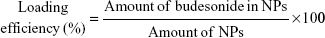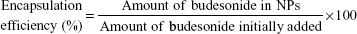Back to Journals » Drug Design, Development and Therapy » Volume 9
Colon-targeted delivery of budesonide using dual pH- and time-dependent polymeric nanoparticles for colitis therapy
Authors Naeem M, Choi M, Cao J, Lee Y, Ikram M, Yoon S, Lee J, Moon HR, Kim M, Jung Y, Yoo J
Received 22 May 2015
Accepted for publication 16 June 2015
Published 21 July 2015 Volume 2015:9 Pages 3789—3799
DOI https://doi.org/10.2147/DDDT.S88672
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Muhammad Naeem,1 Moonjeong Choi,1 Jiafu Cao,1 Yujeong Lee,1 Muhammad Ikram,2 Sik Yoon,2 Jaewon Lee,1 Hyung Ryong Moon,1 Min-Soo Kim,1 Yunjin Jung,1 Jin-Wook Yoo1
1College of Pharmacy, Pusan National University, Busan, 2Pusan National University School of Medicine, Yangsan, South Korea
Abstract: Single pH-dependent drug delivery systems have been widely used for colon-targeted delivery, but their efficiency is often hampered by the variation in gut pH. To overcome the limitation of single pH-dependent delivery systems, in this study, we developed and evaluated the therapeutic potential of budesonide-loaded dual pH/time-dependent nanoparticles (NPs) for the treatment of colitis. Eudragit FS30D was used as a pH-dependent polymer, and Eudragit RS100 as a time-dependent controlled release polymer. Single pH-dependent NPs (pH_NPs), single time-dependent NPs (Time_NPs), and dual pH/time-dependent NPs (pH/Time_NPs) were prepared using the oil-in-water emulsion method. The physicochemical properties and drug release profiles of these NPs in gastrointestinal (GI) tract conditions were investigated. The therapeutic potential and in vivo distribution of the NPs were evaluated in a dextran sulfate sodium (DSS)-induced colitis mice model. The pH/Time_NPs prevented a burst drug release in acidic pH conditions and showed sustained release at a colonic pH. The in vivo distribution study in the mice GI tract demonstrated that pH/Time_NPs were more efficiently delivered to the inflamed colon than pH_NPs were. Compared to the single pH_NPs-treated group, the pH/Time_NPs-treated group showed increased body weight and colon length and markedly decreased disease activity index, colon weight/length ratios, histological damage, and inflammatory cell infiltration in colon tissue. Our results demonstrate that the dual pH/time-dependent NPs are an effective oral colon-targeted delivery system for colitis therapy.
Keywords: colon-specific delivery, dual-sensitive delivery, budesonide, inflammatory bowel disease
Introduction
Inflammatory bowel disease (IBD) affects millions of patients worldwide, and the incidence of the disease is increasing.1 It is considered an intractable disease because its etiology and pathogenesis are not well understood. IBD symptoms range from bloody diarrhea and weight loss to ulceration and complete obstruction of the gastrointestinal (GI) tract, which can negatively affect daily life.2 The general approach toward IBD treatment is to induce remission of outbreaks, facilitate mucosal healing, and reduce the need for surgeries and hospitalizations.3 Symptoms of mild-to-moderate IBD are treated with mesalazine or systemic corticosteroids.4 In many cases, pharmacotherapy for IBD consists of life-long administration of one or more of the aforementioned drugs. Therefore, it is essential to address the quality and severity of adverse effects of these therapeutic regimens. Consequently, innovative strategies for specific and sufficient drug delivery to the inflamed colon for a prolonged period in a sustained manner while reducing the risk of systemic adverse effects are required for successful IBD treatment.5
Various pharmaceutical approaches to colon-targeted drug delivery such as pH-sensitive polymer coatings, time-dependent release systems, and enzyme-dependent release systems have been developed.6 The majority of commercialized systems for local drug delivery to the colon are based on pH changes during GI tract passage.7 Despite the simplicity of pH-dependent systems, their suitability as a single colonic delivery system in different physiological or pathological conditions in the GI tract has been doubtful.8 The pH-dependent release of these formulations means that there is no effective rate-controlling mechanism to ensure extended release of the drug along the entire length of the colon.9 Time-controlled release systems are also limited because of the large variation in the gastric emptying time, resulting in poor time prediction and site specificity.10 Combination of pH-dependent and time-dependent polymers also has been studied. Akhgari et al reported polymeric mixture of pH-dependent and time-dependent polymers as a coating material for colon-targeted drug delivery of indomethacin pellets, suggesting that a drug release profile in colon could be optimized by addition of time-dependent polymer to pH-dependent polymer.11 Therefore, the combination of pH-dependent and time-dependent polymers in a single system to lessen the pH dependency of the former system and to ensure sustained drug release throughout the colon would be an ideal delivery system.
Recently, nanoparticles (NPs)-based systems have emerged as a new strategy for IBD therapy because of their distinctive ability to accumulate in inflamed tissues in the colon.12,13 In our previous study, we developed a polymeric mixture NPs composed of an enzyme-sensitive azo polyurethane and a pH-sensitive methacrylate copolymer, Eudragit S100, for targeted drug delivery to the inflamed colon, to overcome the limitations of single-triggered release systems.14 In this study, NPs were prepared using Eudragit FS30D (EFS) and Eudragit RS100 (ERS), both of which are proven to be biocompatible polymers for oral formulations. EFS was used as a pH-sensitive polymer, and ERS was used as a controlled-release polymer. The objectives of this combination were to minimize early drug release in the stomach and small intestine, to obtain sustained drug release throughout the colon, and to target the inflamed colonic mucosa by using NPs.
The pH/time dual-dependent NPs (pH/Time_NPs) intended for inflamed colon targeted-drug delivery in IBD were prepared using a single oil-in-water emulsion solvent evaporation method. Budesonide, a corticosteroid with high topical activity and low systemic availability because of extensive metabolism by the liver, was loaded into the NPs.15 For comparison, single pH-dependent NPs (pH_NPs) and time-dependent NPs (Time_NPs) were also prepared. The physicochemical properties of the NPs were characterized, and their therapeutic efficacy was evaluated and compared in mouse model of dextran sulfate sodium (DSS)-induced colitis.
Materials and methods
Materials
Budesonide, coumarin-6 (C-6), polyvinyl alcohol (PVA, MW =30,000–70,000), and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) were purchased from Sigma-Aldrich (St Louis, MO, USA). Eudragit® FS30D (EFS) and Eudragit® RS100 (ERS) were generously donated by Evonik Korea Ltd. (Seoul, Korea). Dextran sodium sulfate (DSS, MW = 36,000–50,000) was obtained from MP Biomedicals (Irvine, CA). All other reagents and solvents were of the highest analytical grade commercially available.
Methods
Preparation of NPs
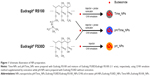
pH_NPs, Time_NPs, and pH/Time_NPs were prepared by an oil-in-water emulsion/solvent evaporation method with some modifications (Figure 1).16 Briefly, for the preparation of pH_NPs, EFS equal to100 mg was taken from a 30% (w/v) dispersion and dissolved with budesonide (10 mg) in 3 mL of acetone/methanol solvent mixture (1:2 v/v). This solution was slowly injected using a syringe pump at a flow rate of 0.5 mL/min into 40 mL of 0.25% w/v PVA solution with 500 rpm stirring. For the preparation of Time_NPs and pH/Time_NPs, a probe sonication method was used. Briefly, 100 mg of ERS was dissolved in 2 mL of dichloromethane (DCM), while 100 mg of a polymeric mixture of EFS and ERS (1:1 w/w) was dissolved in acetone and a DCM co-solvent (1:2). Then, 10 mg budesonide was added to the polymeric solution. The organic phase was added to 40 mL of 0.25% w/v PVA solution at 90 W sonication power and 400 rpm stirring. After evaporating the residual solvent under a fume hood, the NPs were collected by centrifugation at 20,000× g for 30 minutes and washed with deionized water three times. The final dispersion was freeze-dried for 24 hours. Budesonide was replaced by the hydrophobic fluorescent marker C-6 (1 mg) to facilitate the in vivo analysis of NPs distribution and localization in the mice GI tract. The same procedure was followed for the preparation of C-6-loaded NPs.
Physicochemical characterization of NPs
Scanning electron microscopy
The morphology of the NPs was analyzed by scanning electron microscopy. NPs suspended in water were dropped on a carbon tape and air-dried at room temperature in a fume hood or a desiccator. Samples were then coated with platinum for 2 minutes in vacuum and viewed by field emission scanning electron microscopy (S4800, Hitachi, Japan) at an acceleration voltage of 1–5 kV.
Particle size analysis
A qNano size analyzer (Izon Sciences, Christchurch, New Zealand) coupled with an air-based variable pressure module (VPM) was used for the size determination of NPs using 200 nanopore and 200 nm calibration particles. NPs and calibration particles (5 μL) were suspended separately in 1,000 μL of Izon Tris buffer electrolyte and sonicated for at least 30 minutes prior to use. Each recorded measurement was based on at least 500 particles. Particle sizes were determined using Izon control suite 2.2 software and were also evaluated using a Zetasizer.
Drug loading and entrapment efficiency
The budesonide entrapped in the NPs was evaluated using high-performance liquid chromatography (HPLC) according to an established method.17 The HPLC system used for the budesonide analysis was an LC-20AT (Shimadzu, Tokyo, Japan) equipped with an auto sampler processor, an SPD-20A UV detector, and a Luna C18 column (5 μm, 150 mm ×4.6 mm, Phenomenex, Torrance, CA, USA). The ultraviolet (UV) detector wavelength was set to 254 nm, and a combination of methanol and water (70:30) at a flow rate of 0.8 mL/min was used as the mobile phase. A linear calibration curve (R2 =0.9997) was obtained over the range of 0.125–250 μg/mL using standard budesonide solution. Specific amounts of NPs were dissolved in methanol, and the budesonide content was determined using the calibration curve. Samples were prepared in triplicate, and the drug loading efficiency and encapsulation efficiency (%) were calculated using the following equations:
|
|
In vitro drug release study
In vitro drug release was initiated in a buffer system at pH 1.2, and after 2 hours and 6 hours, the pH was changed to 6.5 and 7.4, respectively, corresponding to the pH in the stomach, upper small intestine, and both ileum and colon, respectively.18,19 Drug-loaded NPs (10 mg) were suspended in 30 mL of the release medium and incubated in a shaking water bath (60 rpm, 37°C). Tween-80 (0.2% w/v) was added to the release medium to facilitate the solubilization of budesonide released from NPs. Aliquots of the dissolution medium (150 μL) were withdrawn at predetermined time intervals and centrifuged at 17,000× g for 30 minutes. Supernatants containing budesonide released from the NPs were analyzed using HPLC as described earlier. All experiments were performed in triplicate.
Animal studies
All animal experiments were performed in accordance with the regulations of Pusan National University and Korean legislation on animal studies. Male imprinting control region (ICR) mice (7 weeks of age; body weight, 30–32 g) were purchased from Samtako Bio Korea (Osan, Korea) and acclimatized in the university animal facility at 25°C±3°C under a 12 hours light/dark cycle for 1 week before experimentation.
Induction of DSS-mediated colitis
Colitis was induced in ICR male mice by oral administration of DSS at 2.5% (w/v) in tap water ad libitum for 7 days. Age-matched male ICR mice receiving normal tap water served as controls. Mean DSS water consumption, body weight, and development of clinical symptoms were assessed daily during the treatment period.
Distribution of NPs in the GI tract of mice with induced colitis
In vivo distribution of C-6-loaded pH and pH/Time_NPs in the GI tract was evaluated using a mice model of DSS-induced colitis to mimic IBD conditions. Food was withheld from all mice for 24 hours prior to the administration of NPs. The NPs at a C-6 dose of 0.5 mg/kg were administered by oral gavage under isoflurane esthesia, and the mice were sacrificed 2 hours, 6 hours, and 10 hours after administration. Then, all GI tract segments including luminal contents were collected and divided into four sections, stomach, small intestine, cecum, and colon. The tissue samples were homogenized in PBS and then subsequently extracted with an ethanol/DMSO mixture (1:1 v/v).14 Specimens excised from the nontreated mice were homogenized as mentioned earlier and used as blanks. Samples emitting less than double the blank’s signal were discarded and not included in the quantitation.20 C-6 contents in the samples were analyzed using a fluorescence plate reader (triStar LB 941; Berthold Technologies, Bad Wildbad, Germany).
In vivo therapeutic efficacy
After quarantine for 7 days, as described earlier, the mice were divided into four groups of eight each: healthy control, untreated colitis control, budesonide-loaded pH NPs, and pH/Time_NPs-treated groups. In the healthy control group, mice were given fresh tap water and food ad libitum and changed twice a week for 2 weeks. In the DSS group, 2.5% DSS in tap water was administered 1 week after the beginning of the experiment to induce colitis, and the mice were fed with food. After induction of colitis, DSS administration was stopped and replaced with tap water and drug treatment was started in the treated groups. Each treated group received an equal dose of budesonide (0.168 mg/kg) in the form of suspended NPs administered orally by gavage for 7 days.21
Evaluation of colitis severity by disease activity index
The clinical course of colitis was monitored daily using a disease activity index (DAI) consisting of three parameters: weight loss, stool consistency, and anal bleeding, as described previously.22 Briefly, colitis was scored on a scale from 0 to 4. No weight loss was scored as 0 points, 1%–5% weight loss as 1 point, 5%–10% weight loss as 2 points, 10%–20% weight loss as 3 points, and >20% weight loss as 4 points. For stool consistency, 0 points were assigned for well-formed pellets, 2 points for pasty and semiformed stools, and 4 points for liquid stools that stuck to the anus. Bleeding was scored as 0 points for no blood, 2 points for positive findings, and 4 points for gross anal bleeding.
Macroscopic assessment of colitis
The mice were sacrificed 24 hours after the last drug administration. The entire colon was removed, from the cecum to the anus. Colon length was measured before dividing the colon for histology and evaluation of immune cell infiltration by immunostaining. Resected colon tissue samples were opened longitudinally and rinsed with iced phosphate buffer to remove luminal content. Then, tissue wet weight and colon length were determined and expressed as a colon weight/length quotient.
Histological analysis of colitis
For the microscopic examinations, tissue samples harvested from the healthy, untreated, and treated mice colons were stored in 4% formalin solution at 4°C for 48 hours to fix the tissue. The tissues were washed with PBS to remove excessive formalin solution and then transferred to 30% (w/w) sucrose solution for another 24 hours to protect the tissue from freezing damage during subsequent cryosectioning. Tissue sections with a thickness of 20 were cut with a cryomicrotome (Reichert, Germany).23 Sections (20 μm) were stained with hematoxylin/eosin (H&E), followed by light microscopy analysis (Zeiss, Axioskop, Germany).24,25
Immunofluorescence staining of colon tissue
For fluorescence immunostaining, colon cryosections were prepared as described earlier. Sections were incubated at 4°C overnight with primary antibodies (anti-F4/80; 1:500, abcam) for the detection of macrophages. Sections were then incubated with anti-rabbit IgG labeled with Alexa Flour 568 (Thermo Fisher Scientific, Waltham, MA, USA) for 3 hours at room temperature and then incubated in DAPI (a nuclear dye) solution (5 μg/mL) at room temperature for 20 minutes. Images were obtained using a FV10i FLUOVIEW Confocal Microscope (Olympus, Tokyo, Japan).
Statistical analysis
The student’s t-test in GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was applied for the statistical difference between two treatment groups (one-way analysis of variance [ANOVA] followed by Tukey–Karmar test in case of multiple comparison). P-values <0.05 were accepted as a statistically significant difference. All the results were presented as the mean ± SD.
Results and discussion
NPs preparation and characterization
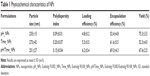
NPs were prepared using a single O/W emulsion solvent evaporation method.16 To determine the optimal NPs fabrication method, we evaluated a peristaltic pump injection method and a probe sonication method. For pH_NPs preparation, polymer was dissolved in a solvent mixture of acetone and methanol (1:2), and NPs were prepared using the injection method. However, for Time_NPs and pH/Time_NPs preparation, the probe sonication method was used because ERS has good solubility in DCM and resulted in a high yield of NPs. All formulations resulted in spherical NPs with high yields, uniform sizes, and high encapsulation efficiencies, as shown in Table 1 and Figure 2A.
Particle size is an important factor in the development of colon-specific drug delivery strategies for IBD treatment because it affects drug accumulation in the inflamed colon.26 Particle size was determined using a qNano size analyzer (Figure 2B) and a zetasizer (Table 1). All the prepared formulations resulted in particles with nanorange sizes with a monodisperse size distribution and polydispersity index values between 0.1 and 0.2. Size histograms of pH_NPs and pH/Time_NPs showed that most NPs fell in the size range of 200–250 nm, while Time_NPs fell within a size range of 250–300 nm (Figure 2B).
In vitro drug release at different pH values
The pH-dependent drug release from NPs was evaluated at pH values of 1.2, 6.5, and 7.4, which resemble the stomach, small intestine, and ileum/colon pH values, respectively. pH_NPs and pH/Time_NPs showed a strong dependence on the pH of their respective buffer system while Time_NPs showed pH-independent release (Figure 3). Time_NPs showed sustained release and the rate was not affected by changes in the release medium pH. Approximately 70% of the drug was released in the first 6 hours at pH 1.2 and 6.5, and the premature drug release would lead to absorption in the small intestine, which resulted in unwanted side effects and less drug availability to the inflamed colon, indicating that single time systems are not suitable for colon delivery. pH_NPs and pH/Time_NPs at pH 1.2 and 6.5 exhibited a slower drug release than that exhibited by Time_NPs. Less than 20% of the drug from pH_NPs and ~30% from pH/Time_NPs was released during the first 6 hours. The slightly increased premature drug release of pH/Time_NPs as compared to pH_NPs at acidic pHs can be explained by the presence of ERS in the NP which make the NPs less resistant to acidic conditions. At pH 7.4 (ie, pH of the ileum and colon); however, pH_NPs and pH/Time_NPs showed markedly different release profiles. pH_NPs showed a sudden burst release (nearly 100%) of the drug owing to complete dissolution of EFS at pH 7.4 as shown in Figure 3, confirming that the single pH-dependent system would not be suitable for colon-specific delivery because of the burst drug release in the ileum. Premature drug release in the ileum can result in systemic absorption, leading to unwanted side effects as well as insufficient drug delivery to the colon. On the other hand, pH/Time_NPs exhibited a sustained drug release profile at pH 7.4 over 24 hours without burst drug release. These results demonstrated that pH/Time_NPs can efficiently retain the entrapped drug until reaching the colon, increasing the drug availability in the colon, which is a desirable approach for colon-targeted delivery.
In vivo distribution of NPs in the mice GI tract
The therapeutic goal of colon-targeted delivery in colitis therapy is to deliver the maximum amount of drug to the inflamed colon while minimizing systemic drug absorption and unwanted side effects in the early parts of the GI tract. Therefore, we sought to evaluate NPs distribution in the GI tract of mice with DSS-induced colitis (Figure 4). The % dose of C-6 in each GI tract segment (stomach, small intestine, cecum, and colon) was assayed 2 hours, 6 hours, and 10 hours after the oral administration of NPs. We compared pH/Time_NPs with pH_NPs, which are most commonly used for colon delivery.7 For in vivo studies, Time_NPs were not evaluated due to their pH-independent burst drug release at pHs of the upper GI tract (stomach and early small intestine) as shown in Figure 3. Distribution results in the colon showed that C-6 levels were significantly higher in the pH/Time_NPs-treated mice than in the pH_NPs-treated mice after 6 hours and 10 hours of administration (Figure 4A and B). The results can be attributed to the different release patterns of the two NPs (Figure 3). pH_NPs released most of their C-6 content in the upper part of the GI tract or the ileum, leading to substantial systemic absorption by the small intestine before reaching the colon. In the case of pH/Time_NPs, the sustained release of C-6 in the alkaline pH of the ileum prevented burst drug release, indicating that compared to single pH systems, dual pH/time systems deliver sufficient amounts of encapsulated drug specifically to the inflamed colon. The in vivo distribution results imply that dual pH/time-dependent systems have greater therapeutic potential than single pH systems for colitis therapy.
In vivo therapeutic efficacy
Next, we studied the therapeutic efficacy of budesonide-loaded NPs in a DSS-induced colitis mice model. Based on the sustained drug release over a wide pH range (Figure 3) and maximum drug delivery potential to the colon (Figure 4B), we speculate that the pH/Time_NPs can avoid complete release of the loaded drug in the small intestine at an alkaline pH and that they may thus reach colitis lesions and have a local therapeutic effect. pH_NPs and pH/Time_NPs were orally administered daily to DSS-induced colitis mice for 7 days. After the treatment, we assessed colitis severity on the basis of DAI, body weight changes, colon length, histological analysis, and immunofluorescence staining of colon tissue sections.
Body weight changes and DAI
In DSS-induced colits mice model, the colitis group (ie, untreated group) exhibited severe diarrhea accompanied by bleeding as indicated by the increased DAI values (Figure 5A). Lower DAI values were observed in mice treated with budesonide-loaded NPs administered orally than in DSS-induced control colitis mice. The pH/Time_NPs-treated groups had significantly lower DAI values than those of the pH_NPs-treated group. Furthermore, compared to the healthy control group, the DSS-induced colitis groups showed significantly decreased body weight (Figure 5B). Before DSS administration, there were no intergroup differences in body weight. However, the administration of 2.5% DSS in drinking water for colitis induction significantly decreased body weights in the colitis groups compared to the body weights in the healthy control group. A faster recovery of body weight was observed in the pH/Time_NPs-treated group than in the untreated and pH_NPs-treated groups.
Colon length and colon length/weight ratio
Colitis with severe bleeding and shortening of the large intestine was induced by DSS administration for 7 days. The colon was significantly shortened in mice with DSS-induced colitis, which is an index of colitis (Figure 6A and B). Healthy mice had a colon length of 11.5 cm, while a significant reduction in colon length was observed after induction of DSS-mediated colitis with values of ~6 cm. Compared to the healthy controls, pH_NPs-treated mice also exhibited colon shortening. However, colon length markedly improved with pH/Time_NPs treatment, and a significant difference in colon length was observed between treated mice and DSS colitis mice. For the pH/Time_NPs-treated group, an increase in colon length to almost 10 cm was observed, a much closer return to baseline than what was observed in the remaining colitis groups. Similar to observations made regarding the clinical activity index, colon weight/length ratios after pH/Time_NPs treatment was found to be lower in comparison to the colitis group (Figure 6C). Again, oral administration of pH_NPs was slightly less efficient, while pH/Time_NPs at the same dose exhibited a significantly enhanced therapeutic effect as compared to pH_NPs.
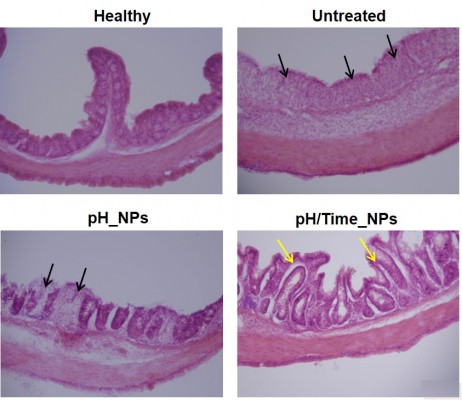
Microscopic assessment of colitis by H&E staining
The therapeutic effect of pH/Time_NPs on colitis was also supported by histological examination of colon tissues in each group. As shown in Figure 7, the mucosa from the healthy control group showed no signs of disrupted morphology. However, the colon sections of DSS-induced colitis control mice exhibited severe mucosal inflammation in all layers of the bowel wall, including a marked increase in muscle layer thickness, severe submucosal edema, goblet cell depletion, inflammatory cell infiltration, and crypt abscesses. Inflammation subsided substantially and less tissue damage was observed in the NPs-treated groups. Interestingly, the pH/Time_NPs-treated group showed signs of re-epithelization, and the histological appearance resembled that of normal mice, indicating the significant therapeutic effect on DSS-induced colitis. Overall, these findings indicate that pH/Time_NPs deliver sufficient drug to the inflamed colon, thereby significantly alleviating inflammation.
Immunostaining
Macrophages produce proinflammatory cytokines such as interleukin-6 and tumor necrosis factor-α at intestinal inflammation sites and are believed to be involved in the pathogenesis of colitis.27 Therefore, an immunohistochemical study of F4/80-positive cells in the colonic mucosa was undertaken to investigate the relationship between macrophage infiltration and colitis severity. Increased macrophage infiltration into the epithelium was induced by DSS intake in colitis control mice, confirming inflammation severity (Figure 8). Compared to colitis control mice, budesonide-loaded NPs-treated mice showed a reduced macrophage counts. More importantly, pH/Time_NPs treatment suppressed the increased macrophage invasion. Treatment with pH_NPs decreased the macrophage invasion into the colonic mucosa much less efficiently than pH/Time_NPs. These results further confirmed the enhanced therapeutic potential of pH/Time_NPs for colitis.
Conclusion
We designed and developed novel budesonide-loaded pH/Time_NPs that can minimize premature drug release in the stomach and small intestine and release the drug in the colon in a sustained manner. An in vitro drug release and in vivo distribution study revealed that pH/Time_NPs exhibited improved colon-specific drug release and distribution while Time_NPs and pH_NPs showed unwanted premature drug release in the stomach and small intestine, respectively (Figure 9). Moreover, assessment of DAI, body weight changes, colon length, and histological and immunohistochemistry investigations of colon tissue demonstrated that pH/Time_NPs alleviated DSS-induced colitis in mice more effectively than pH_NPs. Our findings suggest that pH/Time_NPs are a promising alternative to single pH-dependent systems for colitis therapy.
Acknowledgment
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No 2009-0083538).
Disclosure
The authors report no conflicts of interest in this work.
References
Xavier R, Podolsky D. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. | ||
Frank DN, Amand ALS, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–13785. | ||
Xiao B, Laroui H, Viennois E, et al. Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology. 2014;146(5):e1–e19. | ||
Colombel JF, Sandborn WJ, Reinisch W, et al; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–1395. | ||
Lautenschläger C, Schmidt C, Fischer D, Stallmach A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv Drug Deliv Rev. 2014;71:58–76. | ||
Friend DR. New oral delivery systems for treatment of inflammatory bowel disease. Adv Drug Deliv Rev. 2005;57(2):247–265. | ||
Verma S, Kumar V, Mishra D, Singh S. Colon targeted drug delivery: current and novel perspectives. International Journal of Pharmaceutical Sciences and Research. 2012;3(5). | ||
Ashford M, Fell JT, Attwood D, Woodhead PJ. An in vitro investigation into the suitability of pH-dependent polymers for colonic targeting. Int J Pharm. 1993;91(2):241–245. | ||
Travis SP, Danese S, Kupcinskas L, et al. Once-daily budesonide MMX in active, mild-to-moderate ulcerative colitis: results from the randomised CORE II study. Gut. 2013;63(3):433–441. | ||
Kaus LC, Fell JT, Sharma H, Taylor DC. On the intestinal transit of a single non-disintegrating object. Int J Pharm. 1984;20(3):315–323. | ||
Akhgari A, Sadeghi F, Garekani HA. Combination of time-dependent and pH-dependent polymethacrylates as a single coating formulation for colonic delivery of indomethacin pellets. Int J Pharm. 2006;320(1):137–142. | ||
Ulbrich W, Lamprecht A. Targeted drug-delivery approaches by nanoparticulate carriers in the therapy of inflammatory diseases. J R Soc Interface. 2010;7(suppl 1):S55–S66. | ||
Lamprecht A. IBD: selective nanoparticle adhesion can enhance colitis therapy. Nat Rev Gastroenterol Hepatol. 2010;7(6):311–312. | ||
Naeem M, Kim W, Cao J, Jung Y, Yoo J-W. Enzyme/pH dual sensitive polymeric nanoparticles for targeted drug delivery to the inflamed colon. Colloids Surf B Biointerfaces. 2014;123:271–278. | ||
Tromm A, Bunganič I, Tomsová E, et al; International Budenofalk Study Group. Budesonide 9 mg is at least as effective as mesalamine 4.5 g in patients with mildly to moderately active Crohn’s disease. Gastroenterology. 2011;140(2):425–434. | ||
Yoo J-W, Giri N, Lee CH. pH-sensitive Eudragit nanoparticles for mucosal drug delivery. Int J Pharm. 2011;403(1):262–267. | ||
Krishnamachari Y, Madan P, Lin S. Development of pH-and time-dependent oral microparticles to optimize budesonide delivery to ileum and colon. Int J Pharm. 2007;338(1):238–247. | ||
Vandamme TF, Lenourry A, Charrueau C, Chaumeil J. The use of polysaccharides to target drugs to the colon. Carbohydr Polym. 2002;48(3): 219–231. | ||
Evans D, Pye G, Bramley R, Clark A, Dyson T, Hardcastle J. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29(8):1035–1041. | ||
Beloqui A, Coco R, Alhouayek M, et al. Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. Int J Pharm. 2013;454(2):775–783. | ||
Ali H, Weigmann B, Neurath M, Collnot E, Windbergs M, Lehr C-M. Budesonide loaded nanoparticles with pH-sensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases. J Control Release. 2014;183:167–177. | ||
Dai C, Zheng C-Q, Meng F-J, Zhou Z, Sang L-X, Jiang M. VSL# 3 probiotics exerts the anti-inflammatory activity via PI3k/Akt and NF-κB pathway in rat model of DSS-induced colitis. Mol Cell Biochem. 2013;374(1–2):1–11. | ||
Fu J, Wei B, Wen T, et al. Loss of intestinal core 1–derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 2011;121(4):1657. | ||
Falzarano MS, Passarelli C, Bassi E, et al. Biodistribution and molecular studies on orally administered nanoparticle-AON complexes encapsulated with alginate aiming at inducing dystrophin rescue in mdx mice. Biomed Res Int. 2013;2013:527418. | ||
Becker AJ, Pitsch J, Sochivko D, et al. Transcriptional upregulation of Cav3. 2 mediates epileptogenesis in the pilocarpine model of epilepsy. J Neurosci. 2008;28(49):13341–13353. | ||
Schmidt C, Lautenschlaeger C, Collnot EM, et al. Nano-and microscaled particles for drug targeting to inflamed intestinal mucosa – a first in vivo study in human patients. J Control Release. 2013;165(2):139–145. | ||
Reinecker HC, Steffen M, Witthoeft T, et al. Enhand secretion of tumour necrosis factor-alpha, IL-6, and IL-1β by isolated lamina ropria monouclear cells from patients with ulcretive cilitis and Crohn’s disease. Clin Exp Immunol. 1993;94(1):174–181. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.