Back to Journals » International Journal of Nanomedicine » Volume 15
Combined Delivery of Temozolomide and siPLK1 Using Targeted Nanoparticles to Enhance Temozolomide Sensitivity in Glioma
Authors Shi H, Sun S, Xu H, Zhao Z, Han Z, Jia J, Wu D, Lu J , Liu H , Yu R
Received 27 December 2019
Accepted for publication 15 April 2020
Published 12 May 2020 Volume 2020:15 Pages 3347—3362
DOI https://doi.org/10.2147/IJN.S243878
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mian Wang
Hui Shi,1,2,* Shuo Sun,1,* Haoyue Xu,3,* Zongren Zhao,3 Zhengzhong Han,3 Jun Jia,3 Dongmei Wu,4 Jun Lu,4 Hongmei Liu,3,5 Rutong Yu1,3,5
1Clinical Medical College, Nanjing Medical University, Nanjing, People’s Republic of China; 2The Second People’s Hospital of Lianyungang, Lianyungang, People’s Republic of China; 3Institute of Nervous System Diseases, Xuzhou Medical University, Xuzhou, People’s Republic of China; 4Key Laboratory for Biotechnology on Medicinal Plants of Jiangsu Province, School of Life Science, Jiangsu Normal University, Xuzhou, Jiangsu, People’s Republic of China; 5Department of Neurosurgery, Affiliated Hospital of Xuzhou Medical University, Xuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Rutong Yu; Hongmei Liu Tel +86 13182310079
; +86 17716228111
Email [email protected]; [email protected]
Introduction: Temozolomide (TMZ) is the first-line chemotherapeutic option to treat glioma; however, its efficacy and clinical application are limited by its drug resistance properties. Polo-like kinase 1 (PLK1)-targeted therapy causes G2/M arrest and increases the sensitivity of glioma to TMZ. Therefore, to limit TMZ resistance in glioma, an angiopep-2 (A2)-modified polymeric micelle (A2PEC) embedded with TMZ and a small interfering RNA (siRNA) targeting PLK1 (siPLK1) was developed (TMZ-A2PEC/siPLK).
Materials and Methods: TMZ was encapsulated by A2-PEG-PEI-PCL (A2PEC) through the hydrophobic interaction, and siPLK1 was complexed with the TMZ-A2PEC through electrostatic interaction. Then, an angiopep-2 (A2) modified polymeric micelle (A2PEC) embedding TMZ and siRNA targeting polo-like kinase 1 (siPLK1) was developed (TMZ-A2PEC/siPLK).
Results: In vitro experiments indicated that TMZ-A2PEC/siPLK effectively enhanced the cellular uptake of TMZ and siPLK1 and resulted in significant cell apoptosis and cytotoxicity of glioma cells. In vivo experiments showed that glioma growth was inhibited, and the survival time of the animals was prolonged remarkably after TMZ-A2PEC/siPLK1 was injected via their tail vein.
Discussion: The results demonstrate that the combination of TMZ and siPLK1 in A2PEC could enhance the efficacy of TMZ in treating glioma.
Keywords: co-delivery, polymeric micelle, siPLK1, TMZ, drug resistance, glioma
Introduction
Glioma is the most common and aggressive primary brain tumor in the central nervous system (CNS), accounting for approximately 40% of brain tumors.1 The standard treatments for glioma include surgery, radiation therapy, and chemotherapy.2 However, the presence of the blood-brain barrier (BBB), genetic heterogeneity, and the ability to invade and/or infiltrate adjacent tissues of glioma cells,3,4 has resulted in poor therapeutic effects in glioma.5,6 The median survival time is 14.6 months and the five-year survival rates is less than 10%.7,8 Therefore, it is important to apply chemotherapy drugs to patients with glioma after surgery and to prevent postoperative recurrence.9–11
TMZ is a second-generation imidazotetrazine derivative and is a standard chemotherapeutic drug for gliomas.2 The cytotoxic effect of TMZ acts mainly through the methylation of DNA guanine and the formation of O-6-methylguanine (O6meG).12,13 In the process of DNA replication, O6meG mismatches thymine, resulting in ineffective mismatch repair and persistent G2 checkpoint arrest, eventually leading to apoptosis of tumor cells.13–15 However, the effectiveness of TMZ in clinical treatment is limited because of the following problems: 1) Most gliomas gradually develop drug resistance to TMZ during chemotherapy;5,14 2) the indiscriminate attack on DNA has been shown to cause damage to hematopoietic stem cells;13 3) TMZ is difficult to dissolve and is susceptible to rapid hydrolysis under physiological conditions;1 and 4) the obstruction of the BBB further limits its anti-glioma efficacy.16 In the last few years, many pre-clinical studies have used nanoparticles (NPs) as vehicles to deliver TMZ effectively to gliomas, and have resolved the last three questions above.1,13,16 However, drug resistance to TMZ has not been improved effectively.17 Therefore, there is an urgent need to find a new therapeutic strategies to limit the drug resistance to TMZ in glioma.
Polo-like kinase 1 (PLK1) is a serine/threonine protein kinase that is involved in spindle formation and chromosome segregation during mitosis, and plays a key regulatory role in the cell cycle.18,19 Clinical pathological data showed that the expression of PLK1 in glioma tissues was significantly higher than that in normal brain tissues.20 Inhibition of PLK1 can cause cell cycle arrest and increase apoptosis in glioma cells.21,22 Besides, studies have shown that small-molecule kinase inhibitors of PLK1 increase the sensitivity of tumor cells to chemotherapeutics.23–26 Koncar et al reported that a combination of TMZ and a PLK1 inhibitor, BI2536, significantly suppressed glioma tumor growth.27 Liu et al found that a PLK1 inhibitor combined with TMZ efficiently induced G2/M arrest and suppressed cell proliferation and sphere formation in glioma cells.28 These findings suggested that a combination treatment comprising TMZ and a PLK1 inhibitor could reverse TMZ resistance efficiently in glioma treatment. However, the clinical application of small-molecule kinase inhibitors is greatly affected by off-target and toxic effects.29,30 Knockdown of PLK1 using a small interfering RNA (siRNA) has become a new therapeutic strategy,31–34 and the US Food and Drug Administration (FDA) has approved the application of the siRNA therapy in clinical practice.35 Our previous study successfully delivered siPLK1 into glioma cells using hypoxia-responsive ionizable liposomes, which inhibited the growth of glioma cells efficiently, both in vitro and in vivo.36 However, to date, there has been no study on the combination treatment of TMZ and siPLK1 using a targeted NP delivery system.
In the present study, we constructed an NP drug delivery system to co-deliver TMZ and siPLK1 into glioma cells, with the hope of enhancing TMZ sensitivity and apoptosis in glioma treatment. We used the angiopep-2 (A2) to modify polymeric micelles, because A2-modified polymers can penetrate the BBB through receptor-mediated transport and accumulate in the brain in large quantities. Polymers modified by A2 to deliver drugs through the BBB have achieved certain effects in treating CNS diseases and malignant gliomas.37 TMZ was encapsulated by A2-poly(ethyleneglycol) (PEG)-poly(ethylenimine) (PEI)-poly(Ɛ-caprolactone) (PCL) (A2PEC) micelles through hydrophobic interactions. Then, siPLK1 was complexed with the TMZ-A2PEC micelles through electrostatic interaction. TMZ-A2PEC/siPLK1 could promote the penetration of siPLK1 across the BBB and protect siPLK1 from degradation. In addition, the combined delivery of TMZ and siPLK1 enhanced the sensitivity of glioma cells to TMZ, consequently increasing its anti-tumor activity both in vitro and in vivo.
Materials and Methods
Materials
Ortho-pyridyl disulfide (OPSS)-PEG-succinimidyl valeric acid (SVA) (OPSS-PEG-SVA) was obtained from Laysan Bio, Inc (Tower Drive, Arab, AL, USA). PCL5000-PEI2000 was purchased from Xian Ruixi Biological Technology Co., Ltd (Xian, China). TMZ and D-Luciferin potassium salt were obtained from Dalian Meilun Biotech Co., Ltd (Dalian, People’s Republic of China). 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) was obtained from Nanjing KeyGEN BioTECH Co. Ltd (Nanjing, People’s Republic of China). HypoxyprobeTM-1 Plus Kit was purchased from Hypoxyprobe, Inc. (Burlington, MA, USA). Angiopep-2 (TFFYGGSRGKRNNF KTEEY) was purchased from GL Biochem Ltd (Shanghai, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Beijing Zhongshuo Pharmaceutical Technology Development Co., Ltd. LysoTracker™ red was bought from Invitrogen (Carlsbad, CA, USA). PLK1 (208G4) Rabbit monoclonal antibodies (mAbs) were obtained from Cell Signaling Technology Co., Ltd (Danvers, MA, USA). Beta-actin mAbs were bought from Proteintech Antibodies People Trust (Chicago, IL, USA). DiOC187 (DiR) was brought from Suzhou Biosyntech Co., Ltd (Suzhou, People’s Republic of China). FAM-labeled siRNA (FAM-siRNA), negative control siRNA with a scrambled sequence (nonsense, antisense strand, 5′-ACGUGACACGUUCGGAGAAdTdT-3′), and siRNA targeting PLK1 mRNA (siPLK1, antisense strand, 5′-AGAUCACUCUCCUCAACUAUU-3′) were purchased from GenePharma Co. Ltd. (Shanghai, People’s Republic of China).
Methods
Nanoparticle Preparation
OPSS-PEG-SVA and A2 (molar ratio: 10:1) were dissolved in dimethyl sulfoxide (DMSO, Sigma, Neustadt, Germany). The reaction mixture was stirred gently at room temperature for 36 h, filtered, dialyzed against deionized water (molecular weight cut off: 1 kDa), and lyophilized to obtain A2-modified OPSS-PEG-SVA (A2-OPSS-PEG-SVA).
A2-OPSS-PEG-SVA (1 mg) and PCL5000-PEI2000 (2 mg) were completely dissolved in acetone and vortexed vigorously for 2 min at room temperature. The mixture was dripped into pure water and stirred with a magnetic stirrer for 30 min and purified by membrane dialysis (molecular weight cut off: 8000 Da) against water for 24 h. This process formed A2-PEG-PEI-PCL, which was abbreviated as A2PEC. TMZ-A2PEC was prepared using A2-OPSS-PEG-SVA (1 mg), PCL5000-PEI2000 (2 mg), as TMZ (2 mg) according to the above method.
A predetermined (eg, 0.1 µg) amount of siPLK-1 A or negative control siRNA (NCsiRNA) was mixed with a certain amount of TMZ-A2PEC micelle solution. The mixture was vortexed for 15 s and then left for 30 min at room temperature. By this means, a series of siRNA and TMZ-loaded nanocomplexes (TMZ-A2PEC/siPLK1 and TMZ-A2PEC/NCsiRNA) were formed according to different nitrogen/phosphate (N/P) ratios. Nanocomplexes without TMZ (A2PEC/siPLK1) or containing FAM-labeled siRNA (A2PEC/FAM-siRNA) were prepared in the same way.
Characterization of NPs
The nanocomplexes were freshly prepared. The mean size and zeta potential were determined using a Malvern Zetasizer Nano instrument (Malvern Panalytical, Malvern, UK). For each sample, data obtained from three measurements were averaged to yield the mean size and zeta potential. The morphology of the NPs was detected using transmission electron microscopy (TEM, FEI Tecnai G2 T12; Thermo Fisher Scientific,Waltham, MA, USA).
Gel Retardation Assay
The binding ability of the siRNA to the TMZ-loaded micelles was estimated using agarose gel electrophoresis. Gel electrophoresis was performed using 2% (w/v) agarose gel in TAE buffer with 0.5 µg mL−1 of ethidium bromide (EtBr). Complexation of siRNAs at various N/P ratios 0, 0.3, 0.5, 1, 1.5, and 2 were prepared for electrophoresis. Samples were run at 110 V for 15 min. The result was visualized using the UV exposure.
Drug Loading Efficiency
The TMZ-loading efficiency, defined as the weight percentage of TMZ in the micelle, was determined using a high performance liquid chromatography (HPLC) system equipped with a column (C18, 5 µm, 200 mm, Dikma Technologies Inc., Foothill Ranch, CA, USA). First, the TMZ-loaded micelle was dissolved in acetone, and a calibration curve was obtained using acetone solutions with different TMZ concentrations. Then, the TMZ-loaded micelle solutions were lyophilized, weighed, and redissolved in acetone. The mixture was centrifuged for 30 min using a table-top centrifuge (Micro 17R, HANIL SME, Co., Ltd., Seoul, Korea). After centrifugation, the upper phase of the mixture was measured spectrophotometrically at 328 nm. The loading efficiency and loading content of TMZ were calculated using the following formulas:
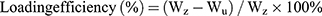
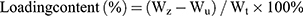
Wz: the weight of the initial TMZ (mg); Wu: the weight of TMZ detected in the upper phase after centrifugation (mg); Wt: the total weight of lyophilized NPs (mg).
Cell Culture
The glioma cell lines LN-229, T98G, and U87 cell lines were obtained from the Shanghai Cell Bank, Type Culture Collection Committee, Chinese Academy of Sciences. U87-GFP-Luci cells were transformed with the luciferase gene. U87R, a TMZ-resistant clone of U87, was established by treating U87 cells with a low dose of TMZ in DMEM for 3 weeks. All cells were routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (heat-inactivated) (FBS, Gibco). The cells were cultured at 37 °C in a humidified atmosphere of 5% CO2.
Cellular Uptake
FAM-siRNA was used as fluorescent probe of the NPs to assess their cellular uptake. Free FAM-siRNA, PEC/FAM-siRNA,and A2PEC/FAM-siRNA were prepared. U87R cells were seeded in 12-well culture plates at a density of 5 × 104 and incubated at 37 °C in a humidified atmosphere of 5% CO2 with DMEM containing 10% FBS (heat-inactivated) for 24 h. The old medium was then replaced with serum-free medium. Free FAM-siRNA, PEC/FAM-siRNA, and A2PEC/FAM-siRNA at a dose of FAM-siRNA 1 µg mL−1 were added, and after the cells were incubated for 4 h, they were trypsinized, centrifuged at 2000 rpm for 5 min, washed three times with cold phosphate-buffered saline (PBS), and resuspended in 500 µL of PBS. Later, the cells were measured using a BD FACSCalibur Flow Cytometer (BD Biosciences, San Jose, CA, USA).
Confocal Laser Scan Microscopy
To assess the cellular uptake and endosomal escape of the NPs, confocal laser scanning microscopy (CLSM) was used. U87R cells were seeded in a 35-mm glass bottom culture dish at a density of 5 × 104 and incubated at 37 °C in a humidified atmosphere of 5% CO2 for 24 h. The old medium was then replaced by serum-free medium. A2PEC/FAM-siRNA at a dose of FAM-siRNA of 1 µg mL−1 was added and the cells were incubated for 2 h and 4 h, respectively. Later, the medium was removed, the cells were stained with Hoechst 33342 (1: 1,000) for 10 min, and then washed three times with PBS. The cells were then stained with LysoTracker Red (1: 10,000). The CLSM imaging was conducted using an FV10i laser scanning microscope (Olympus, Tokyo Japan). Hoechst 33342, FAM-siRNA, and LysoTracker Red were excited at 352, 480, and 577 nm, respectively.
In vitro Cytotoxicity Assay
An MTT assay was performed to assess the cytotoxicity of the nanocomplexes. LN-229 and U87R cells were seeded at a density of 5 × 103 cells/well in 96-well plates and incubated for 12 h. There were six dosage regimens: 1) PBS; 2) free TMZ; 3) free siPLK1; 4) A2PEC micelles complexed with siPLK1 (A2PEC/siPLK1); 5) A2PEC micelles loaded with TMZ and NCsiRNA (TMZ-A2PEC/NCsiRNA); and 6) A2PEC micelles loaded with TMZ and siPLK1 (TMZ-A2PEC/siPLK1). The amount of applied siRNA per well in cell culture was set to 1 µg mL−1, the amount of TMZ per well was 15 µg mL−1. The cells were then incubated for 48 h before 20 μmL of MTT solution (5 mg/mL) in PBS buffer was added to each well and the cells were cultured for another 4h. After the removal of the MTT medium, 150 µL of DMSO was added to each well. The optical density (OD) at 570 nm was measured using spectrophotometric analysis.
Cell Cycle Analysis
LN-229 and U87R cells were seeded at a density of 5 × 104 cells/well in 12-well plate and incubated for 12 h. The cells were then treated with PBS, free TMZ, free siPLK1, A2PEC/siPLK1, TMZ-A2PEC/NCsiRNA, or TMZ-A2PEC/siPLK1. Treatments were given at doses of 15 µg mL−1 TMZ and 1 µg mL−1 siRNA. After the cells were incubated for 48 h, these cells were harvested and washed three times with PBS. Then cells were centrifugated (1,000 rpm, 10 min), the supernatant was discarded, and the cell pellet was fixed in 70% ethanol at 4 °C for 24 h. Before analysis, the cells were washed once in PBS, stained with 500 µL PI/RNase for 15 min in the dark at room temperature. Analyses were performed using a BD FACSCalibur Flow Cytometer. Cell Cycle distributions were calculated using BD FACS Diva software (Verity Software House).
Cell Apoptosis Study
LN-229 and U87R cells were seeded at a density of 1 × 105 cells/well in 6-well plate and cultured at 37 °C in a 5% CO2 for 24 h. The cells were then treated with different micelle formulations (PBS, free TMZ, free siPLK1, A2PEC/siPLK1, TMZ-A2PEC/NCsiRNA, and TMZ-A2PEC/siPLK1). Treatments were given at doses of 15 µg mL−1 TMZ and 1 µg mL−1 siRNA. After the cells were incubated for 48 h, they were trypsinized, collected, washed three times with PBS, and resuspended in 500 μL of binding buffer. Then, 5 μL of Annexin V-FITC and 5 μL PI were added, and cultured with the cells for 15 min in the dark. Finally, cell apoptosis was evaluated using a flow cytometer.
Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction Analysis
The expression levels of PLK1 mRNA in U87R cells of different groups were evaluated using quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR). Total RNA was extracted from cell using the TRIzol reagent (TIANGEN, Beijing, People’s Republic of China) according to the manufacturer’s instructions. The extracted RNA was digested with DNase I to remove possible DNA contamination. First strand cDNA was synthesized with the RNA which had been purified again using the TRIzol reagent, using a reverse transcription kit. Finally, the quantitative analysis of the cDNA was performed using qPCR in an ABI 750 machine (ABI, Foster City, CA, USA). The GAPDH (encoding glyceraldehyde-3-phosphate dehydrogenase) mRNA level was measured as an internal normalization standard. All primers were synthesized by Sangon Biotech (Shanghai, People’s Republic of China).
Western Blotting Analysis
The PLK1 protein was detected in U87R cells using Western blotting. U87R cells (2 × 105) were seeded in a 6-well culture plate and incubated at 37 °C in a 5% CO2 for 24 h. The cells were then treated with PBS, free TMZ, free siPLK1, A2PEC/siPLK1, TMZ-A2PEC/NCsiRNA, or TMZ-A2PEC/siPLK1 at doses of 15 µg mL−1 TMZ and 1 µg mL−1 siRNA for 24 h. The cells were then harvested, washed twice with cold PBS, and lysed. The lysis buffer was centrifuged (12,000 rpm, 10 min) to remove impurities. Quantities and equilibriums of the extracted protein samples were measured using a BCA kit (Beyotime, Shanghai, People’s Republic of China). The proteins were then separated using SDS-PAGE and transferred onto a nitrocellulose membrane using SemiDry blot apparatus (Invitrogen). The membrane was then incubated with primary antibodies overnight and secondary antibodies for 1.5 h. Finally, the membranes were exposed to x-ray films in a dark room. The band densities were quantified using the Image J Software (NIH. Bethesda, MD, USA. The expression of O-6-Methylguanine-DNA Methyltransferase (MGMT) in T98G, LN-229, U87, and U87R cells were evaluated using the same method.
Glioma Model
BALB/c nude mice (male, 5 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All animals received care following the guidelines for the Guide for the Care and Use of Laboratory Animals. All experiment procedures were approved by Xuzhou Medical University of China Animal Care and Use Committee (license no. SYXK 2002–0038; Jiangsu, china). The glioma model was constructed by intracranial injection (striatum, 1.8 mm right lateral to the bregma and 3 mm in depth) of 8 × 105 U87-luci cells suspended in 7 µL of L15 medium into the male nude mice. After 10 days of xenograft glioma, the intensity of luciferase fluorescence was measured using an in vivo imaging system (Caliper, Princeton, NJ, USA) to confirm successful construction of the nude mice glioma model. Thereafter, the nude mice were randomly divided into six groups (n = 6) and housed in a controlled-temperature room with highly sterilized standard feed and water.
Biodistribution Studies
The biodistribution studies were determined after the nude mice glioma model was proven to be constructed successfully. PBS, free DiR, and A2PEC/DiR (the dose of DiR was 1 mg kg−1) were injected through the tail vein, respectively. The nude mice were sacrificed 4 h after administration, and the glioma model brains and their organs (heart, liver, spleen, lung and kidney) were collected. The in vivo imaging system (Caliper) was used to observe the intensity of DiR staining.
In vivo Antitumor Efficacy
After U87 cells were implanted for 10 days, the mice were divided into six groups (n = 6). Each group received an i.v. injection via the tail vein with PBS, free TMZ, free siPLK1, A2PEC/siPLK1, TMZ-A2PEC/NCsiRNA, or TMZ-A2PEC/siPLK1, respectively. The bioluminescence intensity which was used to measure the size of glioma was 9×107 µw cm−2 when the therapy was initiated. All groups were treated on day 12, day 14, day 16, and day 18 at doses of 10 mg kg−1 TMZ and 1 mg kg−1 siRNA. The mice were imaged again using the in vivo imaging system (Caliper) on day 20 and day 30. During the in vivo experiment, the survival time of each group was observed, and the weight of the mice was also recorded every two days.
Organ Safety Evaluation
The U87-Luci tumor-bearing mice received injections of PBS, free TMZ, free siPLK1, A2PEC/siPLK1, TMZ-A2PEC/NCsiRNA, and TMZ-A2PEC/siPLK1 at doses of 10 mg kg−1 TMZ and 1 mg kg−1 siRNA on days 12, 14, 16, and 18. One day after the last treatment, three mice from each group were sacrificed, their organs (heart, liver, spleen, lung and kidney) were collected and sections of the organs were stained with hematoxylin and eosin (H&E) for histological examination.
Statistical Analysis
Statistical analysis of the data was performed using analysis of variance (ANOVA) and Student’s t-tests, P values < 0.05 was considered statistically significant. The results were expressed as mean and mean ± SD in the figures (*P < 0.05, **P < 0.01, ***P < 0.001).
Results
Preparation and Characterization of TMZ-A2PEC/siPLK1
TMZ-A2PEC/siPLK1 was synthesized as outlined in Figure 1. TMZ was encapsulated into the micelle core through hydrophobic interactions to form TMZ-A2PEC. The naked siPLK1 was easily complexed with the TMZ-A2PEC via electrostatic interaction to form TMZ-A2PEC/siPLK1. Agarose gel electrophoresis showed that siPLK1 was completely adsorbed by TMZ-A2PEC when the N/P ratio was 1:1 (Figure 2A). The TEM image showed that TMZ-A2PEC/siPLK1 had a regular spherical shape (Figure 2B). The average particle sizes of A2PEC, TMZ-A2PEC, and TMZ-A2PEC/siPLK1 were 72.94 nm, 76.56 nm, and 82.22 nm, respectively (Figure 2C [a-c]). The zeta potentials of A2PEC, TMZ-A2PEC, and TMZ-A2PEC/siPLK1 were 26.00 mV, 26.60 mV, and 18.80 mV, respectively (Figure 2D [a-c]). The TMZ loading efficiency and loading content of TMZ-A2PEC/siPLK1 were 91.69% and 9.17%, respectively.
Cellular Uptake and Endosomal Escape
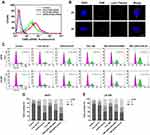
The cellular uptake of the A2PEC/FAM-siRNA was investigated after the U87R cells were treated with PBS, free FAM-siRNA, PEC/FAM-siRNA, and A2PEC/FAM-siRNA (Figure 3A). The flow cytometry results indicated that the cells incubated with A2PEC/FAM-siRNA showed the strongest FAM-siRNA fluorescence among all the groups. This result indicated that the A2 modification enhanced the cellular uptake of A2PEC/FAM-siRNA. In addition, to estimate the effect of endosome escape of the A2PEC/FAM-siRNA in glioma cells, CLSM was used. Cellular endosome escape was monitored after the glioma cells were incubated with A2PEC/FAM-siRNA for 2 and 4 h (Figure 3B). At 2 h, the green FAM-siRNA overlapped with the red endosomes, indicating the uptake of A2PEC/FAM-siRNA into the cytosol. However, at 4 h, the green FAM-siRNA deviated from red endosomes, indicating that the A2PEC/FAM-siRNA had escaped from the endosomes.
Cell Cycle Arrest
We treated U87R and LN-229 cells with PBS, free siPLK1, A2PEC/siPLK1, free TMZ, TMZ-A2PEC/NCsiRNA, and TMZ-A2PEC/siPLK1 for 48 h, separately, and observed the cell cycle arrest using flow cytometry (Figure 3C–E). The percentages of U87R cells in the G2/M were 6.82% (PBS), 10.81% (free siPLK1), 29.96% (A2PEC/siPLK1), 18.7% (free TMZ), 24.14% (TMZ-A2PEC/NCsiRNA), and 40.33% (TMZ-A2PEC/siPLK1). However, in the LN-229 cells, the percentages were 8.47% (PBS), 14.20% (free siPLK1), 32.64% (A2PEC/siPLK1), 19.38% (free TMZ), 25.20% (TMZ-A2PEC/NCsiRNA), and 43.49% (TMZ-A2PEC/siPLK1). These results showed that both siPLK1 and TMZ alone could induce G2/M arrest, and when the cells were treated with the TMZ-A2PEC/siPLK1, the percentage of cells in the G2/M phase was the highest among the groups. These findings indicated that siPLK1 could enhance sensitivity to TMZ by inducing cell cycle arrest, and also explained why more cells could enter the apoptosis phase after incubation with TMZ-A2PEC/siPLK1.
Cell Apoptosis
Annexin V-FITC/PI double-staining assay was used to quantitatively evaluate cell apoptosis by flow cytometry (Figure 4). The control group without treatment showed an extremely low apoptosis rate of 0.70%. In contrast, the cells incubated with the TMZ-A2PEC/siPLK1 showed the highest apoptosis rate of 20.91%. The free siPLK1, A2PEC/siPLK1, free TMZ, and TMZ-A2PEC/NCsiRNA treatments resulted in cell apoptosis rates of only 1.53%, 3.31%, 8.85%, and 10.55%, respectively. These results further demonstrated that the combination of siPLK1 and TMZ could effectively induce glioma cell apoptosis.
Gene Silencing Ability and Establishment of TMZ-Resistant Cell Line U87R
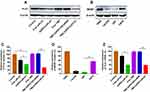
The transcription and translation of PLK1 were evaluated using qRT-PCR and Western blotting, respectively. QRT-PCR showed that treatment with TMZ-A2PEC/siPLK1 and A2PEC/siPLK1 significantly silenced PLK1 gene expression in glioma cells (Figure 5E), leading to 61% and 60% knockdown of PLK1 mRNA, respectively. Meanwhile, incubation of cells with free siPLK1 only resulted in 21% knockdown of PLK1 mRNA. However, the cells treated with PBS, free TMZ, and TMZ-A2PEC/NCsiRNA showed no knockdown efficiency. In the Western blotting experiment (Figure 5A), the TMZ-A2PEC/siPLK1 and A2PEC/siPLK1 groups exhibited significantly decreased PLK1 protein levels (by 68% and 50%) compared with that induced in the free siPLK1 group (30%); the control, free TMZ, and TMZ-A2PEC/NCsiRNA groups showed no effect on the PLK1 protein expression (Figure 5C). These results were consistent with the qRT-PCR data, and both indicated that TMZ-A2PEC/siPLK1 and A2PEC/siPLK1 exerted a strong gene silencing effect on PLK1.
It has been reported that the glioma cell lines with high MGMT levels demonstrate drug resistance in the presence of TMZ.38 To demonstrate that the U87R cells were resistant to the TMZ, the MGMT protein level was evaluated using Western blotting (Figure 5B). The MGMT protein level in U87R cells (59%) was significantly higher than that in U87 (8%) and LN-229 cells (11%), which were relatively sensitive to the TMZ (Figure 5D). These results suggested that the establishment of TMZ-resistant cell line U87R was successful.
In vitro Cell Viability Assay
The in vitro cell viability in U87R and LN-229 glioma cells was evaluated using MTT assays. In the U87R cells (Figure 6A), the cell viability of the TMZ-A2PEC/siPLK1 group (23%) was obviously lower than that of the free TMZ (50%) and TMZ-A2PEC/NCsiRNA groups (48%). However, the cell viability of cells treated with the free siPLK1 and A2PEC/siPLK1 were 89% and 84%, respectively. The results in the LN-229 cells (Figure 6B) were consistent with those in the U87R cells. These results showed that combined treatment with TMZ and siPLK1, mediated by A2-targeted NPs, yielded higher cytotoxicity than TMZ therapy alone.
 |
Figure 6 MTT assay of U87R (A) and LN-229 (B) glioma cells. Data are shown as mean ± standard deviation (n = 3), ***P < 0.001. |
Antitumor Therapy Efficacy
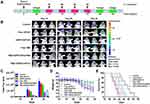
The ability of NPs loaded with TMZ and siPLK1 to cross the BBB and enter into glioma tissues is crucial for the anti-glioma therapy. For this purpose, the brain glioma models were constructed by intracranial injection of 8 × 105 U87-Luci cells into male nude mice. In vivo bioluminescence imaging demonstrated the existence of brain glioma (Figure 7A). The nude mice were injected with PBS, free DiR, and A2PEC/Dir through the tail vein, respectively. After 4 h, in vivo fluorescence imaging was performed (Figure 7B and 7C). The strongest DiR fluorescence was observed in the gliomas of the mice injected with A2PEC/DiR, indicating the accumulation of A2PEC/DiR in the brain gliomas. Meanwhile, little DiR fluorescence was observed after the mice were injected with free DiR. These results indicated that A2PEC could help TMZ and siPLK1 cross the BBB and enter into gliomas effectively.
The tissue biodistribution was examined after the nude mice were injected with PBS, free DiR, and A2PEC/DiR through the tail vein for 4 h, respectively (Figure 7D and 7E). The results showed that both the free DiR and A2PEC/DiR underwent a rapid and widespread distribution in the liver and kidney tissues within 4 h. However, the fluorescence intensity in the liver and kidney in the mice treated with A2PEC/DiR was significantly higher than that in mice treated with free DiR. These results showed that vector A2PEC can extensively prolong the retention time of serum siPLK1, and this increased circulation time would provide TMZ-A2PEC/siPLK1 with a better chance of accumulating in brain gliomas.
To further verify the efficacy of NPs in vivo, we constructed a glioma model using U87-Luci cells. The mice were divided into six groups (n = 6), and received PBS, free TMZ, free siPLK1, A2PEC/siPLK1, TMZ-A2PEC/NCsiRNA, and TMZ-A2PEC/siPLK1 injected via their tail vein, respectively (the dose of siPLK1 was 1 mg kg−1). The U87-Luci tumor-bearing mice were treated four times during the whole experiment (Figure 8A). The bioluminescence intensity was used to measure the glioma growth in the mice (Figure 8B). Tumors in the control group (PBS) grew rapidly. By day 30, the tumors in the control group, as reflected by the bioluminescence measurements (Figure 8C), were 26-fold larger than those at day 10. Following the control group, the bioluminescence measurements of free siPLK1 and A2PEC/siPLK1 groups at day 30 were 22-fold and 20-fold higher than those at day 10, respectively. The tumor growth rate in the mice that were treated with free TMZ, TMZ-A2PEC/NCsiRNA, and TMZ-A2PEC/siPLK1 were 7.8-fold, 5.7-fold and 1.4-fold higher than at day 10, respectively. This result indicated that TMZ-A2PEC/siPLK1 exhibited higher anti-glioma activity than the other treatments, and indicated that the combination of TMZ and siPLK1 could effectively enhance TMZ sensitivity to treat glioma.
To further estimate antitumor efficacy, the U87-Luci tumor-bearing mice were monitored for body weight change and median survival time (Figure 8D and 8E). The median survival times in the PBS, free siPLK1, A2PEC/siPLK1, free TMZ, TMZ-A2PEC/NCsiRNA, and TMZ-A2PEC/siPLK1 groups were 36, 36.5, 38.5, 41.5, 43, and 47.5 days, respectively (Figure 8E). The survival time of U87-Luci tumor-bearing mice treated with the TMZ-A2PEC/siPLK1 was significantly prolonged. These results indicated that TMZ-A2PEC/siPLK1 could effectively inhibit the growth of glioma. The dominance of TMZ-A2PEC/siPLK1 was also reflected by comparing the changes in body weight. The body weight of the mice treated with TMZ-A2PEC/siPLK1 decreased slowly decreased, while all other groups lost weight rapidly (Figure 8D).
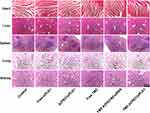
The toxicity of the different formulations toward normal tissues (heart, liver, spleen, lung, and kidney) was investigated (Figure 9). Compared with the control group, the H&E staining images of the major organs in the treatment groups indicated that no noticeable tissue damage or obvious changes in morphology. These results suggested that the combination of TMZ and siPLK1 delivered by the drug-loaded NP is effective in treating glioma and did not induce systemic toxicity in the tumor-bearing mice.
Discussion
Glioma is the most common type of primary CNS tumor, accounting for about 80% of all malignant brain tumors.39–42 Although treatment using surgery, postoperative chemotherapy, and radiotherapy have advanced significantly, patients’ prognosis is still poor, with an average survival rate of approximately one year after diagnosis.43–47 TMZ is the first line chemotherapy for glioma. Although it is used widely to treat patients with glioma, its effectiveness in treatment is not very high because of TMZ resistant glioma.48,49 PLK1 is a serine/threonine protein kinase that is an early trigger for G2/M conversion in the cell cycle.50 Inhibition of PLK1 expression can arrest the cell cycle in the G2/M phase, thereby increasing the effectiveness of chemotherapy drugs.27,28 Combining a PLK1 inhibitor and chemotherapy drugs has already been reported, and can alleviate the drug-resistance of tumor cells.27 To sensitize glioma to TMZ, we devised a vector that could co-deliver TMZ and siPLK1, and both the in vivo and in vitro experiments showed that the effectiveness of TMZ treatment of glioma improved significantly.
In the present study, the copolymer A2PEC was used as the nanocarrier, with TMZ encapsulated in the core through intermolecular hydrophobic interactions and siPLK1 attached to the cationic layer via electrostatic complexation. The average particle size and the zeta potential of TMZ-A2PEC/siPLK1 were 82.22 nm and 18.80 mV, respectively. These results indicated that TMZ-A2PEC/siPLK1 was suitable for brain targeted drug delivery. Besides, A2 was used to modify the nanocarrier to facilitate the cellular uptake of the codelivery system. The results showed that this nanocarrier provided remarkable efficacy in co-delivering TMZ and siPLK1 into U87R cells, and the A2 modified nanocomplexes had a much better delivery efficiency than the non-targeted ones. The CLSM results showed that the NPs had escaped from the endosome after the U87R cells were treated with A2PEC/FAM-siRNA for 4 h, which would result in enhanced gene silencing by siPLK1.
Gene silencing ability is the most important factor in siRNA therapies.33 Our results of qRT-PCR and Western blotting showed that TMZ-A2PEC/siPLK1 could silence PLK1 gene expression significantly, leading to downregulated PLK1 protein levels in glioma cells. Besides, the MGMT protein level in U87R cells, which is consistent with drug resistance to TMZ, was significantly higher than that in the U87 and LN-229 cells, suggesting the successful establishment of the TMZ-resistant cell line U87R. The results were consistent with those of Yang et al.51 PLK1 is an early trigger for G2/M conversion in the cell cycle, and whether the cell cycle can arrest in the G2/M phase by silencing the gene is a crucial factor.24,25 In this study, the results of the cell cycle arrest experiment showed that both U87R and LN-229 cells developed G2/M arrest, and the percentages of cells in G2/M that had been treated with TMZ-A2PEC/siPLK1 were 40.33% and 43.49%, respectively. The MTT and apoptosis assays showed that the combined treatment with TMZ and siPLK1 had a potent effect on cell apoptosis by suppressing the expression of PLK1, which resulted in an elevated in vitro anticancer effect.
To study the targeting effect to U87 cells, we constructed the U87-transplanted tumor model. Fluorescence imaging was performed after injecting DiR into the tail vein of the U87-Luci tumor-bearing mice. The results demonstrated that A2PEC could cross the BBB, and mainly accumulated in the liver and kidney. An in vivo study showed that the tumor growth rate in the mice treated with TMZ-A2PEC/siPLK1 was the lowest among all the groups. The survival time of U87-Luci tumor-bearing mice treated with the TMZ-A2PEC/siPLK1 was prolonged significantly and the body weight of these mice showed the slowest decrease in all groups. The results indicated that co-delivery of TMZ and siPLK1 using A2PEC could effectively enhance TMZ sensitivity, and significantly inhibit glioma growth. Moreover, the H&E staining images of major organs in the mice indicated that TMZ-A2PEC/siPLK1 caused no significant damage to other tissues and organs. Therefore, TMZ-A2PEC/siPLK1 has the potential to treat glioma.
In conclusion, we successfully prepared a safe and highly effective A2-modified nanocarrier, A2PEC, for the codelivery of TMZ and siPLK1. Both in vitro and in vivo studies demonstrated remarkably efficient co-delivery of the two therapeutic agents into glioma cells. Consequently, the PLK1 gene was effectively silenced, and the cell cycle of glioma cells was arrested in the G2/M, which improved the treatment effect of TMZ and led to increased tumor cell apoptosis. The animal study proved that the combined therapy of TMZ and siPLK1 mediated by A2-modified nanocarriers, inhibited tumor growth markedly and dramatically prolonged the survival time of glioma tumor-bearing mice. Thus, co-loading of TMZ and siPLK1 into A2PEC NPs could be an effective targeted delivery strategy for glioma treatment, reversing TMZ resistance and improving therapeutic efficacy.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (No. 81772665). This paper was also financed from Natural Science Foundation of Jiangsu Province (No. BK20150221), Jiangsu Provincial Commission of Health and Family Planning (No. Q201608). Research was supported by Six Talents Peak Foundation of Jiangsu Province (No. 2018-WSW-071), Research and Development Fund Project of Kangda College affiliated to Nanjing Medical University (No. KD2018KYJJYB048), Five-two-one Project Research Fund of Lianyungang (No. LYG52105-2018063).
Author Contributions
Conception and design: R. Yu, H. Liu, J. Lu, H. Shi. Development of methodology: S. Sun, H. Xu. Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): H. Shi, S. Sun, Z. Zhao, J. Jia. Analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis): H. Shi, Z. Han, Z. Zhao, J. Jia. Writing, review, and/or revision of the manuscript: H. Shi, S. Sun, R. Yu, H. Liu. Administrative, technical, or material support (ie, reporting or organizing data, constructing databases): J. Lu, D. Wu, R. Yu, H. Liu. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
No conflicts of interest were disclosed.
References
1. Hart MG, Garside R, Rogers G, Stein K, Grant R. Temozolomide for high grade glioma. Cochrane Database Syst Rev. 2013;4:CD007415. doi:10.1002/14651858.CD007415.pub2
2. Lam FC, Morton SW, Wyckoff J, et al. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat Commun. 2018;9. doi:10.1038/s41467-018-04315-4
3. Fang C, Wang K, Stephen ZR, et al. Temozolomide nanoparticles for targeted glioblastoma therapy. ACS Appl Mater Inter. 2015;7:6674–6682. doi:10.1021/am5092165
4. Bertucci A, Prasetyanto EA, Septiadi D, et al. Combined delivery of temozolomide and anti-miR221 PNA using mesoporous silica nanoparticles induces apoptosis in resistant glioma cells. Small. 2015;11:5687–5695. doi:10.1002/smll.201500540
5. Salazar N, Carlson JC, Huang K, et al. A chimeric antibody against ACKR3/CXCR7 in combination with TMZ activates immune responses and extends survival in mouse GBM models. Mol Ther. 2018;26:1354–1365. doi:10.1016/j.ymthe.2018.02.030
6. Qian L, Zheng J, Wang K, et al. Cationic core-shell nanoparticles with carmustine contained within O6-benzylguanine shell for glioma therapy. Biomaterials. 2013;34:8968–8978. doi:10.1016/j.biomaterials.2013.07.097
7. Liang P, Shi H, Zhu W, et al. Silver nanoparticles enhance the sensitivity of temozolomide on human glioma cells. Oncotarget. 2017;8:7533.
8. Wu M, Fan Y, Lv S, Xiao B, Ye M, Zhu X. Vincristine and temozolomide combined chemotherapy for the treatment of glioma: a comparison of solid lipid nanoparticles and nanostructured lipid carriers for dual drugs delivery. Drug Deliv. 2016;23(8):2720–2725. doi:10.3109/10717544.2015.1058434
9. Messaoudi K, Saulnier P, Boesen K, Benoit JP, Lagarce F. Anti-epidermal growth factor receptor siRNA carried by chitosan-transacylated lipid nanocapsules increases sensitivity of glioblastoma cells to temozolomide. Int J Nanomedicine. 2014;9:1479–1490. doi:10.2147/IJN.S59134
10. Xu X, Wang Z, Liu N, et al. Association between SOX9 and CA9 in glioma, and its effects on chemosensitivity to TMZ. Int J Oncol. 2018;53:189–202. doi:10.3892/ijo.2018.4382
11. Cheng D, Cao N, Chen J, Yu X, Shuai X. Multifunctional nanocarrier mediated co-delivery of doxorubicin and siRNA for synergistic enhancement of glioma apoptosis in rat. Biomaterials. 2012;33:1170–1179. doi:10.1016/j.biomaterials.2011.10.057
12. Clemente N, Ferrara B, Gigliotti C, et al. Solid lipid nanoparticles carrying temozolomide for melanoma treatment. Preliminary in vitro and in vivo studies. Int J Mol Sci. 2018;19:255. doi:10.3390/ijms19020255
13. Kim SS, Harford JB, Moghe M, Rait A, Pirollo KF, Chang EH. Targeted nanocomplex carrying siRNA against MALAT1 sensitizes glioblastoma to temozolomide. Nucleic Acids Res. 2018;46:1424–1440. doi:10.1093/nar/gkx1221
14. Pan H, Wang H, Jia Y, et al. VPA and MEL induce apoptosis by inhibiting the Nrf2-ARE signaling pathway in TMZ-resistant U251 cells. Mol Med Rep. 2017;16:908–914. doi:10.3892/mmr.2017.6621
15. Stupp R, Gander M, Leyvraz S, Newlands E. Current and future developments in the use of temozolomide for the treatment of brain tumours. Lancet Oncol. 2001;2:552–560. doi:10.1016/S1470-2045(01)00489-2
16. Li K, Liang N, Yang H, Liu H, Li S. Temozolomide encapsulated and folic acid decorated chitosan nanoparticles for lung tumor targeting: improving therapeutic efficacy both in vitro and in vivo. Oncotarget. 2017;8. doi:10.18632/oncotarget.22791
17. Yoo B, Ifediba MA, Ghosh S, Medarova Z, Moore A. Combination treatment with theranostic nanoparticles for glioblastoma sensitization to TMZ. Mol Imaging Biol. 2014;16:680–689. doi:10.1007/s11307-014-0734-3
18. Gutteridge REA, Ndiaye MA, Liu X, Ahmad N. Plk1 inhibitors in cancer therapy: from laboratory to clinics. Mol Cancer Ther. 2016;15:1427–1435. doi:10.1158/1535-7163.MCT-15-0897
19. Strebhardt K, Ullrich A. Targeting Polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–330. doi:10.1038/nrc1841
20. Jiang R, Lu X, Yang M, Deng W, Fan Q, Huang W. Monodispersed brush-like conjugated polyelectrolyte nanoparticles with efficient and visualized siRNA delivery for gene silencing. Biomacromolecules. 2013;14:3643–3652. doi:10.1021/bm401000x
21. Jeong SB, Im JH, Yoon J, et al. Essential role of Polo-like kinase 1 (Plk1) oncogene in tumor growth and metastasis of tamoxifen-resistant breast cancer. Mol Cancer Ther. 2018;17:825–837. doi:10.1158/1535-7163.MCT-17-0545
22. Czaplinski S, Hugle M, Stiehl V, Fulda S. Polo-like kinase 1 inhibition sensitizes neuroblastoma cells for vinca alkaloid-induced apoptosis. Oncotarget. 2016;7:8700. doi:10.18632/oncotarget.3901
23. Pezuk JA, Brassesco MS, Morales AG, et al. Inhibition of Polo-like kinase 1 induces cell cycle arrest and sensitizes glioblastoma cells to ionizing radiation. Cancer Biother Radio. 2013;28:516–522.
24. Sakurai Y, Hatakeyama H, Akita H, Harashima H. Improvement of doxorubicin efficacy using liposomal anti-Polo-like kinase 1 siRNA in human renal cell carcinomas. Mol Pharmaceut. 2014;11:2713–2719. doi:10.1021/mp500245z
25. Gleixner KV, Ferenc V, Peter B, et al. Polo-like kinase 1 (Plk1) as a novel drug target in chronic myeloid leukemia: overriding imatinib resistance with the Plk1 inhibitor BI 2536. Cancer Res. 2010;70:1513–1523. doi:10.1158/0008-5472.CAN-09-2181
26. Benoit DSW, Henry SM, Shubin AD, Hoffman AS, Stayton PS. pH-Responsive polymeric siRNA carriers sensitize multidrug resistant ovarian cancer cells to doxorubicin via knockdown of Polo-like kinase 1. Mol Pharmaceut. 2010;7:442–455. doi:10.1021/mp9002255
27. Koncar RF, Chu Z, Romick-Rosendale LE, et al. PLK1 inhibition enhances temozolomide efficacy in IDH1 mutant gliomas. Oncotarget. 2017;8:15827–15837. doi:10.18632/oncotarget.15015
28. Liu NJ, Hu GZ, Wang H, Li ZH, Guo ZG. PLK1 inhibitor facilitates the suppressing effect of temozolomide on human brain glioma stem cells. J Cell Mol Med. 2018;22:5300–5310. doi:10.1111/jcmm.13793
29. Wang Y, Singh R, Wang L, et al. Polo-like kinase 1 inhibition diminishes acquired resistance to epidermal growth factor receptor inhibition in non-small cell lung cancer with T790M mutations. Oncotarget. 2016;7:47998–48010. doi:10.18632/oncotarget.10332
30. Liao G, Wang R, Rezey AC, Gerlach BD, Tang DD. MicroRNA miR-509 regulates ERK1/2, the vimentin network, and focal adhesions by targeting Plk1. Sci Rep. 2018;8:12635. doi:10.1038/s41598-018-30895-8
31. Lerner RG, Grossauer S, Kadkhodaei B, et al. Targeting a Plk1-controlled polarity checkpoint in therapy-resistant glioblastoma-propagating cells. Cancer Res. 2015;75:5355–5366. doi:10.1158/0008-5472.CAN-14-3689
32. Liu DZ, Cheng Y, Cai RQ, et al. The enhancement of siPLK1 penetration across BBB and its anti glioblastoma activity in vivo by magnet and transferrin co-modified nanoparticle. Nanomedicine-Uk. 2018;14:991–1003. doi:10.1016/j.nano.2018.01.004
33. Jiang Y, Tang R, Duncan B, et al. Direct cytosolic delivery of siRNA using nanoparticle-stabilized nanocapsules. Angew Chem Int Ed Engl. 2015;54:506–510. doi:10.1002/anie.201409161
34. Li Y, Liu R, Yang J, Ma G, Zhang Z, Zhang X. Dual sensitive and temporally controlled camptothecin prodrug liposomes codelivery of siRNA for high efficiency tumor therapy. Biomaterials. 2014;35:9731–9745. doi:10.1016/j.biomaterials.2014.08.022
35. Garber K. Alnylam launches era of RNAi drugs. Nat Biotechnol. 2018;36:777–778. doi:10.1038/nbt0918-777
36. Liu HM, Zhang YF, Xie YD, et al. Hypoxia-responsive ionizable liposome delivery siRNA for glioma therapy. Int J Nanomedicine. 2017;12:1065–1083. doi:10.2147/IJN.S125286
37. Chen YC, Chinang CF, Chen LF, Liang PC, Hsieh WY, Lin WL. Polymersomes conjugated with des-octanoyl ghrelin and folate as a BBB-penetrating cancer cell-targeting delivery system. Biomaterials. 2014;35:4066–4081. doi:10.1016/j.biomaterials.2014.01.042
38. Patyka M, Sharifi Z, Petrecca K, Mansure J, Jean-Claude B, Sabri S. Sensitivity to PRIMA-1MET is associated with decreased MGMT in human glioblastoma cells and glioblastoma stem cells irrespective of p53 status. Oncotarget. 2016;7:60245–60269. doi:10.18632/oncotarget.11197
39. Peng Y, Huang J, Xiao H, Wu T, Shuai X. Codelivery of temozolomide and siRNA with polymeric nanocarrier for effective glioma treatment. <![CDATA[International Journal of Nanomedicine]]>. 2018;13:3467–3480. doi:10.2147/IJN.S164611
40. Liu H, Li Y, Mozhi A, et al. SiRNA-phospholipid conjugates for gene and drug delivery in cancer treatment. Biomaterials. 2014;35:6519–6533. doi:10.1016/j.biomaterials.2014.04.033
41. Ozdemir-Kaynak E, Qutub AA, Yesil-Celiktas O. Advances in glioblastoma multiforme treatment: new models for nanoparticle therapy. Front Physiol. 2018;9:170. doi:10.3389/fphys.2018.00170
42. Roger M, Clavreul A, Venier-Julienne M, Passirani C, Montero-Menei C, Menei P. The potential of combinations of drug-loaded nanoparticle systems and adult stem cells for glioma therapy. Biomaterials. 2011;32:2106–2116. doi:10.1016/j.biomaterials.2010.11.056
43. Petovari G, Hujber Z, Krencz I, et al. Targeting cellular metabolism using rapamycin and/or doxycycline enhances anti-tumour effects in human glioma cells. Cancer Cell Int. 2018;18:211. doi:10.1186/s12935-018-0710-0
44. Creixell M, Peppas NA. Co-delivery of siRNA and therapeutic agents using nanocarriers to overcome cancer resistance. Nano Today. 2012;7:367–379. doi:10.1016/j.nantod.2012.06.013
45. Fan Y, Xue W, Schachner M, Zhao W.,Honokiol eliminates glioma/glioblastoma stem cell-like cells via JAK-STAT3 signaling and inhibits tumor progression by targeting epidermal growth factor receptor. Cancers (Basel). 2018;11. doi:10.3390/cancers11010022
46. Parajuli P, Mittal S. Picture of glioma stem cells has become a Notch brighter. Stem Cell Investig. 2018;5:42. doi:10.21037/sci.2018.11.02
47. Zhi T, Jiang K, Xu X, et al. ECT2/PSMD14/PTTG1 axis promotes the proliferation of glioma through stabilizing E2F1. Neuro Oncol. 2019;21:462–473. doi:10.1093/neuonc/noy207
48. Zhang C, Yang X, Fu C, Liu X. Combination with TMZ and miR-505 inhibits the development of glioblastoma by regulating the WNT7B/Wnt/beta-catenin signaling pathway. Gene. 2018;672:172–179. doi:10.1016/j.gene.2018.06.030
49. Wu Q, Cao Z, Xiao W, et al. Study on therapeutic action and mechanism of TMZ combined with RITA against glioblastoma. Cell Physiol Biochem. 2018;51:2536–2546. doi:10.1159/000495923
50. Wang Y, Wu L, Yao Y, Lu G, Xu L, Zhou J. Polo-like kinase 1 inhibitor BI 6727 induces DNA damage and exerts strong antitumor activity in small cell lung cancer. Cancer Lett. 2018;436:1–9. doi:10.1016/j.canlet.2018.08.007
51. Yang B, Ma YB, Chu SH. Silencing SATB1 overcomes temozolomide resistance by downregulating MGMT expression and upregulating SLC22A18 expression in human glioblastoma cells. Cancer Gene Ther. 2018;25:309–316. doi:10.1038/s41417-018-0040-3
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.