Back to Journals » Patient Preference and Adherence » Volume 14
Characteristics of “Hard-to-Use” Press-Through-Package Sheets: An Analysis of Information Collected by Marketing Specialists of a Japanese Medical Wholesaler
Authors Kabeya K, Satoh H , Hori S, Miura Y, Sawada Y
Received 15 March 2020
Accepted for publication 7 July 2020
Published 27 July 2020 Volume 2020:14 Pages 1267—1274
DOI https://doi.org/10.2147/PPA.S254040
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Kenji Kabeya,1 Hiroki Satoh,2,3 Satoko Hori,4 Yasumasa Miura,5 Yasufumi Sawada2
1Faculty of Pharmaceutical Sciences, The University of Tokyo, Tokyo 113-0033, Japan; 2Laboratory of Drug Lifetime Management, Graduate School of Pharmaceutical Sciences, The University of Tokyo, Tokyo 113-0033, Japan; 3Interfaculty Initiative in Information Studies, The University of Tokyo, Tokyo 113-0033, Japan; 4Laboratory of Drug Informatics, Keio University Faculty of Pharmacy, Tokyo, 105-8512, Japan; 5Toho Holdings Co., Ltd, Tokyo 155-8655, Japan
Correspondence: Yasufumi Sawada Tel +81 3-5841-1096
Fax +81 3-5841-1097
Email [email protected]
Background: Press-through-package (PTP) sheets are common forms of packaging for medicines in Japan. However, patients and/or pharmacists have reported difficulty in extracting tablets or capsules from some PTP sheets.
Objective: We used postmarketing surveillance data to identify the characteristics of PTP sheets that patients and pharmacists feel are “hard to use”.
Methods: Marketing specialists of Toho Pharmaceutical Co., Ltd. canvassed patients and medical workers during November 2014–April 2016. Among 1,129 anonymous reports of products being “hard to use”, we identified 39 products with 5 or more reports (Problem group). We compared the sizes of the drugs and PTP pockets, the size ratio, the material used for the front of PTPs, the shape of the pockets, the thickness of the pocket wall, and the force needed to release the drug from the PTP (press-out force: POF) in this Problem group with those in a Control group of 97 problem-free products.
Results: Logistic regression analyses revealed that a bigger pocket, a smaller drug size and a smaller drug-pocket size ratio increase the risk of being “hard to use”. Regarding the material, aluminum, PCTFE and PE increase the risk, while PP and PVC decrease the risk. Other factors had no significant influence.
Conclusion: Pockets in PTP sheets should be designed so as to minimize the gap between the drug and the pocket, and PP or PVC should be used as the front material instead of aluminum, PCTFE or PE. Our results suggest that marketing specialists can play effective roles in postmarketing surveillance.
Keywords: marketing specialist, postmarketing surveillance, PTP, package
Introduction
Press-through-package (PTP) sheets are common packagings for medicines in Japan.1 However, it is sometimes difficult to remove the medicines, and some PTPs have injured patients’ or pharmacists’ fingers.2,3 But, although it is important to optimize the design of PTP sheets, relatively little research has yet been done in this area.4–7 Indeed, most research so far has focused only on the force needed to remove the drug from the PTP (ie, the press-out force: POF), not on evaluation of the practical ease of use.
In Japan, medical representatives (MRs) of pharmaceutical companies usually conduct postmarketing surveillance (ie, the collection of pharmaceutical information from clinical sources). However, MRs are generally employees of individual pharmaceutical companies and are responsible for promoting their companies’ products. Therefore, data collection by MRs has some limitations regarding its effectiveness: (i) MRs do not collect information about other companies’ products and (ii) MRs focus on communicating with doctors and have few opportunities to visit community pharmacies (especially in the countryside) in order to assess pharmacists’ opinions.8
On the other hand, in Japanese medical logistics, medical wholesalers act as brokers between pharmaceutical companies and medical institutions. To ensure an effective and stable supply of medicines, these medical wholesalers stock medicines from pharmaceutical companies, and some of their employees, known as marketing specialists (MSs), deliver ordered medicines to medical institutions on a daily basis. MSs deal in medicines from different pharmaceutical companies and frequently visit medical institutions; therefore, they are not subject to the limitations mentioned above.
In November 2014, Toho Pharmaceutical Co., Ltd. (Tokyo, Japan) developed a postmarketing surveillance system called “Postmarketing Event Monitoring by Marketing Specialists” (PEM-MS). Since its inception, the PEM-MS system has been collecting and storing approximately 400 reports every week. Most of these reports include problems regarding medical tablets and capsules or their packaging.9 Some medical wholesalers have made similar postmarketing surveillance efforts;10,11 however, the collected information has not yielded broadly applicable conclusions.
The aims of this study are: (i) to identify what features of PTP sheets are associated with difficulty in removing drugs from the sheets, based on information collected by MSs, and (ii) to analyze this information and see what lessons can be learnt to improve drug package design.
Materials and Methods
Analyzed Data
Data for this study were retrospectively acquired from Toho Pharmaceutical, which stores opinions and requests related to medical tablets and capsules and their packages received by the company’s MSs during their visits to medical institutions, such as hospitals and pharmacies. In all, 17,533 reports were collected from the Tokyo region during November 2014–April 2016; these reports included the date of data collection, the profession of the medical worker, the name of the product, the nature of the product (such as formulation or package), and details of the medical worker’s opinion or request. The data did not include any confidential or personal information, and ethical approval for this study by the Ethics Committee of the University of Tokyo was not required.
Of these 17,533 reports, the authors firstly extracted 2,191 reports containing negative opinions about PTP sheets, and then extracted 1,129 reports containing indications that PTPs were “hard to use” (Figure 1).
 |
Figure 1 Breakdown of negative PEM-MS reports regarding press-through packages (PTPs). As multiple problems were often included in one report, the sum is not equal to the total number of reports. |
These 1,129 reports mentioned 359 products, and among these, we identified 39 products mentioned in 5 or more reports, and defined these products as the Problem group. As a Control group, we considered 200 products most frequently used in Japan,12 and extracted 97 that had not been mentioned as being “hard to use” in PEM-MS (Control group). The characteristics of the two groups were compared.
Before the following analysis, we compared the two groups in terms of their medicine classification numbers used in Japan,13 as well as their manufacturers, in order to check that there was no bias in the types of products or the manufacturers.
Considered Parameters
To characterize the PTP sheets, the authors considered the following 14 parameters and the POF.
- Major axis of drug [mm]
- Minor axis of drug [mm]
- Major axis of pocket [mm]
- Minor axis of pocket [mm]
- Major axis ratio (=A/C)
- Minor axis ratio (=B/D)
- Projected area of drug [mm2]
- Projected area of pocket [mm2]
- Projected area ratio (=G/H)
- Material of PTP front side
- Shape of pocket section (round or flat?)
- Thickness of pocket top [µm]
- Thickness of pocket side [µm]
- Thickness of pocket bottom [µm]
Data on dimensions were taken from package inserts, PTP material was identified from the Interview Form (regular drug information package in Japan), POF and thickness (Figure 2) were obtained by physical measurement of PTP sheets, and the shape of the pocket was determined by visual inspection. Dimensions of the inside of PTP pockets (Figure 3) were calculated by comparing the official package photographs and the drug size data (A and B). For some drugs whose sheets are opaque, so that the drugs cannot be seen from outside, we purchased and physically measured the PTPs.
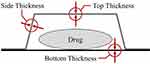 |
Figure 2 Measured thickness of PTP pockets. |
 |
Figure 3 Parameters relating to the inside of PTP pockets. |
The method used to measure POF was based on previous reports.3,4 A plastic (PLA: polylactic acid) base was prepared for measurement (Figure 4: Left). The PTP sheets were placed on the base and the center of the pockets was pressed vertically down with the tip (a metal column of 5 mm diameter) of a digital force gauge (FGP-5: Nidec-Shimpo Co., Kyoto, Japan). The maximum force was recorded at the moment when a hole appeared on the back of the PTP sheet (Figure 4: Right). The force was recorded six times for each PTP sheet and the mean of four records, excluding the largest and smallest values, was calculated as the POF.
 |
Figure 4 Measurement of press-out force. (A) (left): Base for measurement, (B) (right): Method of measurement. |
To determine the thickness of the pockets, the pockets were cut out of the sheets and measured its thickness at three points (top, side, and bottom: Figure 2) with a micrometer (MC-25: Niigata Seiki Co. Ltd., Niigata, Japan).
Physical measurement of the PTP sheets was conducted on 120 products (Problem group: 31 and Control group: 89) that was available at the time of this research.
Analysis Method
The factors related to the inside of PTP pockets (A-I) were compared between the Problem group and the Control group by means of logistic regression analysis, taking the presence of a problem as the objective or dependent variable (Problem: ‘1’, Control: ‘0’). Throughout the analyses, the significance level was set at 5%.
The authors categorized the materials of the PTP front side based on whether or not the PTP sheet includes the material. For example, a PTP sheet made of polyvinyl chloride (PVC) and polyvinylidene chloride (PVDC) was included in both the PVC group and the PVDC group. The authors counted the numbers of products in terms of the materials and groups (Problem/Control) and carried out Fisher’s exact test and residual analysis.
Fisher’s exact test was used to compare the shapes of the PTP pockets in the two groups, and Student’s t-test was used to compare the wall thickness of the PTP pockets (L-N) and POF.
All statistical analyses were carried out with Microsoft Excel 2016 ver.1803 (Microsoft Corporation, Washington, USA) and SPSS Statistics 21 (IBM Japan, Tokyo, Japan).
Results
Suitability of the Problem/Control Groups for the Analysis
The Problem group (39 products) and Control group (97 products) were compared in terms of the medicine classification numbers used in Japan and no marked difference was found. Regarding the manufacturers, the Problem group involved 20 pharmaceutical companies and the Control group involved 38. Each company had 1~8 products, and each group included products from a variety of pharmaceutical companies. As there was no obvious bias in the distributions, we considered that these groups were suitable for the analysis.
Features of the Inside of the PTP Pockets
Table 1 shows the 9 parameters relating to the inside of PTP pockets, and Table 2 shows the results of logistic regression analyses. These analyses revealed that the following parameters had a significant influence on the categorization as “hard to use”: minor axis of drug (B; p<0.001), major axis of pocket (C; p=0.002), major axis ratio (E; p<0.001), minor axis ratio (F; p<0.001), projected area of pocket (H; p=0.030), and projected area ratio (I; p<0.001). Increase of (B), (E), (F), and (I) lowered the risk of “hard-to-use” classification as their estimated β is negative, whereas the increase of (C) and (H) increased the risk as they have positive values of estimated β.
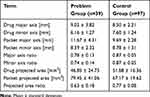 |
Table 1 Parameters Relating to the Inside of PTP Pockets |
 |
Table 2 Results of Logistic Regression |
Material of the Front of the PTP
Materials used in the front of the PTP were significantly different between the Problem group and the Control group (p<0.001, Fisher’s exact test). Residual analysis confirmed significant differences for aluminum and polychlorotrifluoroethylene (PCTFE), as they had adjusted residuals whose absolute values are larger than 1.96, meaning significance. Moreover, polyethylene (PE), polypropylene (PP) and PVC had some influence (Table 3). The results indicate that that aluminum, PCTFE and PE increase the risk of PTP sheets being reported as “hard to use”, while PP and PVC decreased the risk.
 |
Table 3 Cross Table and Adjusted Residuals for PTP Materials |
Shape and Wall Thickness of PTP Pockets
As shown in Table 4, there was no significant difference in the shape of the PTP pockets between the two groups (p=0.283, Fisher’s exact test). Furthermore, there was no significant difference in wall thickness of the PTP pockets at 3 locations (Table 5, Student’s t-test).
 |
Table 4 Shapes of PTP Pocket Sections |
 |
Table 5 PTP Pocket Wall Thickness |
POF
The distributions of POF in the two groups overlapped each other to a large extent (Figure 5). The mean in the Problem group was larger than in the Control group, but the difference was not statistically significant (p = 0.057).
Discussion
In this study, we aimed to identify the characteristics of PTP sheets flagged as being “hard to use” in reports containing information collected by MSs of a medical wholesaler. Logistic regression analyses suggested that larger values of the major axis or projected area of the pocket, and a smaller drug/pocket ratio are risk factors for PTP sheets being “hard to use” (Table 2). Of course, the pockets cannot be smaller than the drugs, so these results suggest that it is critical to make the gap between drug and pocket as small as possible in PTP design. However, the meaning of the finding that a smaller minor axis of the drug is a risk factor is unclear. A further logistic regression analysis of 1-dimensional (A-F) and 2-dimensional (G-I) package elements gave similar results, confirming that pockets too large in relation to the drugs are a risk factor for PTPs being “hard to use”.
The analyses of the materials used on the PTP front side suggested that aluminum and PCTFE significantly increased the risk of being “hard to use”, while PE showed a similar (though not significant) tendency, while PP and PVC tended to decrease the risk (Table 3). Thus, in PTP design process, PP or PVC should be used on the front side of the PTP instead of aluminum, PCTFE, or PE. As for the shape and wall thickness of the PTP pockets, no significant difference was detected between the Problem group and the Control group (Tables 4 and 5).
The authors found that POF tended to be larger in the Problem group than in the Control group (Figure 5), though the difference was not significant (p=0.057). This is consistent with previous studies.3,4,7 As the distributions of POF in the two groups overlapped greatly, there does not appear to be a threshold POF for “hard to use” PTPs.
The results of this study suggest that the following factors are critical in PTP design:
- The gap between the drug and the pocket should be as small as possible.
- PP or PVC should be used for the front side of PTPs, and aluminum or PCTFE should be avoided.
Regarding point 1, manufacturers might wish to reduce costs by using a standardized PTP sheet for various drugs. However, the redesign cost should be balanced against the convenience of patients and pharmacists. In particular, suitably fitted PTP sheets should be designed for new products. Our results also imply that drugs that are too small could be “hard to use” in the PTP format. Previous studies indicate that the diameter of medical tablets should be no larger than 8 mm,14–16 and the sum of the length, width and depth should be less than 21 mm.17 Regarding point 2, some PTP products employ aluminum as a moisture-proof or light-excluding layer to protect chemically unstable ingredients. However, advances in materials engineering and plastic processing technology may offer new possibilities to replace aluminum sheets.
In addition, the transparency of the pockets might be a factor. Although no statistical analysis was conducted in this study, opaque PTP sheets, whose drugs cannot be seen from outside, were found only in the Problem group (9 of 39 products). Moreover, some PEM-MS reports stated that patients or pharmacists could not easily find where to press down because of the pockets’ opaqueness. Thus, opaqueness could contribute to making PTPs “hard to use”.
This study has several strengths. First, the data were collected by MSs employed by a medical wholesaler for postmarketing surveillance; the MSs are expected to be better information collectors than MRs, because they deal with a wider range of medicines and visit medical institutions more frequently. Second, a wide range of information was collected. In particular, the coverage of community pharmacies allowed us to consider a wide range of users’ problems and requests.
Nevertheless, this study also has some limitations. First, information was collected only in Japan, and mainly in the Tokyo region. Therefore, similar studies in other regions of Japan and/or other countries are needed to confirm our conclusions. In this context, the PEM-MS system has covered the whole of Japan since October 2017, so more data should soon be available for analysis. Second, the PEM-MS system does not include detailed personal information about patients and pharmacists, so the influence of such background data as patients’ diseases or pharmacists’ age cannot be taken into account. Third, the reports describe subjective feelings of patients and pharmacists. For instance, some people might feel PTP sheets were “hard to use” if the medical tablets or capsules were fragile and easily damaged, even if the sheets themselves worked well. Finally, the authors selected products with at least 5 negative reports stating that the PTP sheets are “hard to use” for inclusion in the Problem group. This criterion was established to remove coincidental outlier reports, but is nevertheless arbitrary. However, it may be acceptable, because an additional analysis using the criterion of 3 adverse reports and a Problem group of 48 products gave similar results.
The authors believe this study is the first to generate pharmaceutically useful knowledge from information collected by MSs of a medical wholesaler. Although postmarketing surveillance by MSs may not yet fully cover medical institutions, which is an important omission,18 the accumulation of further reports, in addition to enhanced collaboration between MSs and other medical workers, is expected to improve the quality of postmarketing surveillance and contribute to improving drug and packaging design.
Conclusions
Analysis of postmarketing information about “hard-to-use” PTP sheets collected by MSs of a medical wholesaler allowed us to identify key points for improving PTP design. In particular, it is important to minimize the gap between the drug and the pocket, and to use PP or PVC instead of aluminum, PCTFE or PE on the front of the PTP to cover the pockets. The authors anticipate that accumulation of postmarketing surveillance data by MSs will provide an increasingly useful resource for identifying problems and improving drug and packaging design.
Acknowledgments
The authors thank the medical workers, marketing specialists, and staff of the Pharmaceutical Affairs Department of Toho Pharmaceutical Co. Ltd. for their contribution to the postmarketing surveillance in this study, as well as the staff of various pharmaceutical companies for providing product information for this study.
Disclosure
Hiroki Satoh reports Honoraria (lecture fee) from Neopharma Japan Co., Ltd. and Torii Pharmaceutical CO., LTD., outside the submitted work. Hiroki Satoh and Yasufumi Sawada are members and Satoko Hori was a member of the Laboratory of Drug Lifetime Management which was funded by 11 companies including Toho Pharmaceutical Co. Ltd. Yasumasa Miura is employed by Toho Holdings Co. Ltd. The authors report no other conflicts of interest in this work.
References
1. Nakata H, Owaki T. Device to make medicine easy to ingest; difficulty of picking. J Practical Pharm. 2000;51(5):1393–1400.
2. Ueno K, Satake H, Sato Y, Tachiura R, Kobayashi M. Problems and prospects of single-dose packages: a questionnaire survey of pharmacists. Pharm Tech Jpn. 2017;33(10):2113–2117.
3. Hiraoka O, Ando N, Suemune Y, Shimada K, Akamatsu M. Scientific study on ease of taking tablets out of PTP sheets. J Community Pharm and Pharm Sci. 2017;9(2):219–226.
4. Yamatani A, Fukushima S, Hayashi M, Mori Y, Suzuki T. Studies on measurement of pushing out strength and opening of the press through package. Jpn J Pharm Health Care Sci. 2001;27(6):576–582. doi:10.5649/jjphcs.27.576
5. Ito Y, Takeshita H, Shigematsu R, et al.
6. Koinuma M, Murata K, Hirano M.
7. Kita S, Takeshita H, Yabuta A, et al.
8. MR Education and Accreditation Center of Japan. MR circumstances investigation: the investigation on the image of MR playing a part in medical care. Available from: https://www.mre.or.jp/info/guideline.html#guideline09.
9. Sawada Y, Satoh H, Asano T, et al.
10. Sadamatsu N. Drug information and pharmacovigilance: from the viewpoint of a medical wholesaler. Jpn J Drug Inform. 2006;8(1):72–78.
11. Okamato S. Medical supply information activity of wholesalers. Pharm Lib Bull. 2001;46(3):240–247. doi:10.11291/jpla1956.46.240
12. Ministry of health, labour and welfare. 1st NDB open data. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000139390.html.
13. Ministry of Internal Affair and Communication. Japan standard commodity classification. Available from: http://www.soumu.go.jp/toukei_toukatsu/index/seido/syouhin/2index.htm.
14. Sugihara M, Awazu S, Ueda K, Takeda Y, Fukumuro K. Silver science study, show a 62th study report, for ministry of health and welfare. 1988.
15. Miura H, Kariyasu M. Effect of size of tablets on easiness of swallowing and handling among the frail elderly. Jpn J Geriatr. 2007;44(5):627–633. doi:10.3143/geriatrics.44.627
16. Oshima T, Hori S, Maida C, Miyamoto E. Effect of size and shape of tablets and capsules on ease of grasping and swallowing (1): comparison between elderly and students. Jpn J Pharm Health Care Sci. 2006;32(8):842–848. doi:10.5649/jjphcs.32.842
17. Kabeya K, Sato H, Hori S, Miura Y, Sawada Y.
18. Kabeya K, Sato H, Hori S, Miki A, Miura Y, Sawada Y. Problems and solutions on post-marketing surveillance by marketing specialists: outcomes of the workshop using KJ method. Jpn J Drug Inf. 2019;21(2):49–56. doi:10.11256/jjdi.21.49
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

