Back to Journals » Vascular Health and Risk Management » Volume 16
Cardiovascular Protection Variables Based on Exercise Intensity in Stable Coronary Heart Disease Patients After Coronary Stenting: A Comparative Study
Authors Sarvasti D , Lalenoh I, Oepangat E, Purwowiyoto BS , Santoso A , Romdoni R
Received 22 April 2020
Accepted for publication 15 June 2020
Published 6 July 2020 Volume 2020:16 Pages 257—270
DOI https://doi.org/10.2147/VHRM.S259190
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Harry Struijker-Boudier
Dyana Sarvasti,1 Isabella Lalenoh,2 Emanoel Oepangat,2 Budhi Setianto Purwowiyoto,3 Anwar Santoso,3 Rochmad Romdoni4
1Department of Internal Medicine, Faculty of Medicine Widya Mandala Catholic University, Surabaya, Indonesia; 2Department of Cardiology, Siloam Hospital TB Simatupang, Jakarta, Indonesia; 3Department of Cardiology and Vascular Medicine, Faculty of Medicine University of Indonesia National Cardiovascular Center Harapan Kita, Jakarta, Indonesia; 4Department of Cardiology and Vascular Medicine, Faculty of Medicine Airlangga University - Dr. Soetomo District General Hospital, Surabaya, Indonesia
Correspondence: Dyana Sarvasti
Department of Internal Medicine, Faculty of Medicine Widya Mandala Catholic University Jl, Raya Kalisari Selatan No. 1 Tower A, Lt. 6, Pakuwon City, Surabaya 60112, Indonesia
Tel +628563000696
Email [email protected]
Purpose: Our study aimed at determining and comparing the mechanism of cardiovascular protection variables in moderate-intensity continuous training (MICT) and high-intensity interval training (HIIT) in patients with stable coronary heart disease (CHD) after coronary stenting.
Participants and Methods: This experimental study used the same subject and cross-over design, involving eleven stable CHD patients after coronary stenting. These were randomly divided into two groups; MICT for 29 minutes at 50– 60% heart rate reserve and HIIT with 4x4 minute intervals at 60– 80% heart rate reserve, each followed by three minutes of active recovery at 40– 50% heart rate reserve. These were conducted three times a week for two weeks. The participants’ levels of adrenaline, noradrenaline, endothelial nitric oxide synthase (eNOS), extracellular superoxide dismutase (EC-SOD) activity assayed, and flow-mediated dilatation (FMD) were examined before and after treatments were completed.
Results: The HIIT significantly increased the levels of noradrenaline and eNOS compared with MICT (p< 0.05). Also, HIIT was better in maintaining EC-SOD activity and FMD compared with MICT (p< 0.05). Through the noradrenalin pathway, HIIT had a direct and significant effect on eNOS and FMD (p< 0.05) but MICT, through the noradrenaline pathways, had a direct and significant effect on eNOS (p< 0.05), and through the EC-SOD activity pathways had a direct and significant effect on FMD (p< 0.05). MICT reduced EC-SOD activity and also decreased the FMD value.
Conclusion: HIIT is superior to MICT in increasing cardiovascular protection by increasing the concentrations of noradrenalin and eNOS, maintaining EC-SOD activity, and FMD in stable CHD patients after coronary stenting.
Keywords: coronary heart disease, high-intensity interval training, catecholamine, eNOS, SOD, FMD
Introduction
Coronary heart disease (CHD) is currently the leading cause of death worldwide.1 However, regular exercise is considered as one of the preventive and therapeutic measures for CHD.2 High-intensity interval training (HIIT) has some positive effects on the heart such as increasing the blood catecholamine concentration, shear stress, and relative hypoxia.3,4 It also increases the activities of sympathetic nerve, which stimulate the production of higher levels of catecholamine such as adrenaline and noradrenaline, compared with moderate-intensity continuous training (MICT).5 Additionally, increase in the concentration of catecholamine hormone due to HIIT activates β2-adrenergic receptors (β2-ARs) and β3-adrenergic receptors (β3-ARs) which induce the production of nitric oxide (NO) in coronary and peripheral blood vessels, through endothelial nitric oxide synthase (eNOS) activation and expression.6,7 Enhancement of the expression and activity of extracellular superoxide dismutase (EC-SOD) also increase the concentration of NO.8 However, this increase in the expression of NO causes a vasodilating effect on the coronary arteries, shown through flow-mediated dilatation (FMD) examination.9 Based on this background, the purpose of this experimental study was to determine and compare the mechanism of cardiovascular protection variables in HIIT and MICT patients with stable coronary heart disease (CHD) after coronary stenting.
Participants and Methods
Participants
Based on eligibility, 11 participants were enrolled in our study in September 2017, which was completed in December 2018. All the participants had clinical evidence of stable CAD with significant stenosis treated with a percutaneous coronary intervention (PCI) at the Department of Cardiology, National Cardiovascular Center, Harapan Kita, and Siloam Hospital, TB Simatupang, Jakarta, and were screened after successful PCI, defined as complete revascularization.
The inclusion criteria include; age <60 years, left ventricular ejection fraction (LVEF) > 50% (measured within six months of enrolment by echocardiography), sinus rhythm, resting heart rate <100 beats/minute, and willingness to sign the informed consent form.
Then, the exclusion criteria include; patients with orthopedic or neurological limitations to exercise, acute systemic illness (thyrotoxicosis, electrolyte abnormalities, uncontrolled diabetes mellitus, severe anemia Hb < 9 g/dL) or fever, pregnant, severe cardiac valve diseases, fixed-rate pacemaker or implantable cardioverter-defibrillator device, uncontrolled atrial or ventricular dysrhythmias, recent sudden cardiac death syndrome, creatinine serum > 2.5 mg/dL, mental or physical impairment leading to an inability to exercise adequately.
Also, the study protocol was reviewed and approved by the National Cardiovascular Center, Harapan Kita Institutional Review and Ethics Board (No. LB 01.02/VII/KEP. 012/2017), in conformation to the Nuremberg Code and the Declaration of Helsinki on the use of human subjects, and written informed consent was obtained from all participants before being enrolled in the study. The characteristics, medical history, and medications of the participants are shown in Table 1.
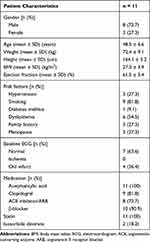 |
Table 1 Baseline Characteristics of the Participants |
Exercise Stress Test
A continuous progressive stress test was performed on a treadmill (GE T2100; idsMED, Jakarta, Indonesia) with the aid of Bruce’s protocol, one day before and one day after the exercise program. The estimated workload in metabolic equivalents (Mets) and ST segments were collected and entered on-line. Also, prescriptions of the exercise and the target heart rates were determined based on the results of the treadmill test, according to the ability of each participant at that time, which was a day before the training. The MICT and HIIT were determined based on the Borg rating scale and the participants’ heart rate reserve (HRR), was calculated using the formula:10 Target heart rate = [(Maximum heart rate - Resting heart rate) × % Intensity desired] + Resting heart rate.
Blood Sampling and Analysis
The blood samples were obtained at rest, one day before and one day after the exercise program using a 19-gauge needle by direct vein puncture. This was drawn into 6-mL vacutainer tubes containing EDTA at room temperature. Then, the resulting platelet-poor plasma was collected in 1.5-mL Eppendorf tubes and frozen at –80°C for biomarker assays. However, all the samples were drawn and analyzed by blinded technicians. Also, the plasma adrenaline and noradrenaline concentrations were determined by enzyme immunoassay method (Adrenaline/noradrenaline ELISA kit; Catalog Number KA1877, Abnova Corporation, Taipei, Taiwan). The endothelial nitric oxide synthase (eNOS) concentrations were measured on the same plasma samples using the Quantitative Sandwich ELISA method (Human endothelial nitric oxide ELISA kit; Catalog Number MBS030177, MyBiosource, Inc., CA, USA). Then, the extracellular superoxide dismutase (SOD) activity was measured using colorimetric based assay, superoxide ions generated from the conversion of xanthine, and oxygen to uric acid and hydrogen peroxide by xanthine oxidase (SOD activity kit; Catalog Number ADI-900-157, Enzo Life Sciences, Inc., New York, USA).
Flow-Mediated Dilatation
The flow-mediated dilatation (FMD) was determined a day before and a day after the exercise program. The participants were allowed to rest for 10 min before initiating the FMD study in the brachial artery, to ensure stable conditions during scanning. Rapid inflation and pneumatic deflation cuff (big ben® sphygmomanometers, Riester, Jungingen, Germany) were positioned on the right forearm, 2 inches below the antecubital fossa. A 10-MHz multi-frequency linear array probes, attached to a high-resolution ultrasound machine (Aloka ProSound Alpha 10, Virginia, USA), was then used to image the brachial artery in the distal third of the upper arm. When an optimal image was obtained, the probe was held stable, and the ultrasound parameters were set to optimize the longitudinal, B-mode image of the lumen-arterial wall interface. After baseline images of the right brachial artery were obtained for 1 minute, the cuff was inflated to 50 mm Hg above the patient’s systolic blood pressure to occlude the right brachial artery and the cuff was kept inflated for 5 minutes. Then, the longitudinal image of the artery was continuously recorded from 30 s before to 2 min after cuff deflation.11 Images of the brachial artery diameters were captured in diastole (gated with electrocardiogram R wave). The FMD is calculated as a percentage change of peak diameter with respect to reactive hyperemia baseline diameter, as shown by this equation:12
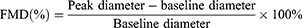
Study Protocol
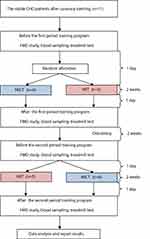
The study subjects were all receiving different treatments at certain time intervals. Also, the cross-over design was used to avoid the residual effects of treatment. The study subjects were 11 stable CHD patients after the implantation of coronary artery stents who met the inclusion criteria, each of which received two treatments; the HIIT and the MICT programs with the same total workload.
All the participants were asked to be in good hydration condition, get enough sleep to avoid fatigue, fever or flu, take the last meal at least two hours before exercise, continue taking their medications, stop taking alcohol and avoid smoking before partaking in the exercise. Additionally, the participants were instructed not to get involve in additional exercise during the study period. Then, the physical examination, measurement of vital signs, and resting ECG recording were carried out before the exercise. Only participants with physical stable condition such as no new complaints, systolic blood pressure <160 mmHg, diastolic blood pressure <100 mmHg, resting heart rate <100 beats/min, respiratory <25 times/min, body temperature 36–37ºC, and no change from the ECG compared to the baseline ECG at the beginning of the examination, were allowed to start the exercise.
The treadmill test procedures were performed before each training to determine its prescriptions, based on the resting and maximum heart rates, according to each patient’s ability. In addition, the treadmill tests were conducted to examine participants’ aerobic capacity before and after exercise program, although not shown in the results of our study. Examinations of the blood for adrenaline, noradrenaline, eNOS, EC-SOD activity, and FMD were also conducted before and after the patient followed the specified training program.
The participants met for training three times weekly for two weeks, amounting to six sessions, for each training program, under the supervision of a physician, trained nurse, and a trainer. Based on previous studies, daily MICT sessions for about 6–10 days could increase the aerobic capacity and mitochondrial enzyme activity of the participants, while seven sessions of HIIT for two weeks could increase subjects’ skeletal muscle capacity for fatty acid oxidation during the exercise.13,14
The training was conducted using a motorized treadmill (Matrix MX-T5x, Wisconsin, USA) where participants walked on it with no inclination. The MICT group went through a 5 minute warm-up period at an intensity corresponding to 40% of HRR before walking for 29 minutes continuously at an intensity 40–60% of HRR. The training session ended with a 3 minute cooling down period at 40% of HRR. Similarly, the HIIT group went through a 5 minute warm-up period at an intensity of 40%, followed by walking on the treadmill four times with 4 minute interval at 60–80% with three active recovery of 3 minute walking at 40–50%, and a 3 minute cool down at 40% of HRR. Each participant had the exercise with an electrocardiography (ECG) telemetry and a heart rate monitoring device (Nihon Kohden ECG Gateway, Tokyo, Japan). Also, the speed of the treadmill was continually adjusted as the training progressed to ensure that all sessions were carried out at the desired heart rate throughout the period. The BORG 6–20 scale was used to measure the rate of perceived exertion after each training session.15
In addition, the participants were detrained for two weeks after going through the first training to eliminate the effects of the previous exercise. Furthermore, each participant was subjected to a physical exercise different from the previous one; the participants who previously received MICT got HIIT, and vice versa.
The flow of participants through each period of the training program is shown in Figure 1.
Statistical Analysis
Participants’ characteristics were subjected to descriptive statistical analysis, with the categorical variables expressed as percentages and continuous variables expressed as mean ± standard deviation (SD). Non-parametric statistics were used due to the small groups involved in the study and to avoid assumptions of normal distributions. Also, changes within each parameter were assessed using the Wilcoxon signed ranks test with SPSS software, version 23.0 (IBM, New York, USA).
Additionally, path analysis was conducted to investigate the effects of each variable, which made it possible for simultaneous conduction of multiple and linear regression analyses. Therefore, it permitted the specification of complex models with multiple variable influences.16 Path analyses were conducted with AMOS software, version 25.0 (IBM, New York, USA) and the level of significance was set at p<0.05 for all tests.
Results
The average age of the participants was 48.5 ± 60.6-years, majority of whom had normal ECG, overweight, and smoking as the most common risk factors for CHD. The average left ventricular ejection fraction based on echocardiography was 61.5 ± 5.4%, and all participants received optimal medical therapy, as shown in Table 1.
Eleven participants completed the two weeks of MICT or HIIT sessions and the average heart rate during the period is shown in Table 2. The HIIT group did not show any statistical significant differences in adrenaline concentration compared to the MICT group, after four weeks of training, as shown in Table 3. There was a significant increase of noradrenalin in the HIIT group compared with MICT at p<0.05; as shown in Table 4. However, there was a significant decrease in EC-SOD activity in the MICT group compared with HIIT at p<0.05; as shown in Table 6. Also, the concentration of eNOS significantly varied before and after HIIT at p<0.05; as shown in Table 5, while a greater improvement was observed in FMD of the HIIT group compared with MICT at p<0.05; as shown in Table 7.
 |
Table 2 The Average of Participants Achieved Improvements in Heart Rate During the Training Program |
 |
Table 3 Changes in Adrenaline Concentration After and Before MICT and HIIT |
 |
Table 4 Changes in Noradrenaline Concentration After and Before MICT and HIIT |
 |
Table 5 Changes in eNOS Concentration After and Before MICT and HIIT |
 |
Table 6 Changes in EC-SOD Activity After and Before MICT and HIIT |
 |
Table 7 Changes in FMD After and Before MICT and HIIT |
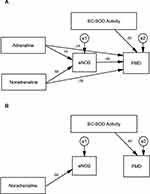
Table 8 shows the standardized regression coefficients of the initial result of path analysis extracted for MICT and HIIT; models from AMOS software shown in Figures 2A and 3A. Positive coefficients mean that the increase in an independent variable leads to a rise in a dependent variable and vice versa. The initial path analysis showed that adrenaline concentration did not affect eNOS concentration since p>0.05, and did not affect FMD as well, since p>0.05. However, the concentration of noradrenaline significantly affected eNOS since p<0.001, with a positive coefficient; but the concentration of noradrenaline did not affect FMD since p>0.05. Similarly, level of eNOS did not have any effect on FMD since p>0.05, but the activity of EC-SOD played a significant role on FMD at p<0.05, with a positive coefficient.
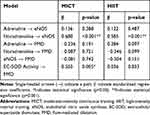 |
Table 8 The Initial Results of Path Analysis for MICT and HIIT |
Results in the HIIT group showed that adrenaline concentration did not affect eNOS and FMD since p>0.05 in both cases. Noradrenalin concentration, on the other hand, influenced eNOS since p<0.001, with a positive coefficient; however, it did not affect FMD since p>0.05. Furthermore, eNOS concentration and the activity of EC-SOD did not affect FMD since p>0.05.
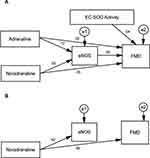
Table 9 summarizes the standardized regression coefficients after non-significant paths in MICT and HIIT were deleted; the final path models are shown in Figures 2B and 3B. The final path analysis showed that in MICT, noradrenalin concentration affected eNOS concentration since p<0.001, and EC-SOD activity affected eNOS since p<0.05, with a positive coefficient.
 |
Table 9 The Final Results of Path Analysis for MICT and HIIT |
Results in the HIIT group showed that noradrenaline concentration affected eNOS since p<0.001, with a positive coefficient, and affected FMD since p<0.05, but with a negative coefficient.
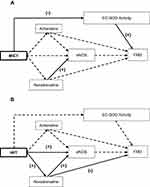
The final diagram of the cardiovascular protection mechanism for MICT and HIIT are shown in Figure 4. MICT reduced EC-SOD activity and the lower the activity, the lower the FMD assay. The higher the noradrenaline in the MICT group, the higher the concentration of eNOS. However, the HIIT further increased noradrenaline and eNOS concentrations compared with MICT. Hence, the higher the noradrenaline concentration in HIIT, the higher the eNOS and the lower the FMD assay, and vice-versa.
Safety Assessment
There was no adverse event in any of the groups.
Discussion
Based on the result, there was no significant difference in the change in adrenaline concentration in both groups. Stimulation of the sympathetic nerves increased during exercise with higher intensity, thereby stimulating the adrenal medulla gland to produce adrenaline hormone. The concentration of this hormone in plasma is also influenced by its clearance but it reduces when the concentration of adrenaline increases more than ten times its basal value.17
This clearance is reduced due to HIIT causing high secretion of adrenaline, which requires a longer time to be eliminated from the body. During MICT also, the clearance of adrenaline concentration is reduced due to its distribution in the organs being more evenly distributed due to the longer duration of exercise (29 minutes). Hence, it takes a long time to eliminate adrenaline in the plasma. Also, there was no significant increase in adrenaline concentrations, presumably because its concentration was more related to psychological stress, hypoglycemia, caffeine consumption, and Valsalva maneuver, compared to noradrenaline concentrations, which are more related to physical exercise and body position (orthostatic).18
The results showed that the level of noradrenaline increased more with HIIT compared with MICT activities. Similarly, a study by Greiwe et al, showed that exercise on a treadmill for 15 minutes in 6 women and 3 men aged 28 years, resulted in higher noradrenaline at rest compared to post-exercise, with an intensity of 60% VO2max. However, the noradrenaline concentration increased when the intensity of the exercise was within 60–85% VO2max.19 In our study, the intensity of MICT was within 50–60% HRR, which is equivalent to 50–60% VO2max, and the intensity of HIIT was within 60–80% HRR (60–80% VO2max), which was almost similar to this previous study. Our study assumes that the higher the intensity of exercise, the stimulation of the sympathetic nervous system increases, thereby resulting in higher noradrenaline. Likewise, Greiwe et al demonstrated the increment in the spillover of noradrenaline during moderate to high-intensity exercise, and increased continuously with time, while the systemic noradrenaline clearance reduced.19
According to Padilla et al, there was a significant relationship between the intensity of aerobic exercise training and the flow-mediated dilatation response. The study showed that the brachial artery shear stress was greatest following high-intensity exercise.20 In vivo exercise has a similar effect as laminar shear stress on endothelial function. Normally, laminar shear stress regulates endothelial structure and function by controlling the expression of mechanosensitive genes and production of vasoactive factors. This shear stress affects eNOS acutely and chronically, thereby activating the enzyme to produce NO within seconds. It also stimulates intracellular calcium, increases calmodulin to bond with eNOS, and increases eNOS activity acutely and transiently.21,22 In addition, shear stress stimulates the phosphorylation of eNOS at serine 635 and 1177 by protein kinase-A. Laminar shear stress which occurs for long time increases eNOS messenger ribonucleic acid (mRNA) and protein; the eNOS expression therefore increases due to increased transcription of temporary eNOS mRNA and sustained improvement in eNOS mRNA stability.22
The HIIT in our study might result in more significant shear stress in the endothelial layer, thereby producing a higher concentration of eNOS compared with MICT. In general, the concentration of eNOS in plasma is an indication of high or low NO synthesis and also equivalent to an improved endothelial function.23
MICT was performed three times a week for two weeks in our study, resulting in decreased EC-SOD activity. This has shown the inability to maintain or increase EC-SOD activity. It is assumed that MICT is unable to elicit an adaptive response to oxidative stress through the activation of nuclear erythroid 2-related factor (Nrf2) signal. Nrf2 is a redox transcription factor and a major regulator of antioxidants. It is also a cytoprotective cofactor responsible for increasing the defense mechanism of an antioxidant.24 Acute exercise could activate Nrf2 signaling through increased reactive oxygen species (ROS) production, thereby increasing the transactivation of antioxidant genes and myocardial cardioprotection.24 According to Gounder et al, increase in the regulation of Nrf2 expression occurs during high-intensity training.25 However, there were only few studies on the adaptive response of Nrf2 to exercise in humans.26
Landmesser et al showed that EC-SOD activity decreased in CHD patients, which was related to endothelial dysfunction.27 The HIIT in our study was performed three times a week for two weeks in stable CHD patients and was able to maintain the EC-SOD activity, compared with MICT. Acute HIIT could increase oxidative stress, which stimulates ROS, including superoxide anions. However, repeated exercise could cause changes in the regulation of the body’s defense system by increasing antioxidants with the capacity of turning pro-oxidants into inactive ones.28
HIIT could create unfavorable conditions with a continuous increase in its intensity, because it could lead to increased ROS and decreased circulating antioxidants.29 This condition could however be prevented with intervals, in the form of active or passive recovery, so as to eliminate its adverse effects on blood vessels.29,30
Also, HIIT could provoke more blood flow in the large blood vessels during exercise where shear stress increases and induces NO bioavailability.31 Moreover, repetitive shear stress due to HIIT could initiate changes at the molecular level. The potassium canal of endothelial cells becomes more sensitive, thereby facilitating the influx of calcium into the cells. This increased in intracellular calcium in endothelial cells increases the activity and expression of eNOS, and NO production, hence resulting in vasodilation.32 The HIIT in our study increased the mean change in FMD such that it was better to stay well maintained, compared with MICT. In general, HIIT is more effective in improving brachial artery endothelial function (FMD) compared with MICT.
Furthermore, the path analysis showed that adrenaline concentration in MICT and HIIT has no significant effect on the concentration of eNOS. This was due to changes in the adrenaline which occurred as the exercise has not been able to activate β2-ARs and β3-ARs in blood vessel as the stimulation and activation on these receptors could induce eNOS activation. Research conducted by Banquet et al, showed that the stimulation in β2-ARs could activate eNOS through Src kinase-PI3K/Akt-dependent.33
Adrenaline affects blood vessel dilatation through its affinity on adrenergic receptors. It has a magnitude of affinity for α1 adrenergic receptors (α1-ARs) and α2 adrenergic receptors (α2-ARs), which is almost the same as noradrenaline, but with a higher affinity for β2-ARs and lower for β3-ARs compared to noradrenaline. Low adrenaline concentrations results to vasodilation, while high adrenaline causes vasoconstriction.34 The concentration of adrenaline in our study, caused by MICT and HIIT had not significant effect on FMD, presumably because its concentration was not up to the level needed to stimulate adrenergic receptors in blood vessels.
Vasodilation of blood vessels is influenced by several mechanisms, including adenosine through the hyperpolarization of calcium-activated potassium channel membrane, or the endothelium-derived hyperpolarizing factor (EDHF) pathway,35 prostacyclin through the cyclic adenosine monophosphate (cAMP) pathway,36 and NO through the cyclic guanosine monophosphate (cGMP) pathway.37 Since eNOS expression increases the bioavailability of NO, this results in vasodilation of blood vessels which increases FMD. In our study, eNOS had no effect on FMD presumably due to the involvement of other pathways which are more dominant in influencing FMD, such as the EDHF or prostacyclin pathway. Hence, there is need for further research in both exercises.
The noradrenaline concentration influences eNOS concentration in both MICT and HIIT. This is a catecholamine hormone more associated with exercise compared with adrenaline. Circulating noradrenaline usually shows a metabolic effect with concentration more than 1.8 ng/mL.18 In our study, the average increase in noradrenaline concentration after the MICT and HIIT programs was 0.038 ± 0.082 and 0.160 ± 0.214 ng/mL respectively, which was able to increase the concentration of eNOS. It is assumed that increasing the concentrations of noradrenaline in both exercise were able to stimulate α-ARs and β2-ARs. Then, the stimulation of these adrenergic receptors resulted in an increase in Akt phosphorylation, which in turn mediates eNOS phosphorylation in Serine 1177, thereby increasing the concentration of eNOS.38,39
The noradrenaline stimulation of β3-ARs is also thought to be the underlying cause of the increase in eNOS after the exercise. Stimulation of β3-ARs has the capacity of activating eNOS through the Rac1-PKA-Akt pathway.40 The results in our study showed that the increase in the concentration of noradrenaline was more significant after performing HIIT than MICT. Similarly, HIIT increased eNOS concentrations compared to MICT. This is due to the fact that the noradrenaline concentration in HIIT was more significant in stimulating α-ARs, β2-ARs, and β3-ARs.
Noradrenaline is a protector against oxidants and known to be a superoxide free radical scavenger, with the capacity of inducing SOD expression.41 The increase in noradrenaline concentration, which is more significant in HIIT, and its function as an antioxidant which induces EC-SOD expression, is thought to be the reason why HIIT is more able to maintain EC-SOD activity than MICT.
Our study showed that the noradrenaline concentration of HIIT affected FMD negatively. This means that increase in noradrenaline results in decrease in FMD, and vice-versa. Noradrenaline stimulation of cardiac β1-ARs is comparable to adrenaline, its effect on peripheral α-ARs is equivalent, but its effect on β2-ARs for peripheral vasodilation is less than that of adrenaline. Noradrenaline significantly increases vascular resistance and reduces blood flow in the muscles compared with adrenaline.42 Although noradrenaline stimulates β2-ARs, its effect on the vasoconstriction of blood vessels is greater. This is because the anatomical location of α1-ARs is closer to the point where noradrenaline is released from terminal neurons compared to the location of β2-ARs. Also, the number and activity of vascular α1-ARs are greater compared with β-ARs, thereby resulting in vasoconstriction.43 This is thought to be the reason why a significant increase in noradrenaline could reduce the value of FMD.
However, noradrenaline has a greater affinity for β3-ARs compared to adrenaline.34 The new β3-ARs is stimulated when the plasma catecholamines are very high. This is considered a “safety valve” when intense adrenergic stimulation occurs at this receptor. In addition, the stimulation of β3-ARs has a cardioprotective effect on the ventricles and atrium of the heart in the form of a positive feedback mechanism against inotropic and chronotropic which increase due to the increase in this catecholamine hormone. Furthermore, the stimulation of β3-ARs reduces the incidence of arrhythmias due to the presence of the catecholamine hormone in β1-ARs.44 Thus, it is assumed that a significant increase in the concentration of noradrenaline in HIIT is expected to stimulate β3-ARs in heart, hence providing a better cardioprotection effect with the capacity of maintaining the balance of oxygen consumption in the heart muscle when there is a high increase in the catecholamine hormone.
Conclusion
Based on the results, HIIT increased the concentrations of noradrenaline and eNOS, as well as maintained the EC-SOD activity and FMD. Although, it was unable show an increase in adrenaline concentration more than MICT. Also, HIIT provided a better effect on cardiovascular protection compared with MICT, as a result of increased in noradrenaline and eNOS, as well as through its ability to maintain EC-SOD activity and FMD in stable CHD patients after coronary stenting.
However, there is need for future research on the effect of HIIT on cardiovascular protection using other means.
Acknowledgments
The authors are grateful to all nurses, staff, and trainers of the Cardiovascular Prevention and Rehabilitation Unit, Vascular Unit, and the Molecular Laboratory and Stem Cell Facility of the National Cardiovascular Center Harapan Kita, Jakarta, for making this project a success.
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO. Cardiovascular diseases (CVDs). Fact sheet. World Health Organization, 2016. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/.
2. Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the million hearts cardiac rehabilitation collaborative. Mayo Clin Proc. 2017;92(2):234–242. doi:10.1016/j.mayocp.2016.10.014
3. Alleman RJ, Stewart LM, Tsang AM, Brown DA. Why does exercise “trigger” adaptive protective responses in the heart? Dose-Response. 2015;13(1):1–19. doi:10.2203/dose-response.14-023.Alleman
4. Roh J, Rhee J, Chaudhari V, Rosenzweig A. The role of exercise in cardiac aging from physiology to molecular mechanisms. Circ Res. 2016;118:279–295. doi:10.1161/CIRCRESAHA.115.305250
5. Zouhal H, Jacob C, Delamarche P, Gratas-Delamarche A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008;38(5):401–423. doi:10.2165/00007256-200838050-00004
6. Calvert JW, Condit ME, Arago´n JP, et al. Exercise protects against myocardial ischemia–reperfusion injury via stimulation of B3-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res. 2011;108:1448–1458. doi:10.1161/CIRCRESAHA.111.241117
7. Tavernier G, Toumaniantz G, Erfanian M, et al. B3-adrenergic stimulation produces a decrease of cardiac contractility 3 ex vivo in mice overexpressing the human B3-adrenergic receptor. Cardiovasc Res. 2003;59:288–296. doi:10.1016/S0008-6363(03)00359-6
8. Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–1639. doi:10.1172/JCI9551
9. Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568(2):357–369. doi:10.1113/jphysiol.2005.089755
10. ACSM. Exercise prescription in patients with cardiovascular disease. In: Ehrman JK, dejong A, Sanderson B, Swain D, Swank A, Womack C, editors. ACSM’s Resource Manual for Guidelines for Exercise Testing and Prescription.
11. Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery. A report of the international brachial artery reactivity task force. J Am Coll Cardiol. 2002;39:257–265.
12. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–1085. doi:10.1161/HYPERTENSIONAHA.110.150821
13. Spina RJ, Chi MM-Y, Hopkins MG, Nemeth PM, Lowry OH, Holloszy JO. Mitochondrial enzymes increase in muscle in response to 710 days of cycle exercise. J Appl Physiol. 1996;80(6):2250–2254. doi:10.1152/jappl.1996.80.6.2250
14. Talanian JL, Galloway SDR, Heigenhauser GJF, Bonen A, Spriet LL. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J Appl Physiol. 2006;102(4):1439–1447. doi:10.1152/japplphysiol.01098.2006
15. Froelicher VF, Myers J. The physiologic response to the exercise Test. In: Froelicher VF, Myers J, editors. Manual of Exercise Testing.
16. Naik AD, Kallen MA, Walder A, Street RL. Improving hypertension control in diabetes mellitus the effects of collaborative and proactive health communication. Circulation. 2008;117:1361–1368.
17. Kjaer M, Christensen NJ, Sonne B, Richter EA, Galbo H. Effect of exercise on epinephrine turnover in trained and untrained male subjects. J Appl Physiol. 1985;59(4):1061–1067. doi:10.1152/jappl.1985.59.4.1061
18. Goldstein DS, McCarty R, Polinsky RJ, Kopin IJ. Relationship between plasma norepinephrine and sympathetic neural activity. Hypertension. 1983;5:552–559. doi:10.1161/01.HYP.5.4.552
19. Greiwe JS, Hickner RC, Shah SD, Cryer PE, Holloszy JO. Norepinephrine response to exercise at the same relative intensity before and after endurance exercise training. J Appl Physiol. 1999;86(2):531–535. doi:10.1152/jappl.1999.86.2.531
20. Padilla J, Harris RA, Rink LD, Wallace JP. Characterization of the brachial artery shear stress following walking exercise. Vascular Med. 2008;13:105–111. doi:10.1177/1358863x07086671
21. Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol. 2003;285:C499–C508. doi:10.1152/ajpcell.00122.2003
22. Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med. 2006;259:351–363. doi:10.1111/j.1365-2796.2006.01621.x
23. Taşolar H, Eyyüpkoca F, Aktürk E, et al. Endothelial nitric oxide synthase levels and their response to exercise in patients with slow coronary flow. Cardiovasc J Afr. 2013;24:355–359. doi:10.5830/CVJA-2013-072
24. Muthusamy VR, Kannan S, Sadhaasivam K, et al. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic Biol Med. 2012;52(2):366–376. doi:10.1016/j.freeradbiomed.2011.10.440
25. Gounder SS, Kannan S, Devadoss D, et al. Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PLoS One. 2012;7(9):e45697. doi:10.1371/journal.pone.0045697
26. He F, Li J, Liu Z, Chuang -C-C, Yang W, Zuo L. Redox mechanism of reactive oxygen species in exercise. FrontPhysiol. 2016;7(486):1–10.
27. Landmesser U, Merten R, Spiekermann S, Bu¨ttner K, Drexler H, Hornig B. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease. Relation to endothelium-dependent vasodilation. Circulation. 2000;101:2264–2270. doi:10.1161/01.CIR.101.19.2264
28. Djordjevic D, Cubrilo D, Zivkovic V, Barudzic N, Vuletic M, Jakovljevic V. Pre-exercise superoxide dismutase activity affects the pro/antioxidant response to acute exercise. Serbian J Exp Clin Res. 2010;11(4):147–155.
29. Goto C, Higashi Y, Kimura M, et al. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans. Role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003;108:530–535. doi:10.1161/01.CIR.0000080893.55729.28
30. Ramos JS, Dalleck LC, Tjonna AE, Beetham KS, Coombes JS. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med. 2015;45(5):679–692. doi:10.1007/s40279-015-0321-z
31. Tjønna AE, Lee SJ, Rognmo Ø, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome. A pilot study. Circulation. 2008;118:346–354. doi:10.1161/CIRCULATIONAHA.108.772822
32. Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol. 2008;104:588–600. doi:10.1152/japplphysiol.01096.2007
33. Banquet S, Delannoy E, Agouni A, et al. Role of Gi/o-Src kinase-PI3K/Akt pathway and caveolin-1 in B2-adrenoceptor coupling to endothelial NO Synthase in mouse pulmonary artery. Cell Signal. 2011;23:1136–1143. doi:10.1016/j.cellsig.2011.02.008
34. Tank AW, Wong DL. Peripheral and central effects of circulating catecholamines. Compr Physiol. 2015;5:1–15. doi:10.1002/cphy.c140007
35. Sato A, Terata K, Miura H, et al. Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease. Am J Physiol Heart Circ Physiol. 2005;288:H1633H1640. doi:10.1152/ajpheart.00575.2004
36. Zoladz JA, Majerczak J, Duda K, Chopicki S. Exercise-induced prostacyclin release positively correlates with VO2max in young healthy men. Physiol Res. 2009;58:229–238.
37. Sausbier M, Schubert R, Voigt V, et al. Mechanisms of NO/cGMP-Dependent Vasorelaxation. Circ Res. 2000;87:825–830. doi:10.1161/01.RES.87.9.825
38. Jiang Q, Ding S, Wu J, Liu X, Wu Z. Norepinephrine stimulates mobilization of endothelial progenitor cells after limb ischemia. PLoS One. 2014;9(7):e101774. doi:10.1371/journal.pone.0101774
39. Yu W, Gu Y, Chen P, et al. Norepinephrine stimulation downregulates the β2-adrenergic receptor–nitric oxide pathway in human pulmonary artery endothelial cells. J Cell Physiol. 2019;234(2):1842–1850. doi:10.1002/jcp.27057
40. Kou R, Michel T. Epinephrine regulation of the endothelial nitric-oxide synthase: roles of RAC1 and beta3-adrenergic receptors in endothelial NO signaling. J Biol Chem. 2007;282:32719–32729. doi:10.1074/jbc.M706815200
41. Ramel A, Wagner K-H, Elmadfa I. Correlations between plasma noradrenaline concentrations, antioxidants, and neutrophil counts after submaximal resistance exercise in men. Br J Sports Med. 2004;38:e22. doi:10.1136/bjsm.2003.007666
42. Leclerc KM, Levy WC. The role of norepinephrine in exercise impairment in congestive heart failure. CHF. 2003;9:25–28. doi:10.1111/j.1527-5299.2002.00948.x
43. Opie LH. Control of the circulation. In: Weinberg RW, Bersin J, Aversa F, editors. Heart Physiology. From Cell to Circulation.
44. Rozec B, Gauthier C. β3-Adrenoceptors in the cardiovascular system: putative roles in human pathologies. Pharmacol Ther. 2006;111:652–673. doi:10.1016/j.pharmthera.2005.12.002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.