Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Characterization of the host–guest complex of a curcumin analog with β-cyclodextrin and β-cyclodextrin–gemini surfactant and evaluation of its anticancer activity
Authors Poorghorban M, Das U, Alaidi O, Chitanda J, Michel D, Dimmock J, Verrall R, Grochulski P, Badea I
Received 9 July 2014
Accepted for publication 3 September 2014
Published 12 January 2015 Volume 2015:10(1) Pages 503—515
DOI https://doi.org/10.2147/IJN.S70828
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Thomas Webster
Masoomeh Poorghorban,1 Umashankar Das,2 Osama Alaidi,1 Jackson M Chitanda,2 Deborah Michel,1 Jonathan Dimmock,1 Ronald Verrall,3 Pawel Grochulski,1,4 Ildiko Badea1
1Drug Discovery and Development Research Group, College of Pharmacy and Nutrition, 2Department of Chemical and Biological Engineering, 3Department of Chemistry, University of Saskatchewan, Saskatoon, SK, Canada; 4Canadian Light Source, Saskatoon, SK, Canada
Background: Curcumin analogs, including the novel compound NC 2067, are potent cytotoxic agents that suffer from poor solubility, and hence, low bioavailability. Cyclodextrin-based carriers can be used to encapsulate such agents. In order to understand the interaction between the two molecules, the physicochemical properties of the host–guest complexes of NC 2067 with β-cyclodextrin (CD) or β-cyclodextrin–gemini surfactant (CDgemini surfactant) were investigated for the first time. Moreover, possible supramolecular structures were examined in order to aid the development of new drug delivery systems. Furthermore, the in vitro anticancer activity of the complex of NC 2067 with CDgemini surfactant nanoparticles was demonstrated in the A375 melanoma cell line.
Methods: Physicochemical properties of the complexes formed of NC 2067 with CD or CDgemini surfactant were investigated by synchrotron-based powder X-ray diffraction, Fourier-transform infrared spectroscopy, and thermogravimetric analysis. Synchrotron-based small- and wide-angle X-ray scattering and size measurements were employed to assess the supramolecular morphology of the complex formed by NC 2067 with CDgemini surfactant. Lastly, the in vitro cell toxicity of the formulations toward A375 melanoma cells at various drug-to-carrier mole ratios were measured by cell viability assay.
Results: Physical mixtures of NC 2067 and CD or CDgemini surfactant showed characteristics of the individual components, whereas the complex of NC 2067 and CD or CDgemini surfactant presented new structural features, supporting the formation of the host–guest complexes. Complexes of NC 2067 with CDgemini surfactants formed nanoparticles having sizes of 100–200 nm. NC 2067 retained its anticancer activity in the complex with CDgemini surfactant for different drug-to-carrier mole ratios, with an IC50 (half-maximal inhibitory concentration) value comparable to that for NC 2067 without the carrier.
Conclusion: The formation of host–guest complexes of NC 2067 with CD or CDgemini surfactant has been confirmed and hence the CDgemini surfactant shows good potential to be used as a delivery system for anticancer agents.
Keywords: inclusion complex, supramolecular arrangement, small-angle X-ray scattering, powder X-ray diffraction, cytotoxic activity
Introduction
With the advances of combinatorial chemistry, many new anticancer agents are synthesized every year in an effort to combat different cancers. The major challenge with some compounds is their low water solubility, which results in poor absorption through biomembranes and leads to low bioavailability. Organic solvents (dimethyl sulfoxide [DMSO]) and surfactants (Cremophor®) are used in laboratory experiments and formulations to enhance the solubilization of these molecules. However, DMSO is not safe for in vivo use because it has its own influence on the cells and the US Food and Drug Administration has not yet approved the use of DMSO as a delivery agent except for the treatment of interstitial cystitis.1 In addition, Cremophor used in conventional chemotherapy formulations is associated with fatal hypersensitivity reactions.2 Therefore, different nanotechnology-based drug delivery systems have been developed to increase the solubility of the hydrophobic anticancer compounds and enhance their efficiency.
Curcumin, the active ingredient of turmeric rhizome, and its analogs are some examples of poorly soluble drug candidates in aqueous medium. While they show strong in vitro anticancer activity toward different cancer cell lines,3–9 their lipophilicity (high log P value) is a limitation for in vivo applications. Various nanotechnology-based drug delivery systems, such as liposomes,10,11 solid lipid nanoparticles,6,12,13 polymer-based nanoparticles7,14,15, and mesoporous nanoparticles,16 have been developed to encapsulate curcumin and its analogs so as to enhance solubilization and deliver them to cancerous cells. Beta-cyclodextrin (CD) (Figure 1A) is a well-known carrier for insoluble drugs in the pharmaceutical industry. It is capable of encapsulating a lipophilic guest molecule in its hydrophobic internal cavity to form a complex and its hydrophilic outer surface leads to improved guest solubility in an aqueous environment.17 Complexes of curcumin or curcumin analogs and CD have been created previously to increase the water solubility of the drug.18–23 Curcumin analogs with the 1,5-diaryl-3-oxo-1,4-pentadienyl pharmacophore structure have been synthesized and their cell toxicity toward various cancer cell lines has been demonstrated.4,24 Among these structures, NC 2067 (Figure 1B) has demonstrated high in vitro cell toxicity toward melanoma and colon cancer cell lines.3,25 Its high log P value of 4.626 and the presence of aromatic ring moieties in its structure suggest that NC 2067 is a good candidate for encapsulation by CD. Beta cyclodextrin–gemini surfactant (CDgemini surfactant) is a novel carrier composed of CD attached by an ester or amide linker to a cationic gemini surfactant (Figure 1C and D, respectively). They were synthesized in order to create a suitable substitute for a solubilizing agent,3 hypothesizing that the hydrophobic CD cavity can accommodate NC 2067 while the gemini surfactant tail possibly could contribute to a supramolecular association that would enhance diffusion into biomembranes.
In a previous study,3 we have shown that NC 2067 in a complex with CD or CDgemini surfactant in a 1:2 mole ratio exhibited high in vitro cell toxicity toward A375 melanoma cell line. Moreover, based on UV spectroscopy findings, we have also suggested a hypothetical model of the interaction of the NC 2067 molecule with CD and CDgemini surfactant.3 However, understanding the nature of the physicochemical interactions between NC 2067 and CD or CDgemini surfactant is necessary to confirm whether inclusion complexes are formed. Furthermore, examination of the structural behavior of the nanoparticles made of NC 2067 in a complex with the CDgemini surfactant should help to optimize the use of these nanoparticles as delivery agents for poorly soluble anticancer agents such as NC 2067.
The objective of the current work is to determine to what degree inclusion of NC 2067 in CD or CDgemini surfactant can occur. Moreover, the nanoparticulate behavior of NC 2067 in a complex with CDgemini is assessed more comprehensively. Finally, the effect of variations in drug-to-carrier mole ratio and linker structure on the size and anticancer activity of the nanoparticulate systems are investigated.
Materials and methods
Preparation of inclusion complexes
Curcumin analog NC 2067 was synthesized as described previously.24 βCD was purchased from Alfa Aesar (Haverhill, MA, USA). CDgemini surfactants with ester or amide linkages were synthesized previously.3 All other chemicals were purchased from Sigma-Aldrich (Oakville, ON, Canada). Complexes of NC 2067 with CD or CDgemini surfactant were created as follows. Solutions of CD and CDgemini surfactant in water (10 mM) and NC 2067 in methanol (2 mM) were combined to obtain mole ratios of drug to delivery agent of 0.5, 1.0, and 2.0 and shaken overnight. The solvent mixture was removed by rotary evaporation under vacuum. These powders were used for the powder diffraction, thermogravimetric analysis (TGA), and Fourier-transform infrared (FTIR) spectroscopy experiments. For all other studies, the residue was reconstituted in water and shaken overnight. Physical mixtures were made by blending the appropriate amount of powders of NC 2067 and CD or CDgemini surfactant.
Characterization of the complex of NC 2067 with CD or CDgemini surfactant
Powder X-ray diffraction measurements
The powder samples of NC 2067 and its physical mixtures and complexes with CD or CDgemini surfactant were loaded into capillaries (MiTeGen, Ithaca, NY, USA). Powder X-ray diffraction patterns were obtained at beamline 08B1-1 (Canadian Macromolecular Crystallography Facility-bend magnet) at the Canadian Light Source Inc (CLS, Saskatoon, SK, Canada) using X-ray energy of 12 keV at room temperature.27 Data collection was performed at a sample- to-detector distance of 280 mm with 30-second exposure time. Data processing was carried out employing X-ray analysis software for partially ordered and disordered systems (Alaidi, personal communication), automating Fit2d.28
FTIR spectroscopy
Infrared spectra of the powder samples of NC 2067 and its physical mixtures and complexes with CD or CDgemini surfactant, embedded in a KBr matrix, were scanned over the 4,000–400 cm−1 region and recorded by using a Bruker IFS 66v/S Fourier-transform spectrometer (Bruker Optics, Billerica, MA, USA) at the CLS.
TGA study
The thermal behavior of NC 2067 and its physical mixtures and complexes with CD or CDgemini surfactant was evaluated using TGA. The scans were carried out employing a TGA Q500 instrument (TA Instruments, New Castle, DE, USA) under nitrogen at a heating rate of 5°C/min.
Small- and wide-angle X-ray scattering measurements
Small- and wide-angle X-ray scattering (SAXS/WAXS) measurements were performed at the BL4-2 beamline at the Stanford Synchrotron Radiation Lightsource (SSRL, Stanford, CA, USA) at an energy of 11 keV and a sample-to-detector distance of 1.1 m at 20-second exposure time. Diffraction intensity versus q (scattering vector) plots were obtained by radial integration of the two-dimensional patterns using X-ray analysis software for partially ordered and disordered systems. The concentration of CDgemini surfactant in all samples was fixed at 10 mM.
Size measurements
Particle sizes in solution were measured using a Zetasizer Nano ZS instrument (Malvern Instruments, Malvern, UK). Results are reported as the mean of three to five measurements ± standard deviation.
Cell viability assay
A375 human amelanotic melanoma cells (American Type Culture Collection, CRL-1619) were seeded at a density of 1×104 cells per well in 96-well tissue culture treated plates. Cells were treated with the formulations at drug concentrations of 0.01–200 μM in quadruplicate wells for 48 hours to produce a balanced four-parameter curve. The experiments were conducted in triplicate. After treatment, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Invitrogen, Burlington, ON, Canada) at 450 μg/mL was added to each well and the plates were incubated for 2 hours at 37°C. Absorbance at 550 nm was recorded using a Synergy BioTek plate reader. The values of the half-maximal inhibitory concentration (IC50) for all samples were calculated using the four-parameter curves generated by the Gen5 software from BioTek.
Statistical analysis
Statistical analysis was performed using SPSS (version 19.0). One-way analysis of variance and Scheffé’s comparison were used. The level of significance was considered at P<0.05 value.
Results
Physicochemical characterization of the host–guest inclusion complexes of NC 2067 in CD and CDgemini surfactant
Powder X-ray diffraction analysis
To confirm the formation of the complexes of NC 2067 and CD or CDgemini surfactant, analysis of powder diffraction patterns of the complexes were compared to the patterns of the physical mixtures of NC 2067 and CD or CDgemini surfactant. Because the structure of the linker (amide or ester) in CDgemini surfactant should have a minimal influence on the host–guest complexes, only CDgemini surfactant with the ester linker was evaluated. Moreover, qualitative analysis of the host–guest complexes will not change with different drug-to-carrier mole ratios and hence, the 1:2 mole ratio was selected for these experiments. Diffractograms of CD, NC 2067, their physical mixture, and the complex they formed are illustrated in Figure 2A. The CD and NC 2067 diffractograms (Figure 2A-a and -b, respectively) display several peaks representative of a crystalline structure. The physical mixture of NC 2067 and CD (Figure 2A-c) shows a pattern resembling the superposition of the NC 2067 and CD peaks, with the most intense peaks of NC 2067, at 2θ angles of 3.9°, 12.8°, 13.2°, 13.9°, and 18.8°, being most evident. Conversely, in the diffraction pattern of the complex of NC 2067 with CD (Figure 2A-d), a new diffraction pattern emerges, with peaks in different positions such as 1.9°, 9.3°, and 12.2°, which is not found in diffractograms of NC 2067 or CD (All the peak positions are presented in Table S1).
Diffractograms of CDgemini surfactant, NC 2067, their simple physical mixture, and their complex are illustrated in Figure 2B. The diffused diffraction pattern of CDgemini (Figure 2B-a) is representative of an amorphous structure. In the physical mixture of NC 2067 and CDgemini surfactant (Figure 2B-c), and as seen in the NC 2067 and CD physical mixture (Figure 2A-c), the strongest peaks of NC 2067 are visible (at 2θ angles of 3.9°, 12.8°, 13.2°, 13.9°, and 18.9°). On the contrary, the diffractogram of the complex of NC 2067 with CDgemini surfactant (Figure 2B-d) illustrates an expected amorphous structure similar to that of CDgemini surfactant, confirming the formation of a complex between NC 2067 and the CDgemini surfactant.
FTIR spectroscopy
Further evaluation of the complex between the drug and CD or CDgemini surfactant (using the ester linker as a model) was carried out by comparison of the FTIR spectra (Figure 3) of NC 2067, CD, CDgemini surfactant, their complexes, and physical mixtures. Generally, in the physical mixtures of NC 2067 and CD or CDgemini surfactant (Figure 3A-c and B-c), the infrared peaks of NC 2067 and CD or CDgemini surfactant are detectable, whereas in the complexes of NC 2067 with CD or CDgemini surfactant (Figure 3A-d and B-d), some peaks of the NC 2067 are missing or are very weak. For example, the three bands corresponding to the aromatic ring conjugated with the alkene C=C bond of NC 2067 in the 1,450–1,600 cm−1 range are still detectable in the physical mixture at 1,468 cm−1, 1,489 cm−1, and 1,512 cm−1, whereas in the complexes, they are very weak or undetectable. The peak of NC 2067 at 1,671 cm−1, corresponding to the α,β-unsaturated ketone stretch, is still present in the physical mixture, whereas it is less intense in the complex. Moreover, there are several peaks of NC 2067 at 694 cm−1, 1,278 cm−1, 1,433 cm−1, and 3,022 cm−1, which disappear after complexation of NC 2067 with CD but are observable in the physical mixture. Similarly, the peaks of NC 2067 at 694 cm−1, 1,433 cm−1, 1,608 cm−1, 1,631 cm−1, and 3,022 cm−1 are lacking in the complex of NC 2067 with CDgemini surfactant but observable in their physical mixture. The disappearance of the NC 2067 peaks at 694 cm−1, corresponding to the monosubstituted aromatic ring, and at 3,022 cm−1, corresponding to the C–H stretch of the aromatic ring, from the complexes supports the theory that one or both aromatic rings conjugated with the alkene bond in the NC 2067 structure (Figure 1B) are involved in the inclusion. On the other hand, a peak at 1,248 cm−1, corresponding to the aryl alkyl ether group (side chain in NC 2067 structure), can be detected in all physical mixtures and complexes with CD and CDgemini surfactant, thus suggesting that this side chain is not involved with the CD moiety in the complex (Figure 3).
TGA study
Thermal behavior of the complexes and physical mixtures of NC 2067 and CD or CDgemini surfactant (using ester linker) was investigated to further corroborate the results obtained from powder X-ray diffraction and FTIR studies. The thermal profile of CD (Figure 4A) showed a water loss of 10% from 30°C to 70°C and degradation occurring in the range of 275°C–315°C, with a concomitant 65% weight loss, which is in agreement with a previous report.29 The physical mixture displayed 8% water loss in the same temperature range as CD, whereas no water loss was recorded in the complex in this range (Figure 4A). Thermal decomposition of NC 2067 began at 218°C, while the complex of NC 2067 with CD showed delayed onset of the degradation, shifting to a higher temperature of 240°C (Figure 4A). The thermogram corresponding to CDgemini surfactant (Figure 4B) did not show the initial water loss at or below 70°C. The complex of NC 2067 with CDgemini surfactant showed marginally less water loss in comparison to CDgemini surfactant alone. Moreover, the degradation temperature of NC 2067 in the complex increased to 232°C (Figure 4B).
Nanoparticulate behavior of NC 2067 in complex with CDgemini surfactant
Size measurements of nanoparticles
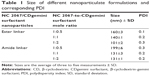
Because a complex of NC 2067 with CD will not form nanoparticles, only the size of nanoparticulate systems of NC 2067 in a complex with the CDgemini surfactant (ester and amide linkers) in water at three different drug-to-carrier mole ratios (1:0.5, 1:1, and 1:2) was measured. All the nanoparticles had sizes in the range of 101–199 nm, with a polydispersity index ≤0.3 (Table 1). The average size of the nanoparticles formed by NC 2067/CDgemini surfactant (ester linker at 1:2 mole ratio) was significantly different (P<0.05) from the size of particles in other formulations. The size of the nanoparticles at 1:0.5 mole ratio was significantly higher than that at the other two mole ratios for both ester and amide linker groups. CDgemini surfactant size measurements provided variable values with high polydispersity index, suggesting the inhomogeneous nature of the aggregations of free CDgemini surfactant in solution.
SAXS/WAXS measurements
To evaluate the lipid phase behavior of the nanoparticles, we assessed the SAXS/WAXS pattern of the complexes of NC 2067 with CDgemini surfactant (ester linker) at three different drug-to-carrier mole ratios (1:0.5, 1:1, and 1:2) (Figure 5). Surprisingly, the CDgemini surfactant, alone, showed a broad peak pattern in the SAXS region (q<0.1 Å−1), corresponding to the formation of aggregates, without showing any peak in the WAXS region (Figure 5A). Moreover, the scattering pattern of different concentrations of CDgemini surfactant (1–30 mM) in the q range of 0.02–0.2 Å−1 did not alter in position (data not shown). Once again, because the nature of the linker has minimal effect on the self-assembling behavior of the gemini surfactants, we focused on the ester linker. Interestingly, adding NC 2067 to the CDgemini surfactant at different mole ratios (1:0.5, 1:1, and 1:2) resulted in a flattening of the peak corresponding to the free CDgemini surfactant phase and, simultaneously, appearance of a peak at q of 0.27 Å−1 in the scattering pattern (Figure 5B–D). Furthermore, at higher q or 2θ angles, some peaks at q of 0.42 Å−1, 0.56 Å−1, 0.66 Å−1, and 0.8 Å−1 (corresponding to 2θ angles of 4.3°, 5.7°, 6.8°, and 8.3°, respectively) are observed for the complexes of NC 2067 and CDgemini surfactant with higher drug-to-carrier mole ratio. These peaks correspond to the most intense peaks observed in the powder X-ray diffraction pattern of the free NC 2067.
Cell activity of NC 2067/CDgemini surfactant formulations
Our aim was to evaluate the effect of various NC 2067/CDgemini surfactant nanoparticulate formulations, made at different drug-to-carrier mole ratios and with different linkers, on the cell viability of the A375 cell line to complete our previous study.3
In vitro activity of NC 2067 in complexes with CDgemini surfactants having different linkers
To evaluate the efficiency of the anticancer agent encapsulated in the carrier, we assessed the ability of NC 2067, in the form of a complex with CDgemini surfactant, to kill A375 melanoma cells (Table 2). NC 2067 in the complex with CDgemini surfactant (having both ester and amide linkers), at 1:2 mole ratio, showed strong cytotoxic effects, with IC50 values of 2.1±0.3 μM and 2.0±0.25 μM, respectively, comparable to the values reported in previous work.3 The IC50 values of NC 2067 in CDgemini surfactants having different linkers were similar (P>0.05).
In order to gain some estimate of the contribution of the delivery agent (CDgemini surfactant) to the overall toxicity shown by the inclusion complexes (Table 2), the toxicity of the CDgemini surfactants (both ester and amide linkers) was evaluated at a 200 μM carrier concentration. This concentration is approximately 50-fold higher compared to the carrier concentration at the IC50 values shown in Table 2 for the complexes. The toxicity of CDgemini surfactant with amide linker (95%±0.5%) was significantly (P<0.05) greater than that of CDgemini surfactant with an ester linker (18%±6%) (Table 2).
In vitro activity of NC 2067 in complex with CDgemini surfactant in different drug-to-delivery agent mole ratios
We next determined whether the ratio of the drug to delivery agent had any influence on the overall efficiency of the nanoparticles. In a previous study, a 1:2 mole ratio of NC 2067/CDgemini surfactant was evaluated.3 Here, we assessed whether lower amounts of the delivery agent could be used for intracellular delivery of the NC 2067. Because the intrinsic toxicity of amide linker was high, we eliminated it from further study. Comparison of the NC 2067 in CDgemini surfactant (ester linker) at three different drug-to-carrier mole ratios (1:0.5, 1:1, and 1:2) showed comparable efficiency, with IC50 values in the range of 2.0–2.4 μM (Table 2). Although the three different drug-to-carrier mole ratio formulations showed significantly higher IC50 in comparison to NC 2067 dissolved in DMSO, they showed high enough toxicity toward A375 melanoma cell line and were significantly more efficient than mephalan (P<0.05).
Discussion
The comparative analysis of the powder X-ray diffraction patterns of complexes formed between NC 2067 and CD or CDgemini surfactant, their physical mixtures, and the individual components leads to the conclusion that the complex is a new structure with altered diffraction pattern in comparison to its components, whereas the physical mixture pattern is the overlay of the guest, NC 2067, and the hosts, CD or CDgemini surfactant. Similar to these results, it has been shown that the inclusion of iprodione in CD displayed new sharp peaks in the diffractogram of the inclusion complex.30 Furthermore, a complex formed by ibuprofen and CD, using supercritical carbon dioxide, coprecipitation, and freeze-drying methods, resulted in new peaks, confirming the formation of complexes.31
Based on the comparison of the FTIR spectra of the complexes of NC 2067 in CD or CDgemini surfactant and their physical mixtures, it has been observed that the peaks corresponding to the aromatic ring conjugated with the alkene C=C bond of NC 2067 are available in physical mixtures, whereas in the complexes, they are weakened or disappear. We hypothesize that the NC 2067 complex with CD or CDgemini surfactant arises from the complexation of the 1,5-diaryl-3-oxo-1,4-pentadienyl pharmacophore moiety and not from the side chain moiety substituted on the 4-piperidinone nitrogen atom.
TGA study of NC 2067 complexed with CD showed no water loss at 30°C–70°C in comparison to CD, which lost 10% of its weight in the same temperature region. This phenomenon could be attributed to the fact that NC 2067, accommodated in the CD cavity, displaced the water from the cavity, similar to a number of inclusion systems in βCD.32,33 Furthermore, it was observed that CDgemini surfactant weight did not decrease due to water loss, supporting the argument that inclusion of the hydrocarbon tail of the gemini surfactant in the CD cavity could have displaced the water molecules commonly located in the CD ring. This finding is consistent with other studies that show that the CD cavity has the affinity to accommodate the tail of the gemini surfactants and other double-chain surfactants.34–39 Moreover, the thermal degradation of NC 2067 complexes formed with CD or CDgemini surfactant started at higher temperatures in comparison to that of free NC 2067, suggesting the formation of a more stable complex. Similar thermal behavior was observed in the case of complexation of sulfamethoxazole with hydroxypropyl-CD, wherein the degradation temperature of the pure compound shifted from 200°C to 243°C in the complex, confirming that the inclusion complex is more stable than the drug.40 It is hypothesized that NC 2067 was shielded by the CD ring and required a higher temperature to degrade due to greater stabilization of the drug in the complexes in comparison to NC 2067 alone.
Overall, it seems that the potential for the gemini surfactant tail to locate within the CD cavity of the CDgemini surfactant competes with inclusion of NC 2067 and creates a more complicated system in comparison to the binary system consisting of NC 2067 and CD. Further studies of the possible inter- and intramolecular self-inclusion behavior of CDgemini surfactant will be carried out by using proton nuclear magnetic resonance spectroscopy to elucidate the intimate interaction between the guest and the host.
Among the formulations, NC 2067/CDgemini surfactant (ester linker) at a 1:2 mole ratio showed an average size of 101 nm, which is optimal for cellular uptake, because particles in the nanometer range can evade the reticuloendothelial system41 and enter the cells by endocytosis.42 An additional benefit of the nanocarriers for anticancer agents is that the presence of nanoparticles in the endosomes prevents the expulsion of the drug through efflux pumps, overcoming the dominant mechanism of resistance toward anticancer agents.43
The SAXS/WAXS data of free CDgemini surfactant showed a diffused pattern that did not change by increasing the concentration. Based on the affinity of the CD cavity to accommodate the gemini tail(s) of the CDgemini surfactant, the gemini moiety will be unavailable to form supramolecular assembly in the traditional manner of the gemini surfactants.44 Moreover, contrary to the case of studies with free gemini surfactants, the constant mole ratio of gemini surfactant to CD moiety in CDgemini surfactant compounds leads to the result that increasing the concentration of CDgemini surfactant does not enhance self-assembly to form micellar structures. This can explain the fact that specific conductivity versus concentration profiles of the CDgemini surfactant (results not shown) do not have a sharp break point, the critical micelle concentration, consistent with that of gemini surfactant, alone. A similar type of aggregate of a long-tailed gemini surfactant with CD molecules was investigated by small-angle neutron scattering.45 In the SAXS/WAXS pattern of NC 2067/CDgemini surfactant complexes (different mole ratios), a new peak emerged. This change in the scattering pattern may be related to the partial displacement of the gemini hydrophobic tail by the NC 2067 from the CD cavity, providing the gemini surfactant moiety freedom to self-assemble as traditional surfactants. This conclusion is consistent with other observations demonstrating that the gemini monomers, in the presence of CD, are initially involved in complexation with CD until the point that the CD cavity is saturated by the gemini surfactant. This phenomenon delays the routine self-assembly of gemini surfactant, resulting in a higher critical micelle concentration.46 Similarly, in our system, addition of NC 2067 competes for inclusion in the CD cavity, possibly replacing the gemini tail(s) from the CD cavity to some extent, and drives the self-assembly behavior of the gemini surfactant moiety. We speculate that the presence of NC 2067 in the cavity of the CDgemini acts as a “promoter” to release the gemini tail from the CD cavity and to form a more organized and repetitive supramolecular structure with interplanar distances of 22.9 Å (corresponding to q of 0.27 Å–1). At this point, the peaks observed in complexes of NC 2067 and CDgemini surfactant are too few for assignment of a specific supramolecular arrangement, resulting in the hypothesis that the new supramolecular arrangement is formed partially and that it coexists with the previous self-inclusion arrangements. Moreover, at 0.5:1 and 1:1 mole ratios of NC 2067/CDgemini surfactant, some peaks related to free NC 2067 became visible. The presence of these peaks is possibly related to the precipitation of NC 2067 in the formulations having lower CDgemini surfactant-to-NC 2067 mole ratio. On balance, characterization of the CDgemini surfactant–based nanoparticles suggests that self-inclusion could occur.
The low IC50 values of the NC 2067 in complexes with CDgemini surfactants having amide or ester linkages indicate a high potency toward cancer cell lines. The intrinsic toxicity of the CDgemini with amide linker was significantly higher than that with the ester linker and could be related to the ability of the amide linker to form hydrogen bonds with the hydroxyl groups of proteins,47,48 interfering with cellular homeostasis and resulting in an increase in cellular death. Because the long-term goal is to design nanoparticulate formulations for targeted cancer therapy, the intrinsic toxicity of a carrier is a major concern. Therefore, the CDgemini surfactant with amide linker was excluded from several of the studies in this work. NC 2067/CDgemini surfactant (ester linker) at different mole ratios showed similar IC50 values, which indicates that mole ratio alteration does not change the formulation’s efficiency.
Although CD is considered a safe excipient and is used in the pharmaceutical industry, some reports suggest that it can remove cholesterol from the cellular membranes of Caco-2 cells, leading to membrane perturbation.49 Moreover, the presence of a guest molecule in the CD cavity could decrease its affinity to attach to cell membrane components.49,50 Gemini surfactants, due to their concentration, spacer length, charge density of the head group, and geometrical packing parameters, have shown different cytotoxicity levels toward normal skin cell lines such as human keratinocytes and human dermal fibroblasts.51 Even though both components of the CDgemini surfactant compound have the capability to be cytotoxic, our study showed that the conjugated CDgemini surfactant is not toxic for A375 melanoma cell line and shows potential as an inert delivery agent.
Conclusion
Different analytical techniques such as powder X-ray diffraction, FTIR spectroscopy, and TGA support the complexation of NC 2067 with CD or CDgemini surfactant. Evidence of the interaction of the aromatic ring(s) of NC 2067 with the interior of the CD cavity was provided from FTIR studies. Although SAXS/WAXS results suggested that CDgemini surfactant alone did not show common micellar self-assembly, the presence of NC 2067 initiated the formation of a new supramolecular arrangement. Attention-grabbing self-inclusion behavior of CDgemini surfactant has been suggested from analysis of TGA thermograms. NC 2067/CDgemini surfactant nanoparticulate formulations containing different linker types and drug-to-delivery agent mole ratios did not affect the anticancer activity toward A375 melanoma cell line. In the future, more in-depth evaluation of the NC 2067/CDgemini surfactant aggregation will be performed; techniques such as two-dimensional nuclear magnetic resonance spectroscopy, rotating frame Overhauser effect spectroscopy, and single-crystal X-ray crystallography will be utilized to provide more geometrical details regarding the host–guest complex structures.
Acknowledgments
Masoomeh Poorghorban is a fellow of the Canadian Institutes of Health Research Training grant in Health Research Using Synchrotron Techniques (CIHR-THRUST) and thanks the program for financial support. We thank Dr Ferenc Borondics, Canadian Light Source, for the infrared data collection. Research described in this study was partly performed using Beamline 08B1-1 at the Canadian Light Source Inc, which is supported by the Natural Sciences and Engineering Research Council of Canada, the National Research Council Canada, the Canadian Institutes of Health Research, the Province of Saskatchewan, Western Economic Diversification Canada, and the University of Saskatchewan. Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource (SSRL), a Directorate of SLAC National Accelerator Laboratory, and an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Stanford University. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, the National Institutes of Health (NIH), and the National Institute of General Medical Sciences (NIGMS; including P41GM103393). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH. We acknowledge the assistance of Dr Thomas Weiss, SSRL, with instrument setting and data collection.
Disclosure
The authors report no conflicts of interest in this work.
References
Parkin J, Shea C, Sant GR. Intravesical dimethyl sulfoxide (DMSO) for interstitial cystitis – a practical approach. Urology. 1997;49 (5 suppl):105–107. | ||
Weiss RB. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8(7): 1263–1268. | ||
Michel D, Chitanda JM, Balogh R, et al. Design and evaluation of cyclodextrin-based delivery systems to incorporate poorly soluble curcumin analogs for the treatment of melanoma. Eur J Pharm Biopharm. 2012;81(3):548–556. | ||
Kudo C, Yamakoshi H, Sato A, et al. Synthesis of 86 species of 1,5-diaryl-3-oxo-1,4-pentadienes analogs of curcumin can yield a good lead in vivo. BMC Pharmacol. 2011;11:4. | ||
Bill MA, Fuchs JR, Li C, et al. The small molecule curcumin analog FLLL32 induces apoptosis in melanoma cells via STAT3 inhibition and retains the cellular response to cytokines with anti-tumor activity. Mol Cancer. 2010;9:165. | ||
Mulik RS, Mönkkönen J, Juvonen RO, Mahadik KR, Paradkar AR. Transferrin mediated solid lipid nanoparticles containing curcumin: enhanced in vitro anticancer activity by induction of apoptosis. Int J Pharm. 2010;398(1–2):190–203. | ||
Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5:3. | ||
Nagaraju GP, Aliya S, Zafar SF, Basha R, Diaz R, El-Rayes BF. The impact of curcumin on breast cancer. Integr Biol (United Kingdom). 2012;4(9):996–1007. | ||
Nagaraju GP, Zhu S, Wen J, et al. Novel synthetic curcumin analogues EF31 and UBS109 are potent DNA hypomethylating agents in pancreatic cancer. Cancer Lett. 2013;341(2):195–203. | ||
Lin YL, Liu YK, Tsai NM, et al. A Lipo-PEG-PEI complex for encapsulating curcumin that enhances its antitumor effects on curcumin-sensitive and curcumin-resistance cells. Nanomedicine. 2012;8(3):318–327. | ||
Kunwar A, Barik A, Pandey R, Priyadarsini KI. Transport of liposomal and albumin loaded curcumin to living cells: an absorption and fluorescence spectroscopic study. Biochim Biophys Acta. 2006;1760(10):1513–1520. | ||
Chirio D, Gallarate M, Peira E, Battaglia L, Serpe L, Trotta M. Formulation of curcumin-loaded solid lipid nanoparticles produced by fatty acids coacervation technique. J Microencapsul. 2011;28(6):537–548. | ||
Kakkar V, Singh S, Singla D, Kaur IP. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol Nutr Food Res. 2011;55(3):495–503. | ||
Ma Z, Haddadi A, Molavi O, Lavasanifar A, Lai R, Samuel J. Micelles of poly(ethylene oxide)-b-poly(ε-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J Biomed Mater Res Part A. 2008;86(2):300–310. | ||
Tsai YM, Chien CF, Lin LC, Tsai TH. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood–brain barrier penetration. Int J Pharm. 2011;416(1):331–338. | ||
Seeta Rama Raju G, Pavitra E, Nagaraju GP, Ramesh K, El-Rayes BF, Yu JS. Imaging and curcumin delivery in pancreatic cancer cell lines using PEGylated α-Gd2(MoO4)3 mesoporous particles. Dalton Trans. 2014;43(8):3330–3338. | ||
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci. 1996;85(10): 1017–1025. | ||
Yallapu MM, Jaggi M, Chauhan SC. β-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B Biointerfaces. 2010;79(1):113–125. | ||
Kazemi-Lomedasht F, Rami A, Zarghami N. Comparison of inhibitory effect of curcumin nanoparticles and free curcumin in human telomerase reverse transcriptase gene expression in breast cancer. Adv Pharm Bull. 2013;3(1):127–130. | ||
Baglole KN, Boland PG, Wagner BD. Fluorescence enhancement of curcumin upon inclusion into parent and modified cyclodextrins. J Photochem Photobiol A Chem. 2005;173(3 special issue):230–237. | ||
Tang B, Ma L, Wang HY, Zhang GY. Study on the supramolecular interaction of curcumin and α-cyclodextrin by spectrophotometry and its analytical application. J Agric Food Chem. 2002;50(6):1355–1361. | ||
Dandawate PR, Vyas A, Ahmad A, et al. Inclusion complex of novel curcumin analogue CDF and β-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm Res. 2012; 29(7):1775–1786. | ||
Rachmawati H, Edityaningrum CA, Mauludin R. Molecular inclusion complex of curcumin-β-cyclodextrin nanoparticle to enhance curcumin skin permeability from hydrophilic matrix gel. AAPS Pharm Sci Tech. 2013;14(4):1303–1312. | ||
Das U, Alcorn J, Shrivastav A, et al. Design, synthesis and cytotoxic properties of novel 1-[4-(2-alkylaminoethoxy)phenylcarbonyl]-3,5-bis(arylidene)-4-piperidones and related compounds. Eur J Med Chem. 2007;42(1):71–80. | ||
Helal M, Das U, Bandy B, Islam A, Nazarali AJ, Dimmock JR. Mitochondrial dysfunction contributes to the cytotoxicity of some 3,5-bis(benzylidene)-4-piperidone derivatives in colon HCT-116 cells. Bioorg Med Chem Lett. 2013;23(4):1075–1078. | ||
Singh RSP, Das U, Dimmock JR, Alcorn J. A general HPLC-UV method for the quantitative determination of curcumin analogues containing the 1,5-diaryl-3-oxo-1,4-pentadienyl pharmacophore in rat biomatrices. J Chromatogr B: Analyt Technol Biomed Life Sci. 2010; 878(28):2796–2802. | ||
Fodje M, Janzen K, Berg R, et al. MxDC and MxLIVE: software for data acquisition, information management and remote access to macromolecular crystallography beamlines. J Synchrotron Radiat. 2012;19(2):274–280. | ||
Hammersley A. FIT2D: an introduction and overview. European Synchrotron Radiation Facility Internal Report ESRF97HA02T. 1997. | ||
Trotta F, Zanetti M, Camino G. Thermal degradation of cyclodextrins. Polym Degrad Stab. 2000;69(3):373–379. | ||
Zhu XL, Wang HB, Chen Q, Yang WC, Yang GF. Preparation and characterization of inclusion complex of iprodione and β-cyclodextrin to improve fungicidal activity. J Agric Food Chem. 2007;55(9):3535–3539. | ||
Hussein K, Türk M, Wahl MA. Comparative evaluation of ibuprofen/β-cyclodextrin complexes obtained by supercritical carbon dioxide and other conventional methods. Pharm Res. 2007;24(3):585–592. | ||
Song LX, Guo XQ, Du FY, Bai L. Thermal degradation comparison of polypropylene glycol and its complex with β-cyclodextrin. Polym Degrad Stab. 2010;95(4):508–515. | ||
Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Formulation and biological evaluation of glimepiride-cyclodextrin-polymer systems. Int J Pharm. 2006;309(1–2):129–138. | ||
Carvalho RA, Correia HA, Valente AJM, Söderman O, Nilsson M. The effect of the head-group spacer length of 12-s-12 gemini surfactants in the host-guest association with β-cyclodextrin. J Colloid Interface Sci. 2011;354(2):725–732. | ||
Faustino CMC, Calado ART, Garcia-Rio L. Interactions between β-cyclodextrin and an amino acid-based anionic gemini surfactant derived from cysteine. J Colloid Interface Sci. 2012;367(1):286–292. | ||
Benk M, Király Z. Thermodynamics of inclusion complex formation of β-cyclodextrin with a variety of surfactants differing in the nature of headgroup. J Chem Thermodyn. 2012;54:211–216. | ||
Leclercq L, Nardello-Rataj V, Rauwel G, Aubry JM. Structure-activity relationship of cyclodextrin/biocidal double-tailed ammonium surfactant host-guest complexes: towards a delivery molecular mechanism? Eur J Pharm Sci. 2010;41(2):265–275. | ||
Qiu XM, Sun DZ, Wei XL, Yin BL. Thermodynamic study of the inclusion interaction between Gemini surfactants and cyclodextrins by isothermal titration microcalorimetry. J Solution Chem. 2007;36(3): 303–312. | ||
Guerrero-Martínez A, González-Gaitano G, Viñas MH, Tardajos G. Inclusion complexes between β-cyclodextrin and a gemini surfactant in aqueous solution: an NMR study. J Phys Chem B. 2006;110(28): 13819–13828. | ||
Garnero C, Aiassa V, Longhi M. Sulfamethoxazole: hydroxypropyl-β-cyclodextrin complex: preparation and characterization. J Pharm Biomed Anal. 2012;63:74–79. | ||
Li SD, Huang L. Nanoparticles evading the reticuloendothelial system: role of the supported bilayer. Biochim Biophys Acta. 2009;1788(10):2259–2266. | ||
Li Y, Wang J, Wientjes MG, Au JLS. Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Adv Drug Deliv Rev. 2012;64(1):29–39. | ||
Constantinides PP, Wasan KM. Lipid formulation strategies for enhancing intestinal transport and absorption of P-glycoprotein (P-gp) substrate drugs: in vitro/in vivo case studies. J Pharm Sci. 2007;96(2):235–248. | ||
Wettig SD, Verrall RE, Foldvari M. Gemini surfactants: a new family of building blocks for non-viral gene delivery systems. Curr Gene Ther. 2008;8(1):9–23. | ||
Alami E, Abrahmsén-Alami S, Eastoe J, Grillo I, Heenan RK. Interactions between a nonionic gemini surfactant and cyclodextrins investigated by small-angle neutron scattering. J Colloid Interface Sci. 2002;255(2):403–409. | ||
Nilsson M, Cabaleiro-Lago C, Valente AJM, Söderman O. Interactions between gemini surfactants, 12-s-12, and β-cyclodextrin as investigated by NMR diffusometry and electric conductometry. Langmuir. 2006;22(21):8663–8669. | ||
Zhang HX, Liu Y. Protein-binding properties of a designed steroidal lactam compound. Steroids. 2014;80:30–36. | ||
Das S, Das U, Michel D, Gorecki DKJ, Dimmock JR. Novel 3,5-bis(arylidene)-4-piperidone dimers: Potent cytotoxins against colon cancer cells. Eur J Med Chem. 2013;64:321–328. | ||
Kiss T, Fenyvesi F, Bácskay I, et al. Evaluation of the cytotoxicity of β-cyclodextrin derivatives: Evidence for the role of cholesterol extraction. Eur J Pharm Sci. 2010;40(4):376–380. | ||
Irie T, Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J Pharm Sci. 1997; 86(2):147–162. | ||
Silva SMC, Sousa JJS, Marques EF, Pais AACC, Michniak-Kohn BB. Structure Activity Relationships in Alkylammonium C12-Gemini Surfactants Used as Dermal Permeation Enhancers. BMC Biology. 2013:1–9. |
Supplementary material
  | Table S1 Most intense peaks in diffractograms of CD, NC 2067, physical mixture, and their inclusion complex powder samples (20 peaks have been shown) |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.