. Serotonin, norepinephrine, histamine, dopamine, and acetylcholine all increase thoracic duct contraction and chyle flow.25
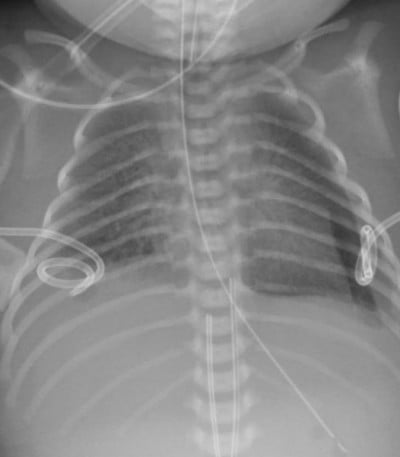
Approximately 10% of the fluid extravasated from interstitial space enters blind lymphatic capillaries and becomes lymph. These capillaries connect to larger lymphatic vessels that arborize into thoracic duct. The thoracic duct is the largest lymphatic vessel in the body, located dorsal to aorta and ventral to thoracic vertebrae. It returns lymph to the vascular system by emptying into the venous system at the junction of left internal jugular and subclavian veins (Figure 1). This maintains fluid balance and prevents tissue edema by returning interstitial fluid to vascular system.26 The lymphatic system plays a key role in many immune functions of the body, including generation of immune cells and defending the body against infection. Thoracic duct acts as a conduit as well as entry point to the systemic circulation.27 Dietary fats are absorbed by intestinal enterocytes in the form of chylomicrons, which predominantly consist of triglycerides, and also contain phospholipids, cholesterol, and other proteins. After they are expelled from enterocytes, they are collected in the villous lacteals and eventually emptied into the cisterna chyli which extends cranially as thoracic duct.
  | Figure 1 Formation, course, and termination of thoracic duct. Reprinted with permission from Earth’s Lab., Dr Joseph H Volker.104 Abbreviation: SVC, superior venacava. |
Clinical manifestations
The chylous pleural effusion impairs normal development of fetal lungs by pressure effects and can lead to pulmonary hypoplasia. It can also lead to mediastinal shift and impair circulation (Figure 2).
  | Figure 2 Congenital chylothorax: clinical effects. |
CC is one of the causes for nonimmune hydrops fetalis (NIHF). The basic mechanism for the formation of fetal hydrops is an imbalance of interstitial fluid production and the lymphatic return. The fetus is particularly susceptible to interstitial fluid accumulation because of its greater capillary permeability, compliant interstitial compartments, and vulnerability to venous pressure on lymphatic return. Increased venous pressure contributes to edema and effusion by increasing the capillary hydrostatic pressure and decreasing the lymphatic return leading to interstitial fluid accumulation. Impaired renal function causes oliguria or anuria, and subsequently hydrops.28 In CC, NIHF may develop from a combination of increased capillary filtration rate (caused by increased capillary hydrostatic pressure) and reduced lymphatic clearance that occurs with elevated central venous pressure secondary to vena caval obstruction or because of interference with cardiac function caused by the mass effect of the chylous effusion.29,30 It may also result from decreased capillary osmotic pressure caused by the loss of albumin with chyle into the pleural space.29
Lymphangiectasia and other congenital lymphatic malformations (lymphatic dysplasia syndrome, lymphangiomas, and cervical or cystic hygromas) can also present with chylothorax/bilateral pleural effusions, and NIHF as a result of obstruction in lymphatic flow.31–33 Cystic hygromas are commonly associated with complex aberrations of the lymphatics and cause mass compression effects that impede both lymphatic drainage and venous return to the heart.34
During the postnatal period, chylous pleural effusion can lead to ongoing pressure effects impeding adequate ventilation of lungs. Pulmonary oxygenation is also affected by increased venous pressure and impending cardiac failure. Therapeutic measures such as thoracocentesis and pleural drainage can lead to loss of fluid and electrolytes resulting in volume depletion and electrolyte imbalance. Loss of albumin can lead to worsening edema, and nutritional depletion can occur from loss of protein and fat. A neonate with CC is at risk of infections from secondary immunodeficiency due to loss of lymphocytes in the chylous fluid.
Management
The treatment goals for chylothorax are to drain the chylous pleural fluid, restrain it from building up again, and determine the underlying cause so that it can be treated and maintenance of nutrition and fluid balance.
Prenatal management
Prenatal diagnosis using ultrasound enables the differentiation between isolated hydrothorax and hydrops fetalis. Chylothorax is diagnosed by analysis of the pleural fluid (absolute cell count >1000/L, lymphocyte count greater than 80%).1,2,5
Infants whose chylothoraces are diagnosed <34 weeks of gestation and who subsequently receive prenatal therapy experience a better perinatal condition and exhibit improved postnatal outcomes.35 Fetuses with CC are at high risk for chromosomal abnormalities, and rapid diagnosis of fetal karyotype36 and any other associated malformations is essential to guide further management and prognosis. Therapeutic measures during prenatal period include maternal dietary modification,37 repeated thoracocentesis,1,5,38 thoraco-amniotic shunting,39 and pleurodesis with OK-432.40–44 Maternal dietary management with low-fat, high medium-chain triglyceride (MCT) diet might help delay the need for thoracentesis in cases of fetal chylothorax.37
The success of prenatal intervention through thoracocentesis and/or pleuroamniotic fluid drainage depends on early diagnosis, the volume and reappearance of chylous effusion, the degree of pulmonary compression, and the existence of hydrops.45 Before 24-week gestation, there is a high risk of development of pulmonary hypoplasia. After this stage, if the effusion is small and there is no displacement of the mediastinum, expectant management with follow-up ultrasound examination is sufficient.3,45,46
Thoracoamniotic shunting was first described in the study by Rodeck et al47 as a method to decompress fetal hydrothoraces allowing more normal development of the fetal lungs, thereby decreasing overall neonatal death. Percutaneous in utero pleuroamniotic shunt is indicated for severe or recurrent chylothorax compromising the fetal lung development and to prevent cardiac failure. Survival past the neonatal period (period not defined in all studies) following the insertion of a pleuroamniotic shunt to drain the pleural effusion has been reported to be between 47% and 70%,48–53 although the severity of effusion and underlying pathology may vary between studies. A German case series of 78 fetuses with hydrothorax treated with pleuroamniotic shunt showed that polyhydramnios, hydrops placentae, shunt-birth interval <4 weeks, and low gestational at birth were associated with mortality. Resolution of hydrops following intervention was associated with improved survival.53 Dislodgement of the shunt into the fetal chest may occur due to growing chest size, fetal movements, or improper placement of the device and can occur in 23% of cases.54 Case series have shown that reinsertion of shunt was required in 6–8%,49,50 and fetal loss was around 10%.48
OK-432 (Picibanil) is a lyophilized preparation of a low-virulence strain of group A Streptococcus pyogenes of human origin, which is used as a sclerosing agent. There are case reports of successful use of OK-432 pleurodesis in fetal therapy for CC.40–44 The mode of action of OK-432 is believed to be cellular and cytokine mediated. The elevated cytokine levels are likely to increase the permeability of the vascular endothelium and accelerate the drainage of accumulated lymph.55 Complete aspiration of pleural fluid prior to OK-432 instillation and subsequent ultrasound demonstration of adhesions are two requirements for a successful outcome.42
Postnatal management
Initial treatment consists of drainage of pleural fluid, adequate ventilation, total parenteral nutrition, dietary modifications, albumin replacement, and medications. Surgery should be considered for patients who fail these initial steps, or in whom complications such as electrolyte and fluid imbalance, malnutrition, or immunodeficiency occur. Appropriate planning of the delivery in tertiary center is important. Neonates with chylothorax often need resuscitation and respiratory support at birth if chylothorax is severe, not well controlled during pregnancy or if associated with pulmonary hypoplasia. It is essential to rule out other specific causes of hydrops fetalis, and any chromosomal or syndromic diagnosis. Small chylothoraces have been described to resorb spontaneously.56 A thoracostomy tube is placed for continuous drainage if the chyle reaccumulates and/or requires frequent intermittent aspirations to keep the pleural space empty.
Strategies for the treatment of CC
- Drainage of chyle from pleural space (thoracocentesis, continuous suction drainage)
- Reduction in chyle volume with dietary modifications (low-fat diet, MCTs) or medication (octreotide)
- Maintenance of nutrition, fluid, and electrolyte balance
- Redirection of chyle elsewhere in the body to relieve respiratory distress (pleuroperitoneal shunt and diaphragmatic fenestration)
- Obstruction of chyle flow into the chest (thoracic duct ligation or thoracic duct embolization [TDE])
Drainage of chylothorax
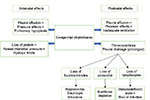
The initial approach to the management of chylothorax involves chest tube drainage of the pleural space (Figure 3). Continuous suction drainage helps to relieve the pressure of chyle on the lungs and re-expand the partially collapsed lungs and permits an accurate measurement of chyle production. Significant volumes drained should be replaced, and attention paid to fluid and electrolyte balance. The response to chest tube drainage depends on severity of chylothorax and underlying etiology.
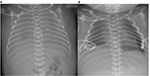  | Figure 3 Bilateral CC, before (A) and after (B) intercostal drain insertion. Abbreviation: CC, congenital chylothorax. |
Medications for chylothorax
In the past decade, new treatment strategies with medications have been predominantly limited to octreotide, to reduce the volume of chyle formation (Table 2).
  | Table 2 Medications used in the management of CC Abbreviations: CC, congenital chylothorax; cGMP, cyclic guanosine monophosphate; NEC, necrotizing enterocolitis. |
Octreotide
Use of octreotide for the management of CC was first reported by Young et al57 from Canada. Octreotide is a somatostatin analog which has been used for a variety of conditions in adults and older children including acromegaly, carcinoid tumor, acute variceal bleeding, gastrointestinal fistulae, and intractable diarrhea.58 In neonates, octreotide is used for the management of persistent hyperinsulinemia states.59 The mechanism of action of octreotide in chylothorax is uncertain. It is proposed that octreotide causes mild vasoconstriction of splanchnic vessels, including hepatic venous flow. This leads to reduction in gastric, pancreatic, and intestinal secretions as well as intestinal absorption. These mechanisms collectively reduce the flow of chyle.60
A Cochrane review by Das and Shah61 looked at the published data to date on the use of octreotide. Although there are multiple case reports, generally demonstrating resolution of chylothorax with octreotide, the time of initiation, dose, duration, and frequency of doses vary significantly between authors and there are no randomized controlled trials identified. They concluded that “no practice recommendation can be made based on the evidence identified and suggested for a multicentre randomised controlled trial to assess the safety and efficacy of octreotide in the treatment of chylothorax in neonates.”
Octreotide is used either subcutaneously at 10–70 µg/kg/day given 6–24 hourly doses, or as continuous intravenous infusion between 0.3 and 10 µg/kg/hour. The duration of administration in cases of successful resolution varies between 4 and 21 days and is mostly guided by response to therapy.61 The safety and efficacy of octreotide in the treatment of chylothorax in neonates has not been evaluated properly. The rarity of the condition is the main rate-limiting step. Octreotide is associated with adverse effects such as arrhythmias, hyperglycemia,62 transient impairment of liver function, transient hypothyroidism, and necrotizing enterocolitis,63 hypoxemia and pulmonary hypertension in preterm neonates.64 However, in the absence of serious/long-term side effects, octreotide continues to be used as off-label medication in the management of CC.
Sildenafil
Malleske and Yoder65 reported successful use of sildenafil in CC in a late preterm infant associated with pulmonary lymphangiectasis, where octreotide use was ineffective.65 In this case, sildenafil was used for the management of pulmonary hypertension associated with lymphangiectasis and was found to benefit CC when octreotide had failed. A mechanism by which sildenafil may facilitate resolution of CC and lymphatic malformations involves generation of new lymphatic vessels. Lymphatic vessel growth and function is regulated, by cyclic guanosine monophosphate (cGMP) which mediates lymphatic endothelial cell proliferation, migration, and tube formation. Sildenafil prevents the degradation of cGMP by selective inhibition of phosphodiesterase-567 and could thereby facilitate lymphatic vessel growth and/or remodeling allowing resolution of lymphatic obstruction and chylothorax.
Sirolimus, also known as rapamycin, is an inhibitor of the mammalian target of rapamycin (mTOR) whose expression is upregulated in a number of vascular anomalies, specifically those demonstrating atypical lymphatic pathology. Case reports have documented effective treatment of chylothoraces associated with lymphatic malformation using sirolimus, in adults and older children.68,69 However, dosing information remains very limited especially for neonates and infants. Recent study by Mizuno et al70 identified age-appropriate dosing regimens for neonates and infants. Further evaluation is necessary before it could be considered in neonatal population.
Supportive management
Ventilation
Most neonates with significant chylothorax and hydrops fetalis need ongoing ventilatory support until chylothorax subsides. Adequate expansion of the lungs is essential to maintain a tamponade effect to prevent re-accumulation of the chylothorax. Ventilation should include lung protective strategies, and use of high frequency ventilation is beneficial.
Nutrition
Use of total parenteral nutrition followed by reestablishment of feeds using MCT-based milk formulae is helpful to eliminate the need for enteral digestion of fats and maintenance of nutrition. MCTs of 8–12 carbon chains are directly absorbed into portal venous system bypassing the lymphatic drainage. There are several enteral formulae available with high MCT percentage such as Monogen, Peptamen, and Portagen. Formula prepared from skimmed milk powder with added coconut oil (rich in MCT) could be a cheap and effective alternative to provide low-fat and high-protein calories in patients with CC in low resource setting, where commercial formulae such as Monogen are not accessible.71 Use of fortified skimmed breast milk was found to be therapeutically equivalent to specialized infant formulae and can be an additional therapeutic option to successfully treat infants with chylothorax.72
Outcome
Usually, the condition resolves in the first few weeks (2–6 weeks) with conservative management.21,73 Conservative management has success rate of 75–80%.1,21 In a review of 204 cases of chylothorax, hydrops was the single most important prognostic factor of a poor outcome in CC.74 Time duration at which conservative management should be considered as failed is not well defined. Some centers use daily drainage as a guide for clinical improvement or failure in pediatric patients (<10 mL/kg/day of pleural drainage is considered as an improvement; >10 mL/kg/day of pleural drainage is considered to be a failure after 4 weeks of nonsurgical management).21 Opinions vary regarding the duration of the period of conservative management. An extended period of conservative management may result in severe immunological and nutritional disturbances.75
Surgical management
Surgical options should be considered when medical management of CC has failed to reduce chyle flow. There is no consensus on the timing of surgery.15 Most recommend an extended period (3–4 weeks) of conservative management in pediatric population before proceeding to surgical management.21,76 Others regard a particular volume, such as >100 mL per year of age in children, as an indication for surgery.77–79 There is no consensus on duration of conservative management in neonatal population before consideration of surgical options. If there is a well-identified site of chyle leak and high flow that precludes spontaneous healing, a case for earlier surgery can be made.80 Successful surgery can shorten hospitalization and reduce the risks of malnutrition and immunosuppression.15
Surgical options include thoracoscopic pleurodesis, pleuroperitoneal shunt, thoracic duct ligation (by thoracoscopy or thoracotomy), TDE, thoracic duct to azygous vein anastomosis, and lung transplantation (lymphangio-leiomyomatosis). Obliteration of the pleural space (pleurodesis) could be performed either chemically or surgically. Chemical pleurodesis may be considered over surgical approach given the concern of risk of blood loss associated with pleurectomy in neonatal patients. Various agents, such as povidone-iodine (Betadine), tetracycline, talc, bleomycin, and fibrin glue, have been used in pediatric and adult patients.81–83 In neonates, there are case reports of successful use of povidone-iodine82,84 and OK-432 for pleurodesis.
Chemical pleurodesis
Povidone-iodine (Betadine)
Brissaud et al82 treated four neonates with CC by intrapleural instillation of povidone-iodine through a chest tube. This procedure was successful in three out of the four cases and appeared to be well tolerated. Betadine 4% scrub (three cases) and Betadine 10% dermique were used. Betadine 10% dermique was diluted with normal saline (3 mL Betadine 10% dermique +7 mL saline), and Betadine 4% scrub was used without dilution. With both products, the chest tube was cleared after the injection with 10–15 mL normal saline, just before chest tube occlusion. The duration of chest tube occlusion was empirical and varied between 3 and 5 hours. Improvement occurred progressively, and the tube was removed 6–16 days after the procedure. Scottoni et al84 also reported the successful resolution of persistent chylothorax in four out of five neonates with single administration of 2 mL/kg of 4% povidone-iodine solution into the pleural space. The median time for resolution was 4 days. None of the patients experienced long-term side effects of the treatment. These results are promising, and povidone-iodine pleurodesis may be considered as an option in the management of refractory chylothorax in neonates.
The mechanism of action of povidone-iodine appears to be related to enhanced sclerosis,73 although the precise mode of action remains unclear. Iodine has strong oxidative and cytotoxic properties, which induces a potent inflammatory response in the wall of any fluid-containing structure. Moreover, povidone-iodine may have anti-exudative properties related to the chelation of proteins.73 However, frequent use of topical cutaneous povidone-iodine in neonates can induce allergic sensitization and impairment of thyroid function.85 Chest pain, metabolic acidosis, hypotension, respiratory and renal failure have been previously observed in adult and pediatric patients who had received high doses of povidone-iodine topically applied in treatment of burns or after continuous mediastinal irrigation. Renal failure following povidone-iodine use has been reported in neonatal cases.82,86 It is hypothesized that certain conditions such as diffuse congenital lymphangiectasis may cause massive iodine absorption and intoxication.86
OK-432 (Picibanil)
OK-432 has been widely used to treat postoperative chylothorax and cystic lymphangioma in children.87,88 Intrapleural administration of OK-432 has been reported to have favorable outcomes in the management of fetal chylothorax.40–43 Kim et al89 and Matsukuma et al90 reported effective use of OK-432 in neonatal cases (two cases each) refractory to conservative management. Fever lasting for 2–4 days and a local inflammatory reaction lasting 3–7 days are the reported side effects.91 However, since there is limited number of case reports, a prospective randomized study in neonatal population will be required to prove its effectiveness, and ascertain side effects and safety of use.
Oxytetracycline
A recent case report by Utture et al92 showed that oxytetracycline was effective after bilateral CC failed to respond to octreotide. Oxytetracycline with 2% lignocaine was administered into the pleural cavity (on day 20) at a dose of 20 mg/kg through the intercostal drainage (ICD), and the ICD was clamped for 2 hours. Due to failure of adequate response, a second dose was given after 48 hours. The ICD output became nil after 2 days. There were no adverse effects noted. Oxytetracycline has been used in adults with malignant chylothorax at a dose of 20 mg/kg.93 The mechanism of action of oxytetracycline is by initiation of the inflammatory cascade by mesothelial cells after contact with the chemical agent leading to activation of the coagulation cascade of the pleura, fibroblast recruitment, activation, and proliferation, and collagen deposition.94
Talc
Hodges et al95 reported a case of a persistent CC associated with a cervical lymphatic malformation successfully treated with thoracoscopic pleurodesis using aerosolized talc. Adjuvant therapies (sirolimus and sclerotherapy) were directed toward the treatment of the macrocystic lymphatic malformation in this case.
Thoracic duct embolization
A retrospective review of 34 patients to assess technical and clinical success of TDE for nontraumatic chylous effusions showed that TDE was most successful in cases of thoracic duct occlusion and extravasation. The clinical success rate was 16% (one of six) in cases of normal thoracic duct, 75% (15 of 20 patients) in occlusions of the thoracic duct.96 Lymphangiography is important for identifying the cause of chylous effusion and selecting patients who might benefit most from TDE. However, lymphatic channel embolization can be technically challenging in the neonatal population.96 Itkin et al97 reported two cases (a 6-month-old and a 1-month-old) with postoperative chylous leaks who were treated successfully with percutaneous TDE using n-butyl cyanoacrylate diluted 1:2 in ethiodized poppy seed oil.
Management of difficult-to-treat or resistant cases
Cases resistant to conservative management and pleurodesis include those with congenital malformations of lymphatics, chromosomal anomalies, and structural abnormalities. Magnetic resonance (MR) imaging including noncontrast MR lymphangiography and dynamic contrast MR lymphangiography will help visualize lymphatic anatomy and flow dynamics.98 Lymphoscintigraphy can identify thoracic duct injury, aplasia/hypoplasia of lymphatic vessels.99 Lung biopsy may be required to diagnose congenital lymphangiectasis. Prolonged parenteral nutrition may be necessary in addition to surgical interventions such as pleuroperitoneal shunts and thoracic duct ligation.
Complications
Several complications can occur in association with the development of CC. These include pulmonary hypoplasia from long-standing chylothorax in utero, malnutrition, hyponatremia, fluid imbalance, increased risk of thrombosis,100 and secondary immunodeficiency.
Incidence of nosocomial infections in patients with CC is about three times higher than that in other neonatal patients.101 Prolonged drainage of large volumes of chylous fluid results in depletion of lymphocytes which puts the neonates at risk of infection. Chylothorax can lead to hypogammaglobulinemia and lymphopenia.101–103 Lymphopenia has been significantly correlated with the duration of lymph drainage.101 Intravenous immunoglobulin has been used in postsurgical infants either prophylactically after development of chylothorax or early in the course of septicaemia.101 In a later study of eight children with hypogammaglobulinemia and lymphopenia attributable to chylothorax, intravenous immunoglobulin G administration did not lead to discernible protection from infectious complications.103
Conclusion
CC is a rare but serious entity in neonates. Early diagnosis and intervention in the prenatal period favor improved postnatal outcome. Postnatal management includes drainage of the pleural fluid, dietary modifications, drug therapy, and rarely surgery. There are no universally accepted guidelines for use of medications in this condition and the duration of conservative management. Overall, outcome of the condition depends on the underlying genetic condition and associated malformations.
Disclosure
The authors report no conflicts of interest in this work.
References
Al-Tawil K, Ahmed G, Al-Hathal M, Al-Jarallah Y, Campbell N. Congenital chylothorax. Am J Perinatol. 2000;17(3):121–126. | ||
De Beer HG, Mol MJ, Janssen JP. Chylothorax. Neth J Med. 2000;56(1):25–31. | ||
Dendale J, Comet P, Amram D, et al. Le chylothorax de découverte anténatale. [Prenatal diagnosis of chylothorax]. Arch Pediatr. 1999;6(8):867–871. French. | ||
Dubin PJ, King IN, Gallagher PG. Congenital chylothorax. Curr Opin Pediatr. 2000;12(5):505–509. | ||
Van Straaten HL, Gerards LJ, Krediet TG. Chylothorax in the neonatal period. Eur J Pediatr. 1993;152(1):2–5. | ||
Rocha G. Pleural effusions in the neonate. Curr Opin Pulm Med. 2007;13(4):305–311. | ||
Van Aerde J, Campbell AN, Smyth JA, Lloyd D, Bryan MH. Spontaneous chylothorax in newborns. Am J Dis Child. 1984;138(10):961–964. | ||
Downie L, Sasi A, Malhotra A. Congenital chylothorax: associations and neonatal outcomes. J Paediatr Child Health. 2014;50(3):234–238. | ||
Attar M, Donn S. Congenital chylothorax. Semin Fetal Neonatal Med. 2017;22(4):234–239. | ||
Smets K. X-linked myotubular myopathy and chylothorax. Neuromuscul Disord. 2008;18(2):183–184. | ||
Huang XZ, Wu JF, Ferrando R, et al. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20(14):5208–5215. | ||
Ma GC, Liu CS, Chang SP, et al. A recurrent ITGA9 missense mutation in human fetuses with severe chylothorax: possible correlation with poor response to fetal therapy. Prenat Diagn. 2008;28(11):1057–1063. | ||
Brodszki N, Lansberg JK, Dictor M, et al. A novel treatment approach for paediatric Gorham-Stout syndrome with chylothorax. Acta Paediatr. 2011;100(11):1448–1453. | ||
Tutor JD. Chylothorax in infants and children. Pediatrics. 2014;133(4):722–733. | ||
Soto-Martinez M, Massie J. Chylothorax: diagnosis and management in children. Paediatr Respir Rev. 2009;10(4):199–207. | ||
Chan SY, Lau W, Wong WH, Cheng LC, Chau AK, Cheung YF. Chylothorax in children after congenital heart surgery. Ann Thorac Surg. 2006;82(5):1650–1656. | ||
Healy F, Hanna BD, Zinman R. Pulmonary complications of congenital heart disease. Paediatr Respir Rev. 2012;13(1):10–15. | ||
Milonakis M, Chatzis AC, Giannopoulos NM, et al. Etiology and management of chylothorax following pediatric heart surgery. J Card Surg. 2009;24(4):369–373. | ||
Chun K, Colombani PM, Dudgeon DL, Haller JA Jr. Diagnosis and management of congenital vascular rings: a 22-year experience. Ann Thorac Surg. 1992;53(4):597–602. discussion 602–603. | ||
Naik S, Greenough A, Zhang YX, Davenport M. Prediction of morbidity during infancy after repair of congenital diaphragmatic hernia. J Pediatr Surg. 1996;31(12):1651–1654. | ||
Beghetti M, La Scala G, Belli D, Bugmann P, Kalangos A, Le Coultre C. Etiology and management of pediatric chylothorax. J Pediatr. 2000;136(5):653–658. | ||
Mosby-Year Book Inc. Mosby’s Medical, Nursing and Allied Health Dictionary. Fourth ed. Maryland Heights, MO: Mosby-Year Book Inc; 1994:335. | ||
Williams KR, Burford TH. The management of chylothorax. Ann Surg. 1964;160:131–140. | ||
Teba L, Dedhia HV, Bowen R, Alexander JC. Chylothorax review. Crit Care Med. 1985;13(1):49–52. | ||
Ferguson M, Shahinian H, Michelassi F. Lymphatic smooth muscle responses to leukotrienes, histamine, and platelet activating factor. J Surg Res. 1988;44(2):172–177. | ||
Marieb E, Hoehn K. Human Anatomy & Physiology. 8 ed. San Francisco, CA: Benjamin and Cummings; 2010. | ||
Kindt T, Goldsby R, Osborne B, et al. Kuby Immunology. New York: W.H. Freeman; 2007. | ||
Bellini C, Hennekam R. Non-immune hydrops fetalis: a short review of etiology and pathophysiology. Am J Med Genet A. 2012;158A(3):597–605. | ||
De Hann TR, Oepkes D, Beersma MF, et al. Aetiology, diagnosis and treatment of hydrops foetalis. Curr Pediatr Rev. 2005;1:63–72. | ||
Ayida GA, Soothil PW, Rodeck CH. Survival in non-immune hydrops fetalis without malformation of chromosomal abnormalities after invasive treatment. Fetal Diagn Ther. 1995;10:101–105. | ||
Huang HR, Tsay PK, Chiang MC, Lien R, Chou YH. Prognostic factors and clinical features in liveborn neonates with hydrops fetalis. Am J Perinatol. 2007;24:33–38. | ||
Prasad C, Rupar CA. Non-immune hydrops: genetic and metabolic causes. Perinatology. 2006;8:164–174. | ||
Jones DC. Nonimmune fetal hydrops: diagnosis and obstetrical management. Semin Perinatol. 1995;19:447–461. | ||
Fahnenstich H, Schmid G, Haverkamp F, et al. Non-immune hydrops fetalis—an analysis of liveborn cases. Pediatr Rev Commun. 1992;6:231–241. | ||
Lee CJ, Tsao PN, Chen CY, Hsieh WS, Liou JY, Chou HC. Prenatal therapy improves the survival of premature infants with congenital chylothorax. Pediatr Neonatol. 2016;57(2):127–132. | ||
Chen CP. Fetal therapy and cytogenetic testing: prenatal detection of chromosome aberration during thoracocentesis for congenital chylothorax by karyotyping from pleural effusion fluid and review of the literature. Genet Couns. 2005;16(3):301–305. | ||
Bartha JL, Comino-Delgado R. Fetal chylothorax response to maternal dietary treatment. Obstet Gynecol. 2001;97(5 pt 2):820–823. | ||
Chen C, Chang T, Wang W. Resolution of fetal bilateral chylothorax and ascites after two unilateral thoracocenteses. Ultrasound Obstet Gynecol. 2001;18:401–406. | ||
Picone O, Benachi A, Mandelbrot L, Ruano R, Dumez Y, Dommergues M. Thoracoamniotic shunting for fetal pleural effusions with hydrops. Am J Obstet Gynecol. 2004;191(6):2047–2050. | ||
Okawa T, Takano Y, Fujimori K, Yanagida K, Sato A. A new fetal therapy for chylothorax: pleurodesis with OK-432. Ultrasound Obstet Gynecol. 2001;18(4):376–377. | ||
Tanemura M, Nishikawa N, Kojima K, Suzuki Y, Suzumori K. A case of successful fetal therapy for congenital chylothorax by intrapleural injection of OK-432. Ultrasound Obstet Gynecol. 2001;18(4):371–375. | ||
Jorgensen C, Brocks V, Bang J, Jorgensen FS, Rønsbro L. Treatment of severe fetal chylothorax associated with pronounced hydrops with intrapleural injection of OK-432. Ultrasound Obstet Gynecol. 2003;21(1):66–69. | ||
Tsukihara A, Tanemura M, Suzuki Y, Sato T, Tanaka T, Suzumori K. Reduction of pleural effusion by OK-432 in a fetus complicated with congenital hydrothorax. Fetal Diagn Ther. 2004;19(4):327–331. | ||
Chen M, Chen CP, Shih JC, et al. Antenatal treatment of chylothorax and cystic hygroma with OK-432 in nonimmune hydrops fetalis. Fetal Diagn Ther. 2005;20(4):309–315. | ||
Reece E, Goldstein I, Hobbins J. Fundamentals of Obstetric and Gynecologic Ultrasound. Stamford,CT. Appleton and Lange; 1994. | ||
Watson WJ, Munson DP, Christensen MW. Bilateral fetal chylothorax: results of unilateral in utero therapy. Am J Perinatol. 1996;13(2):115–117. | ||
Rodeck CH, Fisk NM, Fraser DI, et al. Long-term in utero drainage of fetal hydrothorax. N Engl J Med. 1988;319(17):1135–1138. | ||
Smith RP, Illanes S, Denbow ML, et al. Outcome of fetal pleural effusions treated by thoracoamniotic shunting. Ultrasound Obstet Gynecol. 2005;26(1):63–66. | ||
Nicolaides KH, Azar GB. Thoraco-amniotic shunting. Fetal Diagn Ther. 1990;5(3–4):153–164. | ||
Thompson PJ, Greenough A, Nicolaides KH. Respiratory function in infancy following pleuro-amniotic shunting. Fetal Diagn Ther. 1993;8(2):79–83. | ||
Wilson RD, Baxter JK, Johnson MP, et al. Thoracoamniotic shunts: fetal treatment of pleural effusions and congenital cystic adenomatoid malformations. Fetal Diagn Ther. 2004;19(5):413–420. | ||
Mussat P, Dommergues M, Parat S, et al. Congenital chylothorax with hydrops: postnatal care and outcome following antenatal diagnosis. Acta Paediatr. 1995;84(7):749–755. | ||
Mallmann M, Graham V, Rosing B, et al. Thoracoamniotic shunting for fetal hydrothorax: predictors of intrauterine course and postnatal outcome. Fetal Diagn Ther. 2017;41:58–65. | ||
Sepulveda W, Galindo A, Sosa A, Diaz L, Flores X, de la Fuente P. Intrathoracic dislodgement of pleuro-amniotic shunt. Three case reports with long-term follow-up. Fetal Diagn Ther. 2005; 20(2):102–105. | ||
Samuel M, McCarthy L, Boddy SA. Efficacy and safety of OK-432 sclerotherapy for giant cystic hygroma in a newborn. Fetal Diagn Ther. 2000;15(2):93–96. | ||
Levine C. Primary disorders of the lymphatic vessels – a unified concept. J Pediatr Surg. 1989;24(3):233–240. | ||
Young S, Dalgleish S, Eccleston A, Akierman A, McMillan D. Severe congenital chylothorax treated with octreotide. J Perinatol. 2004;24(3):200–202. | ||
Lamberts SW, van der Lely AJ, de Herder WW, et al. Octreotide. N Engl J Med. 1996;334(4):246–254. | ||
Glaser B, Hirsch HJ, Landau H. Persistent hyperinsulinemic hypoglycemia of infancy: long-term octreotide treatment without pancreatectomy. J Pediatr. 1993;123(4):644–650. | ||
Rasiah SV, Oei J, Lui K. Octreotide in the treatment of congenital chylothorax. J Paediatr Child Health. 2004;40(9–10):585–588. | ||
Das A, Shah P. Octreotide for the treatment of chylothorax in neonates. Cochrane Database Syst Rev. 2010;(9):CD006388. | ||
Mohseni-Bod H, Macrae D, Slavik Z. Somatostatin analog (octreotide) in management of neonatal postoperative chylothorax: is it safe? Pediatr Crit Care Med. 2004;5(4):356–357. | ||
Reck-Burneo CA, Parekh A, Velcek FT. Is octreotide a risk factor in necrotizing enterocolitis? J Pediatr Surg. 2008;43(6):1209–1210. | ||
Arevalo RP, Bullabh P, Krauss AN, Auld PA, Spigland N. Octreotide-induced hypoxemia and pulmonary hypertension in premature neonates. J Pediatr Surg. 2003;38(2):251–253. | ||
Malleske DT, Yoder BA. Congenital chylothorax treated with oral sildenafil: a case report and review of the literature. J Perinatol. 2015;35(5):384–386. | ||
Kajiya K, Huggenberger R, Drinnenberg I, Ma B, Detmar M. Nitric oxide mediates lymphatic vessel activation via soluble guanylate cyclase alpha1beta1-impact on inflammation. FASEB J. 2008;22(2):530–537. | ||
Jeon Y, Heo Y, Kim C, et al. Phosphodiesterase: overview of protein structures, potential therapeutic applications and recent progress in drug development. Cell Mol Life Sci. 2005;62(11):1198–1220. | ||
Hammill AM, Wentzel M, Gupta A, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer. 2011;57(6):1018–1024. | ||
McCormick A, Rosenberg S, Trier K, et al. A case of a central conducting lymphatic anomaly responsive to sirolimus. Pediatrics. 2016;137(1). | ||
Mizuno T, Fukuda T, Emoto C, et al. Developmental pharmacokinetics of sirolimus: implications for precision dosing in neonates and infants with complicated vascular anomalies. Pediatr Blood Cancer. Epub 2017 Feb 16;64(8). | ||
Gupta V, Mahendri NV, Tete P, Santhanam S. Skimmed milk preparation in management of congenital chylothorax. Indian Pediatr. 2014;51(2):146–148. | ||
Lopez G. Use of fortified skimmed breast milk to feed infants with postoperative chylothorax. Scholar Arch. 2015:3658. | ||
Cohan RH, Saeed M, Schwab SJ, Perlmutt LM, Dunnick NR. Povidone-iodine sclerosis of pelvic lymphoceles: a prospective study. Urol Radiol. 1988;10(4):203–206. | ||
Aubard Y, Derouineau I, Aubard V, Chalifour V, Preux PM. Primary fetal hydrothorax: a literature review and proposed antenatal clinical strategy. Fetal Diagn Ther. 1998;13:325–333. | ||
Engum SA, Rescorla FJ, West KW, Scherer LR 3rd, Grosfeld JL. The use of pleuroperitoneal shunts in the management of persistent chylothorax in infants. J Pediatr Surg. 1999;34(2):286–290. | ||
Buttiker V, Fanconi S, Burger R. Chylothorax in children: guidelines for diagnosis and management. Chest. 1999;116(3):682–687. | ||
Hargus EP, Carson SD, McGrath RL, Wolfe RR, Clarke DR. Chylothorax and chylopericardial tamponade following Blalock-Taussig anastomosis. J Thorac Cardiovasc Surg. 1978;75(4):642–645. | ||
Murphy MC, Newman BM, Rodgers BM. Pleuroperitoneal shunts in the management of persistent chylothorax. Ann Thorac Surg. 1989;48(2):195–200. | ||
Stringel G, Mercer S, Bass J. Surgical management of persistent postoperative chylothorax in children. Can J Surg. 1984;27(6):543–546. | ||
Soto-Martinez ME, Clifford V, Clarnette T, Ranganathan S, Massie RJ. Spontaneous chylothorax in a 2-year-old child. Med J Aust. 2009;190(5):262–264. | ||
Aoki M, Kato F, Saito H, et al. Successful treatment of chylothorax by bleomycin for Gorham’s disease. Clin Orthop Relat Res. 1996;(330):193–197. | ||
Brissaud O, Desfrere L, Mohsen R, et al. Congenital idiopathic chylothorax in neonates: chemical pleurodesis with povidone-iodine (Betadine). Arch Dis Child Fetal Neonatal Ed. 2003;88(6):F531–F533. | ||
Stenzl W, Rigler B, Tscheliessnigg KH, Beitzke A, Metzler H. Treatment of postsurgical chylothorax with fibrin glue. Thorac Cardiovasc Surg. 1983;13(1):35–36. | ||
Scottoni F, Fusaro F, Conforti A, Morini F, Bagolan P. Pleurodesis with povidone-iodine for refractory chylothorax in newborns: personal experience and literature review. J Pediatr Surg. 2015;50(10):1722–1725. | ||
Ancona A, Suarez de la TR, Macotela E. Allergic contact dermatitis from povidone-iodine. Contact Dermatitis. 1985;13(2):66–68. | ||
Mitanchez D, Walter-Nicolet E, Salomon R, et al. Congenital chylothorax: what is the best strategy? Arch Dis Child Fetal Neonatal Ed. 2006;91(2):F153–F154. | ||
Okazaki T, Iwatani S, Yanai T, et al. Treatment of lymphangioma in children: our experience of 128 cases. J Pediatr Surg. 2007;42(2):386–389. | ||
Katanyuwong P, Dearani J, Driscoll D. The role of pleurodesis in the management of chylous pleural effusion after surgery for congenital heart disease. Pediatr Cardiol. 2009;30(8):1112–1116. | ||
Kim JE, Lee C, Park KI, Park MS, Namgung R, Park IK. Successful pleurodesis with OK-432 in preterm infants with persistent pleural effusion. Korean J Pediatr. 2012;55(5):177–180. | ||
Matsukuma E, Aoki Y, Sakai M, et al. Treatment with OK-432 for persistent congenital chylothorax in newborn infants resistant to octreotide. J Pediatr Surg. 2009;44(3):e37–e39. | ||
Ogita S, Tsuto T, Nakamura K, Deguchi E, Iwai N. OK-432 therapy in 64 patients with lymphangioma. J Pediatr Surg. 1994;29(6):784–785. | ||
Utture A, Kodur V, Mondkar J. Chemical Pleurodesis with Oxytetracycline in Congenital Chylothorax. Indian pediatrics. 2016;53(12):1105–1106. | ||
Walker-Renard PB, Vaughan LM, Sahn SA. Chemical pleurodesis for malignant pleural effusions. Ann Intern Med. 1994;120(1):56–64. | ||
Antony VB, Rothfuss KJ, Godbey SW, Sparks JA, Hott JW. Mechanism of tetracycline-hydrochloride-induced pleurodesis. Tetracycline-hydrochloride-stimulated mesothelial cells produce a growth-factor-like activity for fibroblasts. Am Rev Respir Dis. 1992;146(4):1009–1013. | ||
Hodges MM, Crombleholme TM, Meyers M, et al. Massive fetal chylothorax successfully treated with postnatal talc pleurodesis: a case report and review of the literature. J Pediatr Surg Case Rep. 2016;9(suppl C):1–4. | ||
Nadolski GJ, Itkin M. Thoracic duct embolization for nontraumatic chylous effusion: experience in 34 patients. Chest. 2013;143(1):158–163. | ||
Itkin M, Krishnamurthy G, Naim MY, Bird GL, Keller MS. Percutaneous thoracic duct embolization as a treatment for intrathoracic chyle leaks in infants. Pediatrics. 2011;128(1):e237–e241. | ||
Dori Y. Novel lymphatic imaging techniques. Tech Vasc Interv Radiol. 2016;19(4):255–261. | ||
Bellini C, Boccardo F, Campisi C. Lymphatic dysplasias in newborns and children: role of lymphoscintigraphy. J Pediatr. 2008;152(4):587–589. | ||
Bernet-Buettiker V, Waldvogel K, Cannizzaro V, Albisetti M. Antithrombin activity in children with chylothorax. Eur J Cardiothorac Surg. 2006;29:406–409. | ||
Wasmuth-Pietzuch A, Hansmann M, Bartmann P, Heep A. Congenital chylothorax: lymphopenia and high risk of neonatal infections. Acta Paediatr. 2004;93(2):220–224. | ||
Garty BZ, Levinson AI, Danon YL, Wilmott R, Douglas SD. Lymphocyte subpopulations in children with abnormal lymphatic circulation. J Allergy Clin Immunol. 1989;84:515–520. | ||
Orange J, Geha R, Bonilla F. Acute chylothorax in children: selective retention of memory T cells and natural killer cells. J Pediatr. 2003;143:243–249. | ||
Earth’s Lab. Thoracic duct, Dr Joseph H Volker. [webpage on the Internet].Available from https://earthslab.com/anatomy/thoracic-duct-formation-course-connection-tributaries-and-development/. Accessed December 4, 2017. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
