Back to Journals » Clinical Interventions in Aging » Volume 14
Colonoscopy and colorectal cancer rates among octogenarians and nonagenarians: nationwide study of US veterans
Authors Virk GS, Jafri M, Ashley C
Received 28 October 2018
Accepted for publication 18 January 2019
Published 26 March 2019 Volume 2019:14 Pages 609—614
DOI https://doi.org/10.2147/CIA.S192497
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Gurjiwan Singh Virk,1 Mikram Jafri,2 Christopher Ashley3
1Department of Medicine, Albany Medical Center, Albany, NY 12208, USA; 2Department of Geriatrics, VA Stratton Medical Center, Albany, NY 12208, USA; 3Department of Gastroenterology, VA Stratton Medical Center, Albany, NY 12208, USA
Background: According to Surveillance, Epidemiology and End Results (SEER) database, colorectal cancer (CRC) is the fourth most common type of cancer and second highest in cancer-related death after lung cancer. The SEER database is geographically limited, currently present in only 10–12 states. Though this gives a good approximation about the overall direction of CRC incidence and prevalence, we need more nationwide data to compare numbers. Furthermore, colonoscopies and CRC rates in the Veterans Affair (VA) geriatric population have not been studied.
Objectives and methods: Our aim was to study the rate of colonoscopies and CRC in octogenarians and nonagenarians and to find the prevalence of CRC in this population. The goal was to obtain data in this subset of patients in order to further expand CRC screening guidelines. A retrospective data analysis was performed consisting of US male veterans who underwent colonoscopy in the VA Health Care System from 2000 to 2015.
Results and conclusion: A total of 458,224 patients aged ≥80 years were identified from the database between years 2000 and 2015. This was divided into three groups of age 80–84 years (89,621 patients), 85–90 years (248,155 patients), and >90 years (120,448 patients). A total of 81,946 patients underwent colonoscopies of which 9,365 were diagnosed with CRC. There was a statistically significant linear increase in rate of colonoscopies with increase in age suggesting that these veterans who end up living to a higher age eventually get a colonoscopy for one reason or the other. The drop in CRC percentage and prevalence observed in age group 85–90 years is statistically different when compared to that in 80–84 years and >90 years groups; however, its clinical significance remains to be elucidated.
Keywords: colonoscopy, octogenarians, nonagenarians, veterans
Background
Colorectal cancer (CRC) is a common and lethal disease. Approximately 135,430 new cases of CRC are diagnosed every year with over 50,000 cancer-related deaths.1 According to Surveillance, Epidemiology and End Results (SEER) database, CRC is the fourth most common type of cancer and second highest in cancer-related death after lung cancer.2 CRC incidence is close to zero before 40 years of age and rises thereafter to ~282/100,000 by 80 years of age. Median age at diagnosis is 68 years with peak around 65–74 years, and median age of death is 73 years with peak death around 75–84 years of age.2 CRC originates as adenomas or flat dysplasia and evolves into cancer with slow progression over a decade. Colorectal screening is the process of detecting early-stage CRCs and precancerous lesions in asymptomatic individuals. Since the implementation of CRC screening, the death rate from CRC has been declining at the rate of 2.7% each year between 2004 and 2013, resulting in a 53% reduction in CRC mortality.3 The US Multi-Society Task Force (MSTF) for colorectal cancer, a panel of gastroenterologists, recommends screening asymptomatic average risks persons for colorectal cancer beginning at age 50 up until age 75.
A recent study by van Hees et al4 has shown that screening older patients who have never been screened before is cost-effective up to 86 years of age for fecal immunochemical test, 83 years of age for colonoscopy, and 84 years of age for sigmoidoscopy without comorbidities and at an average risk for CRC.3 Although this study showed limited benefit for continued screening, it was based on data obtained through a microsimulation. A similar study by Garcia et al on Medicare beneficiaries showed prospective data suggesting statistical significant decrease in risk of CRC between those receiving screening vs those not screened over an 8-year period between 70 and 79 years of age.5 Incidence and stage of CRC were studied for this age group but not CRC-specific mortality. Furthermore, patients aged ≥80 years were excluded from the study. Another study by Tang et al has also shown that patients who have life expectancy >10 years would benefit from CRC screening.6 The previously aforementioned studies all have similar conclusion that age should not be the sole criteria for defining CRC screening.
CRC screening is not recommended in octogenarians and nonagenarians. However, most colonoscopies in this age group are performed for a diagnostic purpose. There is lack of national data about colonoscopies and CRC rates in these age groups. Previously reported data on CRC have excluded patients who are aged ≥80 years, and thus, limited data are available for the rate of colonoscopy in octogenarians and nonagenarians. Furthermore, colonoscopies and CRC rates in the Veterans Affair (VA) geriatric population have not been studied.
Objectives and methods
Our aim was to study the rate of colonoscopies and CRC in octogenarians and nonagenarians, and to find the prevalence of CRC in this population. The goal was to obtain data in this subset of patients in order to further expand CRC screening guidelines. Furthermore, it will help us to compare CRC prevalence in the VA vs the general population.
A retrospective data analysis was performed consisting of US male veterans who underwent colonoscopy in the VA Health Care System. We searched Veterans’ Health Administration (VHA) databases containing extracted medical record information on all VHA patients. Using VA Informatics and Computing Infrastructure (VINCI) database, we created a cohort of male veterans who were 80 years of age and underwent a screening or diagnostic colonoscopy at any VA hospital between 2000 and 2015. Females were excluded from the study, as there are only a limited number of females who underwent colonoscopy during this time.
We acquired our data using SQL programming and data query language. We applied Current Procedural Technology (CPT) codes for diagnostic and screening colonoscopy on our target population to determine percentage of colonoscopy in our cohort. We used the most commonly used CPT codes for colonoscopy as identified by our staff gastroenterologist. To determine individual frequency among octogenarians and nonagenarians, we divided this cohort into three main groups according to age: 80–84, 85–90, and >90 years of age. Subsequently, we then used International Statistical Classification of Diseases-9 (ICD-9) codes for CRC (153.0–153.9 and 154) on this cohort to identify the patients with CRC. A flowchart showing our study methods is shown (Figure 1).
We then calculated percentages of colonoscopies in each group by dividing colonoscopies in that age group by the total number of patients in that group. Similarly, the percentage of CRC in each group was calculated by dividing CRC cases in each group by total colonoscopies in that age group. Prevalence of CRC in each group was calculated by dividing patients with CRC in that age group over total number of patients in that group multiplied by 100,000. Each veteran included in our study had individual identifiers and was counted only once. If a veteran underwent multiple colonoscopies during the duration of the study, he was counted only once by the VINCI algorithm.
Statistical analysis
We used XLSTAT 2017 software to run chi-squared and Cochran–Armitage tests for trend on patients receiving colonoscopies to test for an association and to find a trend in the percentage of colonoscopies. Statistical significance was accepted at P<0.05. We calculated the ORs for colonoscopies in these age groups with respect to each other. The OR, its standard error, and 95% CI were calculated according to Altman 1991.7 Similarly, we ran the above statistics algorithm for patients diagnosed with CRC during these colonoscopies to see any significant difference in percentage of CRC within the age groups and test for a significant trend, if any, among the three age groups. Then, we also calculated odds for percentage of CRC in these age groups with respect to each other.
Ethical statement
Our study was approved by the Samuel S. Stratton VA Medical Center Institutional Review Board (FWA #00002073; IRB #00000950). This study was a retrospective review; hence, informed consent was not obtained from the subjects.
Results
Table 1 shows total population involved in the study. Table 2 shows colonoscopy rate and Table 3 shows CRC prevalence. A total of 458,224 patients aged ≥80 years were identified from the database between 2000 and 2015. We divided this into three age groups (80–84, 85–90, and >90 years) to determine individual frequencies. Group 1 (80–84 years) had 89,621 patients, group 2 (85–90 years) had 248,155 patients, and group 3 (>90 years) had 120,448 patients (Table 1).
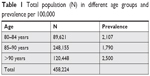  | Table 1 Total population (N) in different age groups and prevalence per 100,000 |
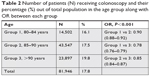  | Table 2 Number of patients (N) receiving colonoscopy and their percentage (%) out of total population in the age group along with OR between each group |
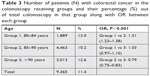  | Table 3 Number of patients (N) with colorectal cancer in the colonoscopy receiving groups and their percentage (%) out of total colonoscopy in that group along with OR between each group |
The total number of patients who underwent colonoscopy were 81,946 (17.88%) and, of these, 9,365 (11%) were diagnosed with colon cancer. As expected, the group with the highest number of patients (group 2) also had the highest number of colonoscopies performed. However, there was a surprisingly high percentage of patients above 90 years of age who received a colonoscopy (Table 2). The number of veterans diagnosed with CRC after receiving a colonoscopy is shown in Table 3. More veterans in group 2 were diagnosed with CRC than in other groups as they had highest patient population. However, when calculating the percentage of CRC, group 2 had the lowest percentage as compared to both group 1 and group 3. Prevalence of CRC was calculated per 100,000 veterans (Table 3).
Statistical analysis
There was a statistically significant difference in patients receiving colonoscopies among different age groups (P<0.001; chi-squared test; Table 2). Cochran–Armitage trend analysis also demonstrated a significant trend for an increase in proportion of patients receiving colonoscopies as age increases (P<0.001) with a linear trend (linear regression, R2=0.98, Figure 2). In order to quantify this trend even further, we calculated the OR (Table 2). As the group number increased, the odds of having colonoscopies also increased which was statistically significant.
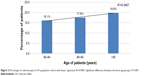  | Figure 2 Percentage of colonoscopies in VA population cohort with linear regression R2=0.9807. Significant difference between the three age groups, P<0.001. |
Analysis of CRC percentages using chi-squared test was also significant for P-value <0.001, suggesting overall statistical difference between different rows and column (Table 3), but during Cochran–Armitage trend analysis among different age groups, group 1 and group 3 were not statistically different. This was further confirmed after calculating the OR between different age groups. Odds of having CRC in group 1 compared to group 2 was statistically significant as was odds of having CRC in group 3 compared to group 2. The data showed that odds of having CRC in group 2 was statistically lower to both groups 1 and 3 whereas groups 1 and 3 were not statistically different from each other.
Discussion
It is estimated that by 2030, the US population aged ≥65 years increases to ~72 million, almost double its estimated population of 43.1 million in 2012.8 Demographic changes in the United States will result in a marked increase in the number of cancer diagnoses over the next 20 years. Age is a major risk factor for sporadic CRC, and incidence of CRC increases at 40 years of age.2 CRC screening of asymptomatic people can result in the early diagnosis of CRC and pre-cancerous lesions.9 Early stage detection of CRC results in improved 5-year survival.10 CRC screening via the use of colonoscopy is highly sensitive for precancerous lesions, single-session diagnosis and treatment.11 Previously reported data on CRC have excluded patients who are aged ≥80 years, and thus, limited data are available for the rate of colonoscopy in octogenarians and nonagenarians.
Our study demonstrates a large population of octogenarians and nonagenarians who underwent colonoscopy in VA healthcare system despite the United States Preventive Services Task Force (USPTF) recommendation for cessation of screening at 75 years of age. The highest number of veterans in our study population was between 85 and 90 years of age. This is due to an increase in the Veteran population during the Second World War (1939–1945). The above data show that rate of colonoscopies increases with age. There is statistically significant gradual increase in the percentage of colonoscopies performed in groups 1–3, suggesting that these veterans who end up living to a higher age eventually get a colonoscopy for one reason or the other. Even though most of these were diagnostic colonoscopies, they resulted in large number of veterans aged >80 years to be diagnosed with CRC.
Although our study showed increasing colonoscopy rates in octogenarians and nonagenarians, our major limitation is that we are unable to determine individual complications from the procedure. The data were obtained via the use of automated codes using VINCI national database and thus limit the details that would have been available by individual chart review.
The SEER database is geographically limited, currently present in only 10–12 states. Although this gives a good approximation about the overall direction of CRC incidence and prevalence, we need more nationwide data to compare numbers. Our data suggest almost equal prevalence of CRC as indicated by SEER database around 2.5%–3% for age >90 years, but we showed a decline in prevalence for 85–90 years age group rather than gradual increase as shown before. This statistically significant drop observed in CRC percentage in age group 85–90 years when compared to 80–84 and >90 years is intriguing, but its clinical significance remains to be elucidated. Veterans who received colonoscopies up to the age of 75 years may have been covered for the next 10–15 years as evolution of adenomas to cancers is of the same duration. We hypothesize that after passing this decade, veterans may have been symptomatic with signs or symptoms of CRC which resulted in an increase in colonoscopies frequency and prevalence in nonagenarians.
One limitation of the study is that we were unable to review previous colonoscopies performed prior to the inclusion in our study period. Also, colonoscopies performed outside the VA hospital would not have been included in the VINCI database. Non-VA care is very common, especially for Veterans aged >65 years who have Medicare. It would have been beneficial to see if there is a possible missed lesion on prior colonoscopy or this may have been the result of cessation of screening.
Another limitation in our study is that CPT and ICD-9 codes cannot reliably ascertain colonoscopy indications and cancer outcomes respectively as discussed by Ko et al.12 More often, due to the billing and coding nature of these colonoscopies it sometimes becomes challenging to establish if the patient received screening or diagnostic colonoscopy. Ko et al showed that sensitivity for average risk screening colonoscopies vary from 55% to 86%, whereas sensitivity for diagnostic colonoscopy varied between 77% and 89%. Although we tried to include the major CPT codes for both diagnostic and screening colonoscopies, its overall sensitivity may be varied. We recognize that ICD-9 codes and CPT codes are standardized, and in certain patients, there may be more than one indication for colonoscopy.
In conclusion, our study has the largest cohort of any study published on octogenarians and nonagenarians. We believe our large sample size and integrated national health records indicate that there are large numbers of colonoscopies being performed in patients aged ≥80 years. The data also show that there are a large number of elderly veterans diagnosed with CRC in the study duration. Even though guidelines recommend against continuing screening colonoscopy after 75 years of age, there is a significant increase in colonoscopy percentage as age increases. Hence, these elderly patients are undergoing colonoscopies and being diagnosed with CRC. There is a significant drop in CRC percentage in group 85–90 years of age as compared to 80–84 years of age and then significant increase in >90 years of age for which the reasoning is unclear. We need to keep these numbers in mind when making screening guidelines especially in this modern era where due to advancement of medical technologies patients are living longer. Future studies showing survival and staging data in octogenarians and nonagenarians may assist patients and clinicians in making better-informed decisions about continuation of screening and treatment in the elderly. Large randomized controlled trails and prospective studies in these age groups should be considered to determine whether screening and treatment at these ages result in overall benefit.
Acknowledgments
We are thankful to Dr Sajid Hussain for helping during the initial designing stage of the study.
The abstract of this paper was presented at the Digestive Disease Week (DDW) conference as a poster with interim findings. The poster’s abstract was published in “Poster Abstracts” section in the GIE Journal entitled “Screening for Colorectal Cancer (CRC) in Octogenarians and Nonagenarians: Nationwide Study of US Veterans.” DOI: https://doi.org/10.1016/j.gie.2017.03.832.
Disclosure
The authors report conflicts of interest in this work.
References
Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. | ||
SEER STAT fact sheet; colon and rectum. National Cancer Institute. Available from: http://seer.cancer.gov/statfacts/html/colorect.html. Accessed June 28, 2016. | ||
Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. | ||
van Hees F, Habbema JD, Meester RG, Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG. Should colorectal cancer screening be considered in elderly persons without previous screening? A cost-effectiveness analysis. Ann Intern Med. 2014;160(11):750–759. | ||
García-Albéniz X, Hsu J, Bretthauer M, Hernán MA. Effectiveness of screening colonoscopy to prevent colorectal cancer among Medicare beneficiaries aged 70 to 79 years: a prospective observational study. Ann Intern Med. 2017;166(1):18–26. | ||
Tang V, Boscardin WJ, Stijacic-Cenzer I, Lee SJ. Time to benefit for colorectal cancer screening: survival meta-analysis of flexible sigmoidoscopy trials. BMJ. 2015;350:h1662. | ||
Altman DG. Practical statistics for medical research. CRC press, 1990. | ||
Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–2765. | ||
Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Canc Inst. 2017;109(9):djx030. | ||
Zauber AG. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig Dis Sci. 2015;60(3):681–691. | ||
Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. New Engl J Med. 2014;370(14):1287–1297. | ||
Ko CW, Dominitz JA, Neradilek M, et al. Determination of colonoscopy Indication from administrative claims data. Med Care. 2014;52(4):e21–e29. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.