Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 10
Cohesive Polydensified Matrix® hyaluronic acid volumizer injected for cheek augmentation has additional positive effect on nasolabial folds
Authors Gauglitz G, Steckmeier S, Pötschke J , Schwaiger H
Received 16 May 2017
Accepted for publication 1 November 2017
Published 14 December 2017 Volume 2017:10 Pages 507—513
DOI https://doi.org/10.2147/CCID.S141906
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Gerd Gauglitz,1 Stephanie Steckmeier,1 Julian Pötschke,2 Hannah Schwaiger,1
1Department of Dermatology and Allergy, Ludwig-Maximilian University, Munich, Germany; 2Department of Plastic and Hand Surgery, Klinikum St Georg gGmbH, Leipzig, Germany
Purpose: Cohesive Polydensified Matrix® hyaluronic acid (CPM-HA) volumizer has been used successfully for several years to reverse biometric volume loss during facial aging. This observational study explored the additive effect on nasolabial folds when CPM-HA volumizer is injected into the neighboring cheek area.
Patients and methods: In this open-label, prospective, postmarketing noninterventional study, 18 adult patients seeking esthetic enhancement of the lateral cheek hollows and cheekbone area were injected with CPM-HA volumizer integrated with lidocaine (CPM-HA-VL) in the upper or lower cheek area. Safety and performance of CPM-HA-VL up to 12 months after injection with follow-up visits at week 4 and month 3, 6, and 12 were assessed. The primary endpoint was improvement of cheek fullness on the validated Merz Aesthetics Scales. Additionally, changes in nasolabial folds were quantified using a phaseshift rapid in vivo measurement of skin optical three-dimensional (3D) in vivo measurement device.
Results: Patients (94.4% female, median age 52 years, age range 39–69 years) were injected with a mean volume of 2.5±1.1 mL CPM-HA-VL per side. Immediately after injection, mean severity for upper and lower cheek fullness assessed on the validated MAS improved from 2.5±0.6 and 2.8±0.5, respectively, to 1.0±0.0, and remained unchanged through month 12. Improvement in relation to baseline was attested on the Global Aesthetics Improvement Scale for all assessments. Compared with baseline, the following assessments offered a statistical significance in the reduction of wrinkle depth of nasolabial folds (maximum depth reduction by 30.4% at 3 months) according to optical 3D in vivo measurements. Pain during injection was minimal and abated within 30 minutes. Treatment was well tolerated and led to great patient satisfaction.
Conclusion: CPM-HA-VL injected into the upper and lower cheeks led to long-lasting satisfactory cosmetic results in cheek augmentation as well as in reducing depth of nasolabial folds adjacent to the injection site.
Keywords: CPM-HA-VL volumizing filler, facial rejuvenation, esthetic procedure, dermal filler, PRIMOS 3D
Introduction
Alterations in the craniofacial skeleton are a common accompanying effect of the aging process. Changes in the relative dynamics of bone expansion and bone resorption lead to a significant loss of facial bone with age.1 In consequence of the resulting biometric volume loss, the jaw line becomes less pronounced, the skin in cheeks, under the eyes, and around the nose and mouth yields to gravity and starts sagging. This process is further promoted by the decrease in muscle tone and increasing laxity of ligaments. In addition, wrinkle formation intensifies with the deceleration of collagen and elastin production.2 Prompted by the wish to maintain a youthful appearance, increasingly more individuals seek esthetic correction of this facial aging process by minimally invasive procedures.
In previous decades, facial rejuvenation focused on the treatment of wrinkles alone. However, with growing understanding of the facial aging process in recent years, the primary focus has shifted to the reversion of volume loss. Enhancement of the lateral cheek hollows and cheekbone area recreates midface volume, smoothens nasolabial folds, and restores the youthful V-form of the face.3 Therefore, the current treatment strategies aim at restoring juvenile proportions of the entire face by injection of a soft-tissue filler.
Volume fillers based on hyaluronic acid (HA) have become increasingly popular because of their good performance and favorable safety profile.4 Depending on the individual treatment goal and area of application, available HA fillers vary in their clinical characteristics which are determined by particle size, the type of cross-linking agent used, the degree of cross-linking, the percentage of cross-linked HA, the amount of free unlinked HA, and the elastic modulus (G′).5 For the enhancement of the lateral cheek hollows and cheekbone area, the use of Cohesive Polydensified Matrix® (CPM)-HA volumizer is supported by 5 years of clinical experience. Resulting from CPM technology, it is characterized by variable cross-linking densities within the gel – denser areas for the volumizing effect and areas of lesser density for cohesivity of the matrix. This property allows for better integration into soft tissues and prevention of the Tyndall effect.6 Furthermore, the CPM-HA-specific plasticity of this filler allows the practitioner to mold and sculpt the product easily into the desired shape after the injection in order to achieve optimal esthetic results. Clinical data have confirmed the safety and efficacy of the CPM-HA volumizer with regard to the respective areas that have been injected.7–10
While concomitant correction of the nasolabial fold has been observed by volumizing the cheek and the lost malar fat pads,10 there is little information on the extent and duration of the additive effect a dermal filler has on the areas adjacent to the injection site. Therefore, the present observational study further explored the effect on nasolabial folds when a filler is injected into the cheek.
Materials and methods
The study was designed as an open-label, prospective, non-interventional study performed at a single center in Germany. It was conducted from July 2014 to November 2015 in accordance with the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of the Ludwig-Maximilian University. All patients provided written informed consent before enrollment in the study.
The primary endpoint was improvement of cheek fullness on the validated Merz Aesthetics Scales (MAS). Enhancement of the lateral cheek hollows and cheekbone area by a CPM-HA volumizer with integrated lidocaine (CPM-HA-VL, Belotero® Volume Lidocaine; Merz Pharmaceuticals GmbH, Frankfurt, Germany) was expected to lead to a reduction of the depth of nasolabial folds after injection in the cheek area. These changes were objectified and evaluated. Additional secondary endpoints included the assessment of safety and patient satisfaction of CPM-HA-VL up to 12 months postinjection.
A total of 18 adult patients seeking esthetic treatment in the upper or lower cheek area were recruited from the investigator’s patient pool. Prior to treatment at the first visit (V1) baseline characteristics were documented, including a severity assessment of the area to be treated using the validated 5-point MAS for upper and lower cheek fullness.11
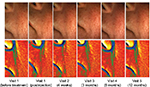
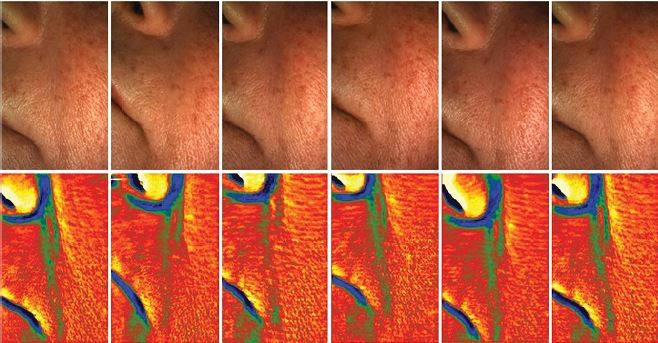
Furthermore, depth of nasolabial folds was measured using a phaseshift rapid in vivo measurement of skin (PRIMOS) optical three-dimensional (3D) in vivo measurement device (GFMesstechnik GmbH, Teltow, Germany).12–16 To observe changes in fold depth, a parallel stripe pattern of light was projected which was afterwards captured by a camera system and plotted in a color-coded height map of the measured surface.12 At each visit, measurements were performed by the same investigator.
During the first visit, patients were treated in the lower and upper cheek area with CPM-HA-VL according to the investigator’s usual practice and patients’ needs. The CE-marked dermal filler consists of 26 mg/mL HA from biofermentation origin cross-linked with 1,4-butanediol diglycidyl ether. The volumes to be injected as well as the injection techniques were subject to the investigator’s discretion. During and 30 minutes after the injection, pain was assessed on an 11-point visual analog scale (VAS) ranging from “0” (no pain) to “10” (extreme pain). Immediately after the injection and at follow-up visits 4 weeks (V2), 3 (V3), 6 (V4), and 12 months (V5) postinjection the following assessments were conducted: changes in 1) severity of cheek hollowness and 2) depth of nasolabial folds were documented. Performance was assessed using the MAS. The cosmetic result was rated in comparison to baseline using the Global Aesthetics Improvement Scale (GAIS). To verify the comparison, photographs were taken at each visit. Written informed consent was obtained from each patient. The informed consent included the scientific application and publication of the fully identifiable pictures. Patient satisfaction was assessed on a 6-point Likert scale (very good, good, satisfactory, sufficient, poor, insufficient). Patients also stated whether they would undergo treatment with CPM-HA-VL again. Tolerability was assessed on a 5-point Likert scale (very good, good, satisfactory, sufficient, poor). Reactions at the injection site were documented at each visit.
Statistical methods
The analyses were descriptive based on the observed cases. Continuous variables were summarized by number, mean, SD, median, minimum, and maximum. For quantitative variables, absolute and percent frequencies (N, %) were calculated. Evaluation of parameters measuring the clinical course was performed by intraindividual difference analysis (first vs last examination) using the Wilcoxon signed rank test. Difference was calculated per patient and subsequently averaged. Patients with missing data for one or both variables were not imputed. All tests were two-sided, and significance was declared at the 0.05 level.
Results
A total of 17 females and 1 male were enrolled between July and November 2014. Median age was 52 years (range 39–69). At baseline, mean severity on the MAS for upper and lower cheek fullness was 2.5±0.6 and 2.8±0.5, respectively. Mean depth of nasolabial folds at baseline was 3.2±1.6 mm (right) and 3.7±2.1 mm (left). There was no statistically significant difference between the left and right side. Therefore, values were pooled for the analysis of the clinical course.
Mean injected volume was 2.5±1.1 mL per side of the face. The fanning technique using a blunt cannula 22G 50 mm provided within the package was applied in most cases (72.2%), while CPM-HA-VL was administered using bolus and fanning technique in 3 patients (16.7%, n=2 not reported). No additional anesthetic or cooling was applied. Mean pain during and 30 minutes postinjection was rated 1.5±1.1 (range 0–3) and 0.1±0.4 (range 0–1.5) on the VAS, respectively. Five patients (27.8%) felt no pain at all during the injection. Thirty minutes postinjection, only 2 patients still felt minimal pain (0.5 and 1.5 on the VAS, respectively).
Efficacy
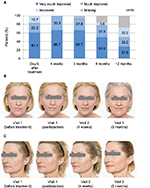
All patients showed significant increase of cheek volume and decreases in wrinkle depth at all postinjection measurements compared with baseline. All patients experienced lasting improvement on the MAS throughout the study period. Mean severity of the upper and lower cheek on the MAS improved to 1.0±0.0 after treatment and remained unchanged through month 12. Improvement in relation to baseline was attested on the GAIS for all postinjection assessments (Figure 1A). Results on the GAIS were consistent with the photo documentation (Figure 1B, C).
Matching of PRIMOS 3D measurements indicated that the effect on nasolabial folds occurred immediately after the injection. Wrinkle depth of nasolabial folds continued to decrease during the first 3 months postinjection (Figure 2). Months 6 (2.799±2.004 mm) and 12 (3.067±1.713 mm) showed slight increases in wrinkle depth compared with month 3 (2.769±1.849 mm), but the reduction compared with baseline remained significant (Figure 3).
  | Figure 3 Nasolabial folds: reduction of wrinkle depth. Measurements of the left and right side were pooled. *p<0.01; **p<0.001. |
Patient reported outcomes
The clear majority of patients (94.4%) rated their satisfaction with the treatment result as very good immediately after the injection as well as 4 weeks and 3 months postinjection (Figure 4). The rating continued to be favorable (very good or good) during the remaining visits. One patient assessed satisfaction with the result as good immediately and 4 weeks after the injection. With one exception, all patients stated that they would undergo the treatment again.
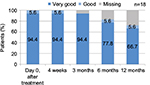  | Figure 4 Patient satisfaction rated on a 6-point Likert scale. |
Safety
The investigator rated the tolerability of the product as good or very good in all patients (Figure 5). Mild transient redness was observed in 1 patient for a brief period immediately after injection. No other adverse events or reactions at the injection site were reported. In total, 13 patients completed the 12-month follow-up visit. Five patients dropped out of the study (N=1 at month 3, N=2 at month 6, N=2 at month 12) due to the time required for the assessments.
  | Figure 5 Global tolerability assessment on a 5-point Likert scale. |
Discussion
The study with 18 patients confirmed the good performance and safety of lidocaine containing CPM-HA volumizer for augmentation of the cheeks. Improvement was visible immediately after the injection and lasted up to 12 months. During the entire postinjection period, patients showed a mean improvement of 1.5 points on the MAS when compared with baseline. The positive results achieved in cheek augmentation are in line with previous studies.17,18 The long-lasting treatment success in cheek augmentation may be attributed to the high capacity of CPM-HA-VL to resist vertical and dynamic compression which makes CPM-HA-VL ideal for achieving an excellent volumizing effect following deep implantation.19 Clinical studies and postmarketing clinical observations have shown a lasting effect without touch-up up to 18 months.18,20,21 Compared to other dermal fillers, CPM-HA-VL has an intermediate elastic modulus (G′) of 300 Pa19 which leads to greater tissue supporting capacity and consequently better volume efficiency.22 Furthermore, CPM-HA-VL benefits from the high compression parameters E′ and FN, which confer the ability to apply mechanical pressure on fibroblasts in the extracellular matrix, thus stimulating the synthesis of de novo collagen.19 This recently discovered connection between HA injection and induction of collagen synthesis may contribute to explaining the clinical persistence of the treatment effect which outlasts the bioavailability of the filler product in the dermis.23 During the process of optimal tissue integration over the first weeks after implantation, CPM-HA-VL continues to develop, thus achieving a pleasing 3D effect. Furthermore, the filler binds water, leading to an additional volumizing effect not restricted to the treated area, but extending to the adjacent regions as well, such as the nasolabial folds in this study. The good integration into the tissue24 also ensures that the filler material remains at the injection site without caudal migration.
Beyond the beneficial effects observed in cheek augmentation, CPM-HA-VL injected into the upper and lower cheeks also had an impact on the nasolabial folds.25 This result was to be expected because deflation of the midface is the primary cause of nasolabial folds.22 However, to the best of our knowledge, this is the first study that objectively quantified the extent of the effect on a location adjacent to the injection site. There was a significant long-term reduction in wrinkle depth. The added positive impact on nasolabial folds spares the patient the necessity to undergo further treatment for this indication, thus reducing treatment costs as well as inconveniences and risks associated with minimally invasive procedures involving injectables. Accordingly, these objective results reflected in the subjective patient assessment: all patients were satisfied with the cosmetic result.
Due to the lidocaine content in the filler product, additional anesthetics were not required. After 30 minutes, pain had abated in almost all patients. Studies have shown that lidocaine integrated into the filler reduced the perception of pain during the injection and correlated with higher patient satisfaction.26 Although injection site reactions such as redness, swelling, and bruising are expected with any type of dermal filler injection,17 only 1 event of redness was reported. Consistently, most patients in the present study stated their willingness to repeat the procedure.
This study is limited by its noninterventional design and lack of a control group as well as the low number of patients. However, by use of standardized objective assessment measures, such as the PRIMOS optical 3D in vivo measurement, solid data were obtained. The technique has been repeatedly employed in the analysis of subtle wrinkles and other indications such as scars.14–16 Lateral solution in the two-digit micrometer range enables high precision imaging which allows the analysis of subtlest irregularities on the skin surface. The use of photo documentation also increased data quality and ruled out recall bias when comparing the cosmetic result with baseline.
From investigations of the aging face, experts have come to the conclusion that changes occur in all tissue structures and that a change in one area may influence changes in neighboring tissues.27 This observation may also be applicable for the reverse process of restoring youthful proportions. Therefore, the additive effect on nasolabial folds observed in this study supports the paradigm shift toward considering all structural changes in the aging face and the interdependency between them instead of focusing treatment on individual lines and folds.
Conclusion
CPM-HA-VL injected into the upper and lower cheeks led to satisfactory cosmetic results in cheek augmentation as well as in reducing depth of nasolabial folds adjacent to the injection site. The effect lasted up to 12 months and was associated with minimal levels of transient injection-related pain, leading to a high degree of patient satisfaction.
Acknowledgments
The authors would like to thank Dr Petra Jöstingmeyer (medunit GmbH, Cologne, Germany) for support in writing the manuscript and Merz Pharmaceuticals GmbH (Frankfurt,, Germany) for financial writing support.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
GG serves as speaker and advisor for Merz Pharmaceuticals, Galderma, Sinclair Pharma, Lumenis, Candela, Asclepion, Cynosure, Pollogen, Johnson & Johnson, Almiral, Biofrontera, Urgo GmbH, and MediWound. The other authors report no conflicts of interest in this work.
References
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.