Back to Journals » Clinical Ophthalmology » Volume 9
Comparative analysis of the development of collateral vessels in macular edema due to branch retinal vein occlusion following grid laser or ranibizumab treatment
Authors Kokolaki AE, Georgalas I , Koutsandrea C, Kotsolis A, Niskopoulou M, Ladas I
Received 25 January 2015
Accepted for publication 7 April 2015
Published 3 September 2015 Volume 2015:9 Pages 1519—1522
DOI https://doi.org/10.2147/OPTH.S81576
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Afroditi Eleni Kokolaki, Ilias Georgalas, Chryssanthi Koutsandrea, Athanasios Kotsolis, Maria Niskopoulou, Ioannis Ladas
Department of Ophthalmology, University of Athens, Athens, Greece
Purpose: To evaluate the differences in the development of collateral vessels in patients with macular edema due to branch retinal vein occlusion (BRVO) after treatment with either grid laser or ranibizumab (RNB).
Methods: Comparative study including patients with macular edema due to acute BRVO and best-corrected visual acuity (BCVA) between 20/40 and 20/200. The sample was divided into two groups according to the treatment applied: laser group, including eyes treated with Argon laser when retinal hemorrhages were sufficiently absorbed to perform the treatment, and RNB group, including patients treated initially with one monthly intravitreal injection for a period of 3 months of RNB and more injections according to need thereafter.. Before treatment patients in both groups, received a complete ophthalmic examination, including BCVA, fundus examination, optical coherence tomography, fundus color photography, and fundus fluorescein angiography (FA). This same protocol of examination was repeated in every visit after treatment, except FA that was only repeated every 3 months. The detection of the collateral vessels was done by two experienced examiners based on the analysis of the early phase of the FA. If there was a discrepancy in their judgment, the criterion of a third examiner evaluating the FA was considered.
Results: Mean baseline BCVA was 0.86±0.26 and 0.82±0.25 (logMAR [logarithm of the minimum angle of resolution]) in the RNB and laser groups, respectively (P=0.83). At the end of the follow-up, mean BCVA was 0.38±0.18 and 0.64±0.33 (logMAR) in the RNB and laser groups, respectively. The difference in the final BCVA between both groups was statistically significant (P=0.002). Collaterals developed in both groups; 66.67% of patients (14 out of 21 patients) developed collaterals at a mean time of 6.14±2.60 months after diagnosis in the RNB group, and 68.18% (16 out of 22 patients) developed collaterals in the laser group at a mean time of 6.2±1.97 months after diagnosis. No statistically significant differences between groups were found in the number of cases developing collateral vessels (P=1.00) as well as in the time required for such development (P=0.947).
Conclusion: The use of RNB for the treatment of macular edema due to BRVO does not seem to alter the development of collateral vessels. Future studies with larger samples are required to confirm these outcomes.
Keywords: collateral vessels, macular edema, branch retinal vein occlusion, laser, ranibizumab treatment
Introduction
Retinal vein occlusion is the second most common retinal vascular disorder after diabetic retinopathy and is considered to be an important cause of visual loss.1
Especially for branch retinal vein occlusion (BRVO), compression of an arteriosclerotic artery over a subjacent vein is thought to be the most common pathophysiologic mechanism in this disease, leading to venous engorgement, hemorrhages, and retinal edema.2 In this specific condition, collateral vessels, that drain the venous blood into adjacent areas, often develop in the initial months or years that drain the venous blood into adjacent areas and may result in an anatomical or even functional improvement.3
The Branch Retinal Vein Occlusion Study showed that grid laser photocoagulation of the leaking area was beneficial for the treatment of macular edema due to BRVO.4 Additionally, recent studies have shown the efficacy of anti-VEGF agents as a therapy for BRVO.5–8 Despite the good results of this treatment option, concerns have been raised on whether anti-VEGF drugs may have a negative impact on the development of collateral vessels, which would be an unfavorable factor for this therapeutic option.
The purpose of this study was to compare the development of collateral vessels in patients with macular edema due to BRVO treated with either grid laser or ranibizumab (RNB) (an anti-VEGF agent).
Methods
Patients
This comparative study included a total of 43 patients with macular edema due to BRVO treated with intraocular injections of 0.5 mg RNB or grid laser at the University Ophthalmology Clinic of the University of Athens, Greece. The inclusion criteria for the study were the presence of macular edema with central retinal thickness (CRT) of at least 250 μm due to acute (1–3 months) BRVO and best-corrected visual acuity (BCVA) between 20/40 and 20/200. Patients with any previous treatment for the BRVO or with any other retinal disease were excluded. The study was approved by the institutional review board. The patients were informed about the study and gave their consent, following the tenets of the Declaration of Helsinki of 1975 (revised in Tokyo in 2004).
Patients were randomized into two groups according to the treatment option used: the laser group and the RNB group. In the first group, patients were treated with Argon green laser when the retinal hemorrhages had been sufficiently absorbed to perform the treatment. Spots of 100 μm were applied in a grid pattern over the leaking area outside the foveal avascular zone that was defined according to the fluorescein angiography (FA) pattern. If BCVA remained below 20/40 and CRT was of more than 250 μm at 3 months after the initial treatment, then the grid laser treatment was repeated. In the RNB group, patients were treated initially with one monthly intravitreal injection for a period of 3 months of RNB 0.5 mg and examined every month thereafter. Retreatment was performed if BCVA after treatment was below 20/40 and CRT was more than 250 μm.
Examination protocol
Before treatment in each group, patients received a complete visual and ocular examination, including BCVA measured with standard Snellen charts, dilated fundus examination with slit lamp biomicroscopy, optical coherence tomography (OCT) exam (Stratus, V. 4.0, Carl Zeiss Meditec AG, Jena, Germany), fundus color photography, and fundus FA (TRC-50DX, Topcon, Tokyo, Japan). This same protocol of examination was repeated in every visit after treatment, except FA that was only repeated every 3 months. The detection of the collateral vessels was done by two experienced examiners based on the analysis of the early phase of the FA. If there was a discrepancy in their judgment, the criterion of a third examiner evaluating the FA was considered. If no collaterals were detected during the initial 12 months of follow-up in any patient, this patient was considered as not having developed collaterals.
Statistical analysis
All visual acuities were converted to logMAR (logarithm of the minimum angle of resolution) prior to analysis for statistical purposes. The Wilcoxon matched paired test and the Fisher’s exact test were used for the statistical analysis of the data. A P-value of less than 0.05 was considered to be the threshold for statistical significance.
Results
A total of 43 patients with BRVO were included and divided into two groups according to the treatment received: the RNB group and the laser group between January 2009 and March 2010. Both groups were similar at baseline with respect to age, sex, severity and duration of disease, CRT and medical risk factors for retinal vascular disease. The mean follow-up time was 26.29±11.42 months and 26.73±12.93 months in the RNB and laser groups, respectively. The RNB group received a mean number of injections of 7.14±4.75 during the whole follow-up. In the laser group, the mean number of laser treatment sessions was 1.45±0.51 during the follow-up.
Visual outcomes
Mean baseline BCVA ± standard deviation was 0.86±0.26 logMAR and 0.82±0.25 logMAR in the RNB and laser groups, respectively (P=0.83). At the end of the follow-up period, mean BCVA was 0.38±0.18 logMAR and 0.64±0.33 logMAR in the RNB and laser groups, respectively (Table 1). The difference of this final BCVA for the two groups evaluated was statistically significant (P=0.002).
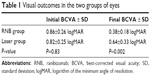  | Table 1 Visual outcomes in the two groups of eyes |
Development of collaterals
Collaterals developed in both groups; 66.67% of patients (14 out of 21 patients) developed collaterals at a mean time of 6.14±2.60 months after diagnosis of BRVO in the RNB group, and 68.18% (16 out of 22 patients) developed collaterals in the laser group at a mean time of 6.2±1.97 months after diagnosis (Table 2). In both groups, all collateral vessels developed within the retina. There was no statistically significant difference between groups in the mean number of patients that developed collaterals (P=1.00) as well as in the mean time at which collaterals developed during the follow-up (P=0.947).
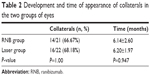  | Table 2 Development and time of appearance of collaterals in the two groups of eyes |
Discussion
Retinal collateral vessels in BRVO act as an alternative pathway of the blood flow from the obstructed to an adjacent unobstructed area. Collaterals are pre-existing vessels that do not carry any significant flow in normal eyes. However, in cases of vein occlusion, and due to the pressure alteration within the retinal vessels, the blood volume that flows in the collaterals increases.9 This bypass of the obstruction is considered to be very relevant for the natural course of the disease, as it leads to a reduction of edema and subsequently to a visual improvement at least in cases without a severely ischemic fovea. In the current study, we evaluated and compared the development of collateral vessels in patients with macular edema due to BRVO treated with two different treatment modalities: grid laser or RNB (anti-VEGF agent).
As VEGF is a significant factor for angiogenesis, anti-VEGF treatment may inhibit the development of collaterals, leading to a prolongation of the duration of the disease. However, in our series, the use of RNB did not alter the development of collaterals, as there was no statistically significant difference between the laser and RNB groups. The most probable explanation for this finding is that these collateral vessels were pre-existing vessels, as previously mentioned. These pre-existing vessels are engorged due to the increase of the intraluminal blood flow and, presumably, are not affected by a probable inhibition of the angiogenesis. Future studies are needed to confirm this hypothesis with a larger sample of cases. Im et al evaluated the outcomes of laser treatment in 45 patients with BRVO and found that collateral vessels were present in the angiographic analysis in 60% of patients during a follow-up ranging from 6 to 60 months, a value which is very similar to that found in the current series in the laser group (66.67%).10
To date, there is very limited scientific evidence concerning the development of collateral vessels in BRVO after treatment with anti-VEGF drugs. Ferrara et al reported the outcomes after treatment with bevacizumab of six eyes with central retinal vein occlusion (CRVO).11 In this series, none of the patients developed collateral vessels at the optic nerve head after a mean follow-up of 12 months. The authors suggested that due to the beneficial anatomical results of bevacizumab, collateral vessels might have been unnecessary to improve or maintain flow. This may explain the absence of collaterals after treatment in their series.11 Recently, Hayreh et al have concluded in a prospective study evaluating the cause of CRVOs that if there are no retinociliary collaterals, there are plenty of venous tributaries within the optic nerve and therefore there is no need for collaterals to develop on the disc.12 In any case, as CRVO is significantly different to BRVO with regards to pathogenesis and natural course, the results from the aforementioned studies cannot be compared with those obtained in our series. Weinberg et al showed that the treatment with intravitreal triamcinolone did not seem to influence the development of collaterals in BRVO or CRVO.3 The authors assume that the influence of steroids on the retinal vasculature is multifactorial. They concluded that it was not clear whether such influence might have any type of impact on the formation of collaterals.3
This is the first study comparing the development of collaterals in BRVO between two treatment modalities, grid laser and the use of an anti-VEGF drug. To date, only a comparison of the visual outcomes and macular thickness changes between these two modalities of treatment had been performed.13 Our study has some limitations that should be mentioned, such as the limited number of cases and the absence of a control group with patients receiving no treatment. Likewise, the length of the follow-up is approximately 26 months on average which may be considered as not sufficient. However, it has been shown that the development of the collateral vessels in BRVO occurs over a period of 6–24 months after the onset of the disease.9 In conclusion, the treatment of BRVO with RNB does not seem to inhibit the formation of retinal collaterals. Indeed, with this treatment modality the formation of collaterals is similar as in eyes treated with grid laser. Studies with larger series of patients are required to confirm this preliminary scientific evidence.
Disclosure
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
Orth DH, Patz A. Retinal branch vein occlusion. Surv Ophthalmol. 1978;22(6):357–376. | ||
Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina. 2013;33(5):901–910. | ||
Weinberg DV, Wahle AE, Ip MS, et al. Score Study Report 12: development of venous collaterals in the Score Study. Retina. 2013;33(2):287–295. | ||
Branch Retinal Vein Occlusion Study Group. Argon laser photocoagulation for macular oedema in branch retinal vein occlusion. Am J Ophthalmol. 1984;98(3):271–282. | ||
Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118(8):1594–1602. | ||
Brynskov T, Kemp H, Sørensen TL. Intravitreal ranibizumab for retinal vein occlusion through 1 year in clinical practice. Retina. 2014;34(8):1637–1643. | ||
Thach AB, Yau L, Hoang C, Tuomi L. Time to clinically significant visual acuity gains after ranibizumab treatment for retinal vein occlusion: BRAVO and CRUISE trials. Ophthalmology. 2014;121(5):1059–1066. | ||
Pacella E, Pacella F, La Torre G, et al. Testing the effectiveness of intravitreal ranibizumab during 12 months of follow-up in venous occlusion treatment. Clin Ter. 2012;163(6):e413–e422. | ||
Christoffersen NL, Larsen M. Pathophysiology and hemodynamics of branch retinal vein occlusion. Ophthalmology. 1999;106(11):2054–2062. | ||
Im CY, Lee SY, Kwon OW. Collateral vessels in branch retinal vein occlusion. Korean J Ophthalmol. 2002;16(2):82–87. | ||
Ferrara DC, Koizumi H, Spaide RF. Early bevacizumab treatment of central retinal vein occlusion. Am J Ophthalmol. 2007;144(6):864–871. | ||
Hayreh SS, Zimmerman MB, Podhajsky PA. Retinal vein occlusion and the optic disk. Retina. 2012;32(10):2108–2118. | ||
Tan MH, McAllister IL, Gillies ME, et al. Randomized controlled trial of intravitreal ranibizumab versus standard grid laser for macular edema following branch retinal vein occlusion. Am J Ophthalmol. 2014;157(1):237–247. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
