Back to Journals » Patient Preference and Adherence » Volume 14
Comparison of Different Adherence Measures in Adolescent Outpatients with Depressive Disorder
Authors Mok YE , Lee J , Lee M
Received 14 February 2020
Accepted for publication 26 May 2020
Published 24 June 2020 Volume 2020:14 Pages 1065—1072
DOI https://doi.org/10.2147/PPA.S249728
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Young Eun Mok,1 Jong-ha Lee,2 Moon-soo Lee1
1Division of Child and Adolescent Psychiatry, Department of Psychiatry, Korea University Guro Hospital, Guro-gu, Seoul, Republic of Korea; 2Department of Psychiatry, Korea University Ansan Hospital, Ansan, Gyeonggi Province, Republic of Korea
Correspondence: Moon-soo Lee
Division of Child and Adolescent Psychiatry, Department of Psychiatry, Korea University Guro Hospital, 148, Gurodong-ro, Guro-gu, Seoul 08308, Republic of Korea
Tel +82 2 2626 3163
Fax +82 2 852 1937
Email [email protected]
Purpose: Adolescent depression can have a chronic course; hence, the importance of adherence to antidepressant medication for successful treatment outcomes is emphasized. This study aimed to examine different adherence measures and identify clinical factors that influence adherence in adolescent depression.
Patients and Methods: A prospective study was conducted for patients diagnosed with depressive disorder according to the Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition from outpatient psychiatric settings at Korea University Medical Center, Guro Hospital. Patient demographics were obtained from a questionnaire, interview, and review of chart records. Adherence was assessed by four methods (Medication Event Monitoring System [MEMS], pill count, clinical rating scale, and patient’s self-report). The Toronto Side Effect Scale was used to evaluate side effects, and specific depressive symptoms were assessed using the Hamilton Rating Scale for Depression and Childhood Depression Inventory–Korean version. The Multidimensional Scale of Perceived Social Support was administered to analyze social support, and the Parenting Stress Index-Short Form was used to evaluate parental stress levels. We used concordance correlation analysis to evaluate the relationship among the four adherence measures and the relationship between adherence level and clinical factors.
Results: Overall, the study enrolled 48 outpatients (mean age 16.33± 1.93 years). The mean duration of illness was 1.27± 2.17 years. Adherence rates for MEMS, clinician rating scale, pill count, and self-report after conversion to dichotomous measures were 67.5%, 48.9%, 60.0%, and 56.3%, respectively. Only the duration of illness remained significantly correlated with MEMS (r = 0.510, p =0.001).
Conclusion: Pill count exhibited a higher degree of agreement with MEMS adherence than the other two adherence measures, possibly indicating that pill count may be a considerably reliable measure of adherence. Furthermore, MEMS adherence was positively correlated with disease duration, suggesting that the longer the duration of illness, the higher the adherence.
Keywords: medication event monitoring system, pill count, duration of illness, self-report, clinician rating scale, symptom severity, parental stress
Introduction
Major depressive disorder (MDD) is one of the most common psychiatric disorders and is the leading cause of disability worldwide.1,2 The disorder is a common mental health problem even in adolescents, affecting 4 to 5% of mid to late adolescence.3 Adolescents with depression have a major risk of suicide, which is the leading cause of death in this population.4 Core symptoms of persistent and pervasive sadness, along with a loss of interest or pleasure in activities, are diagnostic criteria applied independently of age. Moreover, associated symptoms including low self-esteem, excessive guilt, suicidal thoughts or behaviors, changes in sleep and appetite, and psychomotor agitation retardation are also diagnostic criteria for all ages.5 However, marked irritability is also recognized as a cardinal mood symptom for children and adolescents in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criterion set.5 Depression in adolescents is often challenging to recognize in the clinical setting, possibly due to prominence of mood reactivity, irritability, and fluctuation in symptoms.6 Furthermore, depression in adolescents can be missed when the chief complaints are unexplained physical symptoms, other psychiatric symptoms, refusal to attend school, or behavioral problems.7 Such a variety of depressive symptoms in adolescents may cause social and educational impairments as well as an increased rate of comorbidities.8,9
As challenging as its recognition, depression in adolescents may have unfavorable long-term consequences. Clinical studies of depression in adolescents show that it is a chronic and recurrent condition. Although most episodes remit within a year, 50–70% of patients likely develop a further episode within five years.10 Depression in adolescence often proceeds through a process of chronification and predicts a broad range of difficulties in social functioning and psychiatric comorbidities, such as anxiety disorder persisting into adulthood.5,10
For successful treatment and prevention of relapse and recurrence, adherence to antidepressant medication is crucial.11 However, medication adherence rates in chronic childhood illnesses vary from 10–89%.12 As far as the depressive disorder in adolescence is concerned, the rates of adherence to medication have been reported to be 49.5% and 42% during the acute and continuation phase, respectively.13 Since low adherence to medication in depressive disorder often leads to treatment failure and chronification of the disorder into adulthood, evaluating the factors associated with adherence in adolescents seems essential. Most of the previous studies have mainly focused on the evaluation of treatment adherence in adults with psychopathological or other medical problems.14–16 Up until now, there have been limited number of studies regarding the relationship between medication adherence in adolescents with depression, especially using the Medication Event Monitoring System (MEMS), the objective reference standard.14
The currently used assessment methods to examine medication adherence in adolescents are the child report, parent report, pill count, and measurement of medication concentrations in urine or blood samples.17 Indirect methods such as self-report questionnaires or pill count often show overestimation of adherence.12 Direct methods of assessment, such as the measurements of the concentrations of medication in urine or blood samples, maybe more accurate but are resource consuming and invasive.17 The MEMS is a device that records the time and date that a pill bottle was opened through an electronic computer chip implanted into the cap of the pill bottle. Data obtained from the MEMS are downloaded directly to a computer program for analysis.18 This device allows objective assessment of medication use both in the clinical and research settings, and is currently regarded as the objective reference standard for measuring adherence.14 In this study, MEMS was used to evaluate antidepressant adherence in adolescents with depression.
The primary aim of this prospective study was to examine differences in adherence to antidepressants as estimated by self-report, the clinician’s rating scale, pill count, and MEMS in adolescents with depressive disorder. The secondary aim was to explore the relationships between medication adherence and clinical factors, including duration of illness, symptom severity, side effects, perception of social support, and parental stress level, to identify the possible point of intervention to enhance medication adherence.
Patients and Methods
Study Population and Procedures
We recruited a total of 48 patients who were diagnosed with depressive disorder according to the DSM-IV from outpatient psychiatric settings at Korea University Medical Center, Guro Hospital in Seoul, Korea, from 2012 to 2018. Our inclusion criteria were as follows: (1) adolescents between the age of 12 and 18 years who (2) met the diagnostic criteria for the depression-related disorder as specified in the DSM-IV, ascertained by their doctors, and (3) were treated with a single antidepressant. Clinicians at Korea University Medical Center, Guro Hospital informed the parents/guardians and participants about the study. Written informed consent was obtained from each parent or guardian of each adolescent study participant as well as from each adolescent participant. After enrollment, the participants were given a single antidepressant medication in a bottle with an electronic monitor cap (the MEMS). Antidepressants prescribed in the study were fluoxetine, sertraline, escitalopram, duloxetine, mirtazapine, venlafaxine, and desvenlafaxine. Some patients also received trazodone, melatonin, and antipsychotics in addition to the antidepressant. However, only the antidepressant was given in MEMS. They were then asked to take their medication once daily and return for a follow-up visit after 1 month. The patients or guardians were not instructed to intervene in the process of taking medication any more than they usually do. At the follow-up visit, an interview was performed, and the medication adherence of each participant was assessed using the MEMS, as well as by other adherence measures. Patients that were excluded from our study included: (1) those with any disease resulting in cognitive dysfunction such as intellectual disability, (2) those who had received electroconvulsive therapy in the previous 6-month period, and (3) acutely suicidal patients. This study was approved by the Institutional Review Board of Guro Hospital, Korea University Medical Center. This study was conducted in accordance with the Declaration of Helsinki.
Assessments
Patient demographics, including age, sex, education level, and housing status, were obtained from a questionnaire, interview, and review of chart records. The duration of illness was defined as the period between the onset of depressive symptoms and the time of diagnosis. The duration of illness was obtained from a review of chart records as well. Historical information regarding the depressive disorder of each participant was also investigated.
Adherence
Adherence was assessed using the MEMS, a pill count, a clinician rating scale, and a patient’s self-report. Each variable, except the clinician’s rating, was also treated as a dichotomous variable using a threshold of 80%.19 For the clinician’s rating, a specific scale score of 5 or higher was designated as adherence.
MEMS
The primary outcome measure was the MEMS since it is known as the objective reference standard for the measurement of adherence. The downloaded data were used to evaluate whether the participants’ bottles were opened according to the prescribed number of times daily. The proportion of the times the medication vial caps were opened in a given month, relative to the prescribed doses for that month, was obtained as a measurement of adherence to the antidepressant medication. The patients were informed of the MEMS cap’s function before the start of the study. The results were dichotomized into adherence and nonadherence, using a threshold of 80%.
Pill Count
The ratio of the actual pill count, as recorded by the investigator at the follow-up visit, was utilized to derive a pill count adherence index. The results were also dichotomized into adherence and nonadherence, using a threshold of 80%.
Clinician Rating Scale of Compliance
The clinician’s assessment of adherence was conducted with no information given about the MEMS cap data. We also realized that utilizing an absolute value from 0 to 100% was not realistic for the clinicians, so instead, we adopted an assessment scale – the clinician rating scale of compliance.15 Measuring the patient’s adherence by the researcher usually took approximately 10 minutes. General questions involving the patient’s symptoms and sense of well-being, the patient’s functional status, attitude towards taking medication, and the medications’ side effects were evaluated. Moreover, a specific question, asking how many days the patient was adherent to the medication in the past month, was included as well. During the assessments, the clinician ensured that statements regarding the desirability of adherence were not made. The clinician rating scale is an ordinal scale of 1–7 (1 = complete refusal, 2 = partial refusal or only accepts minimum dose, 3 = accepts only because compulsory, or very reluctant/requires persuasion, or questions the need for medication often [every two days], 4 = occasional reluctance [questions the need for medication once a week], 5 = passive acceptance, 6 = moderate participation, some knowledge and interest in medication and no prompting required, 7 = active participation, readily accepts, and shows some responsibility for the regimen) with higher numbers reflecting better adherence.16 In previous studies that used this scale, a score of 5 or higher was used to designate adherence.15,16 Accordingly, we employed a score of ≤4 as the threshold for clinically meaningful nonadherence.
Patient Self-Report
Participants were asked to estimate their adherence to antidepressant treatment between 0 and 100% at the study’s endpoint. The results were dichotomized into adherence and nonadherence by using a threshold of 80%.
Side Effects
The Toronto Side Effect Scale (TSES) was used to evaluate the side effects experienced by the participants. The Toronto Side Effect Scale is a 32-item instrument utilizing direct physician inquiry to elicit adverse events.20 The TSES was used to evaluate the incidence, frequency, and severity of the central nervous system (CNS), gastrointestinal (GI), and sexual side effects.20 Frequency and severity were measured on a 5-point Likert scale, and an intensity score was calculated by multiplying the frequency by the severity.20
Clinical Symptoms
Specific depressive symptoms were assessed using the Hamilton Rating Scale for Depression (HRSD) and the Childhood Depression Inventory–Korean version (K-CDI).21,22
Social Support
Participants were asked to complete the Multidimensional Scale of Perceived Social Support (MSPSS). The MSPSS evaluates the perceptions of social support using a 12-item scale.23 Items are rated on a 7-point scale (1 = very strongly disagree and 7 = very strongly agree), with each of the three subscales (friends, family, or significant other), assessed by four individual items written in the present tense. The patients were asked to answer each question by reflecting on how they usually feel. The psychometric properties of the MSPSS were previously investigated in a non-Western country.24
Parenting Stress Index-Short Form (PSI-SF)
Assuming that parental stress may affect adherence, we used the Parenting Stress Index-Short Form to evaluate stress levels. The Parenting Stress Index (PSI) is a 120-item parent-report questionnaire. A brief measure, known as the PSI-Short Form (PSI-SF), which consists of 36 items from the full PSI, was developed to reduce the burden of the lengthy PSI.25 The PSI-SF is based on several exploratory factor analyses of the full PSI and is comprised of three subscales, each consisting of items including the Parental Distress (PD), Parent-Child Dysfunctional Interaction (PCDI) and Difficult Child (DC) subscales, as well as a Total Stress scale.26
Statistical Analysis
To analyze the demographic characteristics of the participants, we calculated the means and standard deviations for the continuous variables, and frequencies and percentages for the categorical variables. We used a concordance correlation analysis that allows the evaluation of the degree to which pairs of observations fall on the 45° line through the origin, to assess agreement among the three continuous adherence measures.27 This correlation coefficient accounts for a measure of precision, as well as a measure of accuracy. To evaluate agreement among the three dichotomized adherence measures, we utilized Kappa statistics. Concordance correlation coefficients were calculated to analyze the relationship between MEMS, demographic variables, and clinical scale scores.
Results
Demographic Characteristics and Other Clinical Characteristics of the Participants
A total of 48 outpatients were enrolled in the study. Their mean age was 16.33±1.93 years, and 35 of the participants were female (72.9%), while the remaining 13 were male (27.1%). The mean duration of illness of the participants was 1.27±2.17 years. The most common diagnosis among the participants was MDD, single episode (68.7%). Clinical scales evaluating side effects, clinical symptoms, social support, and parenting stress index were evaluated at each visit. Table 1 provides the details of our participants’ demographics.
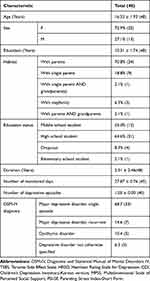 |
Table 1 Participants' Demographic Data and Clinical Variables (n=48) |
Adherence
We evaluated adherence in two ways. First, we treated adherence as a continuous variable. The mean values for the various measures of adherence were as follows: MEMS 84.06±19.84%, pill count 80.3±17.50%, and self-report 74.1±23.94%. The mean score of the clinician rating scale scores for the cases was 4.71±1.52. We then converted the continuous results of this adherence into dichotomous variables (adherent/non-adherent). Employing this approach, the adherence rates for the MEMS, the clinician rating scale of adherence, the pill count, and the self-report were 67.5%, 48.9%, 60.0%, and 56.3%, respectively (Table 2).
 |
Table 2 Adherence Scales |
All the adherence measures were significantly correlated with each other (Table 3). To evaluate how these adherence measures agreed with the MEMS when these variables were dichotomized, the Kappa coefficients were calculated to be 0.142 (clinician rating scale of compliance vs MEMS), 0.885 (pill count vs MEMS), and 0.204 (self-report vs MEMS) (Table 4)
 |
Table 3 Spearman Correlation Coefficient Among the Adherence Scales |
 |
Table 4 Kappa Coefficients Among the Adherence Scales |
We performed further analysis of the relationships between these adherence variables and other demographic variables (age, total number of years of education, and duration of illness). Age was not significantly correlated with any of the adherence measures. Duration of illness was correlated with adherence as measured by MEMS (r = 0.510, p =0.001) (Table 5). Other demographic and clinical variables, including clinical scales, were not significantly correlated with adherence measured by the MEMS (Table 5).
 |
Table 5 Correlation Coefficient with MEMS |
Discussion
Low adherence in the adolescent population can be understood in the light of psychosocial and environmental factors. First, adolescence is a crucial time for development and change. During this period, not only do adolescents mature physically but cognitively as well. Despite such cognitive maturation, however, adolescents tend to believe more what they can see or have experienced, and cannot fully understand the long-term or unseen consequences of nonadherence. They often remain self-centered and feel invulnerable to consequences. Also, during this phase, they strive to gain independence and want to have control over their lives. Adolescents try to gain a sense of control over themselves by not following the rules set by authority figures; thus, not taking medication or adhering to appropriate treatment can be a way of confronting the authority of parents and professionals. The other crucial factor associated with adherence in adolescents is their relationship with parents, peers, and caregivers. They struggle to be accepted, and therefore, the negative perception of those around them may hinder adherence.28
Adherence, as assessed by the MEMS, seemed higher than adherence recorded with the self-report and the pill count in our study. Likewise, the rate of adherence, as measured by the MEMS, seemed higher in the adherent group than the rates estimated by the three other measures when adherence was defined as a dichotomous variable. However, the differences were not statistically significant. A possible explanation for such finding is that opening the bottle does not necessarily mean swallowing the pills. The participants’ awareness of the study may have encouraged them to open the bottle even when they were not taking the medication. Such discrepancy may have resulted in what appears as greater adherence assessed by the MEMS compared to that of the rates measured by other adherence measures.
Compared to the objective reference standard of adherence measurement (the MEMS), pill count exhibited a higher degree of agreement than did the other two adherence measures. The relatively higher Kappa coefficient of pill count reflects the compatibility of the pill count method. Our finding is supported by a similar study which also proposed pill count as an acceptable proxy for evaluating medication adherence in adolescents with depressive disorder.29 Taken together, our results indicate that pill count may be a considerably reliable measure of adherence. Although the MEMS is considered the objective reference standard of measurement, it is time and resource consuming, expensive, and therefore, may not be suitable for all.30 Considering the high degree of agreement with the MEMS (67.5% vs 60%), the pill count measurement may serve as a relatively reliable method of adherence measurement in a clinical setting.
The rate of adherence measured by MEMS was positively correlated with the duration of illness (0.510, p = 0.001), and we can infer that the longer the duration of illness, the better the adherence. The relationship between the degree of adherence and the duration of disease may be explained by the growing awareness of having a disorder.31 Another possibility is that parents’ belief in the seriousness of illness may have increased over time and led to enhanced adherence.14 Such findings, however, need careful interpretation since a longer duration of illness may concurrently be a longer treatment duration. Those patients having a long treatment duration may have a greater tendency to adhere, and will also be more likely to engage in research.32
All the adherence measures were significantly correlated with each other. The clinician rating scale score showed a relatively lower Kappa coefficient than did the other two adherence measures (the pill count and even self-report). Furthermore, in the non-adherent group, the clinician rating scale scored higher than that of the other adherence measures. These findings are in line with the results from a previous study and suggest that it may be difficult for a clinician to detect medication nonadherence in a clinical setting.13 This finding underscores the need to utilize more accurate and objective measurement in practice.
Age was not significantly correlated to adherence measures. However, in a previous study based on the adult population, age was significantly correlated with some adherence measures. There are a few possible explanations for such differences. First, cognitive decline, as well as physical decline, is often associated with a change in adherence level in the older population.33 Adolescence, on the other hand, is a time of maturation, and factors associated with lower adherence due to advancement in age do not influence them. Another possibility is that the age range included in our study was relatively narrow. Such a narrow age range is less likely to provide statistically significant results. Also, when we evaluated the direct relationship between medication adherence and social support, parenting stress index, and side effects, the correlation was not significant. This finding was also seen in previous studies highlighting various individual, psychosocial, and environmental factors that influence a patient’s adherence.28 The influence of these factors may also vary within the same person throughout different stages of development.34 Therefore, such factors may require longitudinal observation in order to allow immediate and appropriate intervention over the years.
Based on reports of previous studies, we have inferred that parental stress, nevertheless, impacts the onset and course of depression in adolescents.25 Surprisingly, there was no correlation between adherence and parental stress level apparent in our study. Such findings may be a sign of the acquisition of independence in adolescents. Parenting stress level seems to have less significant influence during the phase of transition from dependence to independence. Although environmental factors do influence adolescents, individual factors such as the perception of the disease or treatment may play an important role as well. This underscores the importance of including and letting adolescents play an active role in the treatment process.35
In a previous study, adolescents that are adherent to medication had lower severity of depressive symptoms throughout treatment.29 The participants in this study were, however, in a relatively stable state because they showed relatively low mean HRSD scores, and they did not need to change their medication for at least 1 month before the study. In this sense, we can speculate that such factors believed to be important in the management of depression do not show a clear and simple direct relationship with adherence in stable patients with depressive disorders.
These results, however, need to be interpreted in the context of the following limitations: (1) Although the patients were not informed about the exact results of the MEMS measurements, they were made aware that this study intended to understand adherence. This might have encouraged adherence during the study’s relatively short duration, and so the adherence rates might have been inflated by this. (2) The relatively small number of participants and the use of different antidepressants could have influenced the results. Therefore, our findings must be replicated by future studies with larger samples based on more extended follow-up periods.
Conclusion
Subjective measures of adherence and the adherence measured by the MEMS do not show a significant correlation. This suggests that relying on the patient’s subjective reports regarding treatment adherence may be unreliable. Application of objective measures of adherence, such as the MEMS and pill count, may be needed to accurately interpret adherence, allowing clinicians to better guide their patients with appropriate education, hence improving adherence. Longer duration of illness seems to better influence and improve adherence. In this sense, patients’ improved awareness and understanding of the disease may be the key to better adherence. For better adherence and, eventually, a better clinical outcome, more variables for adolescents with a depressive disorder should be studied in the future.
Acknowledgment
The abstract of this research has been presented at the 66th annual meeting of the American Academy of Child and Adolescent Psychiatry.This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant numbers NRF- 2017R1D1A1B03028672) and a grant from Boryung Pharmaceutical Co., Ltd.
Disclosure
Dr Young Eun Mok reports grants from National Research Foundation of Korea, grants from Boryung Pharmaceutical Co., during the conduct of the study. Prof. Dr. Moon-soo Lee reports grants from Korea Research Foundation, grants from Boryung Pharmaceutical company, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547.
2. Gore FM, Bloem PJ, Patton GC, et al. Global burden of disease in young people aged 10–24 years: a systematic analysis. The Lancet. 2011;377(9783):2093–2102. doi:10.1016/S0140-6736(11)60512-6
3. Jane Costello E, Erkanli A, Angold A. Is there an epidemic of child or adolescent depression? J Child Psychol Psychiatry. 2006;47(12):1263–1271. doi:10.1111/j.1469-7610.2006.01682.x
4. Windfuhr K, While D, Hunt I, et al. Suicide in juveniles and adolescents in the United Kingdom. J Child Psychol Psychiatry. 2008;49(11):1155–1165. doi:10.1111/j.1469-7610.2008.01938.x
5. Maughan B, Collishaw S, Stringaris A. Depression in childhood and adolescence. J Can Acad Child Adolescent Psychiatry. 2013;22(1):35.
6. Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. The Lancet. 2012;379(9820):1056–1067. doi:10.1016/S0140-6736(11)60871-4
7. Thapar A, Collishaw S, Pine DS, Thapar AKJTL. Depression in adolescence. 2012;379(9820):1056–1067.
8. Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolescent Health. 2007;41(3):256–262. doi:10.1016/j.jadohealth.2007.03.015
9. Hasler G, Pine D, Kleinbaum D, et al. Depressive symptoms during childhood and adult obesity: the Zurich Cohort Study. Mol Psychiatry. 2005;10(9):842–850. doi:10.1038/sj.mp.4001671
10. Bhatia SK, Bhatia SC. Childhood and adolescent depression. Am Fam Physician. 2007;75(1):73–80.
11. Keller MB, Hirschfeld R, Demyttenaere K, Baldwin D. Optimizing outcomes in depression: focus on antidepressant compliance. Int Clin Psychopharmacol. 2002;17(6):265–271. doi:10.1097/00004850-200211000-00001
12. Brown RT, Borden KA, Clingerman S. Adherence to methylphenidate therapy in a pediatric population: a preliminary investigation. Psychopharmacol Bull. 1985;21(1):28–36.
13. Fontanella CA, Bridge JA, Marcus SC, Campo JV. Factors associated with antidepressant adherence for Medicaid-enrolled children and adolescents. Ann Pharmacother. 2011;45(7–8):898–909. doi:10.1345/aph.1Q020
14. Byerly MJ, Thompson A, Carmody T, et al. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatric Serv. 2007;58(6):844–847. doi:10.1176/ps.2007.58.6.844
15. Byerly M, Fisher R, Whatley K, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133(2–3):129–133. doi:10.1016/j.psychres.2004.11.002
16. Kemp R, Hayward P, Applewhaite G, Everitt B, David A. Compliance therapy in psychotic patients: randomised controlled trial. BMJ. 1996;312(7027):345–349. doi:10.1136/bmj.312.7027.345
17. Yang J, Yoon B-M, Lee M-S, Joe S-H, Jung I-K, Kim S-H. Adherence with electronic monitoring and symptoms in children with attention deficit hyperactivity disorder. Psychiatry Investig. 2012;9(3):263. doi:10.4306/pi.2012.9.3.263
18. Quittner AL, Espelage DL, Drotar D. Measuring adherence to medical treatments in childhood chronic illness: considering multiple methods and sources of information. J Clin Psychol Med Settings. 2000;7(1):41–54. doi:10.1023/A:1009545319673
19. Remington G, Kwon J, Collins A, Laporte D, Mann S, Christensen B. The use of electronic monitoring (MEMS®) to evaluate antipsychotic compliance in outpatients with schizophrenia. Schizophr Res. 2007;90(1–3):229–237. doi:10.1016/j.schres.2006.11.015
20. Vanderkooy J, Kennedy SNH, Bagby RMC. Antidepressant side effects in depression patients treated in a naturalistic setting: a study of bupropion, moclobemide, paroxetine, sertraline, and venlafaxine. Can J Psychiatry. 2002;47(2):174–180. doi:10.1177/070674370204700208
21. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56. doi:10.1136/jnnp.23.1.56
22. Bang YR, Park JH, Kim SH. Cut-off scores of the children’s depression inventory for screening and rating severity in Korean adolescents. Psychiatry Investig. 2015;12(1):23. doi:10.4306/pi.2015.12.1.23
23. Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the multidimensional scale of perceived social support. J Pers Assess. 1990;55(3–4):610–617.
24. Eker D, Arkar H. Perceived social support: psychometric properties of the MSPSS in normal and pathological groups in a developing country. Soc Psychiatry Psychiatr Epidemiol. 1995;30(3):121–126. doi:10.1007/BF00802040
25. Haskett ME, Ahern LS, Ward CS, Allaire JC. Factor structure and validity of the parenting stress index-short form. J Clin Child Adolescent Psychol. 2006;35(2):302–312. doi:10.1207/s15374424jccp3502_14
26. Solis ML, Abidin RR. The Spanish version parenting stress index: A psychometric study. J Clin Child Adolescent Psychol. 1991;20(4):372–378. doi:10.1207/s15374424jccp2004_5
27. Lawrence I, Lin K. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;255–268.
28. Taddeo D, Egedy M, Frappier J-Y. Adherence to treatment in adolescents. Paediatr Child Health. 2008;13(1):19–24. doi:10.1093/pch/13.1.19
29. Nakonezny PA, Hughes CW, Mayes TL, et al. A comparison of various methods of measuring antidepressant medication adherence among children and adolescents with major depressive disorder in a 12-week open trial of fluoxetine. J Child Adolesc Psychopharmacol. 2010;20(5):431–439. doi:10.1089/cap.2009.0108
30. Shi L, Liu J, Fonseca V, Walker P, Kalsekar A, Pawaskar M. Correlation between adherence rates measured by MEMS and self-reported questionnaires: a meta-analysis. Health Qual Life Outcomes. 2010;8(1):99. doi:10.1186/1477-7525-8-99
31. Nigro G, Angelini G, Grosso SB, Caula G, Sategna-Guidetti C. Psychiatric predictors of noncompliance in inflammatory bowel disease: psychiatry and compliance. J Clin Gastroenterol. 2001;32(1):66–68. doi:10.1097/00004836-200101000-00015
32. Riekert KA, Drotar D. Who participates in research on adherence to treatment in insulin-dependent diabetes mellitus? Implications and recommendations for research. J Pediatr Psychol. 1999;24(3):253–258. doi:10.1093/jpepsy/24.3.253
33. Lee M-S, Lee H-Y, Kang S-G, et al. Variables influencing antidepressant medication adherence for treating outpatients with depressive disorders. J Affect Disord. 2010;123(1–3):216–221. doi:10.1016/j.jad.2009.10.002
34. Donohoe G, Owens N, O’donnell C, et al. Predictors of compliance with neuroleptic medication among inpatients with schizophrenia: a discriminant function analysis. Eur Psychiatry. 2001;16(5):293–298. doi:10.1016/S0924-9338(01)00581-8
35. Nevins TE. Non‐compliance and its management in teenagers. Pediatr Transplant. 2002;6(6):475–479. doi:10.1034/j.1399-3046.149.ptr1s077.1.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
