Back to Journals » Drug, Healthcare and Patient Safety » Volume 12
Burden of Healthcare-Associated Infections and Associated Risk Factors at Adama Hospital Medical College, Adama, Oromia, Ethiopia
Authors Chernet AZ, Dasta K , Belachew F, Zewdu B, Melese M , Ali MM
Received 28 February 2020
Accepted for publication 7 October 2020
Published 14 October 2020 Volume 2020:12 Pages 177—185
DOI https://doi.org/10.2147/DHPS.S251827
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Rajender R Aparasu
Adinew Zewdu Chernet,1 Kassu Dasta,2 Feleke Belachew,3 Baharu Zewdu,4 Mengistu Melese,5 Musa Mohammed Ali6
1Oromia Public Health Laboratory, Adama, Ethiopia; 2College of Health Science, School of Medical Laboratory Science, Addis Ababa University, Addis Ababa, Ethiopia; 3International Centers for AIDS Care and Treatment Program (ICAP), Addis Ababa, Ethiopia; 4Washington Medical Center, Addis Ababa, Ethiopia; 5Adama Hospital Medical College, Adama, Ethiopia; 6College of Medicine and Health Sciences, School of Medical Laboratory Science, Hawassa University, Hawassa, Ethiopia
Correspondence: Adinew Zewdu Chernet Email [email protected]
Introduction: Healthcare-associated infection (HCAI) is a type of infection that is acquired while receiving healthcare services in a hospital or other healthcare settings. The objective of this study was to determine the incidence of HCAI and associated factors at Adama Hospital Medical College (AHMC), Adama city, Ethiopia.
Method: A hospital-based longitudinal study was conducted among 300 participants at AHMC from February to May 2017. The study participants’ clinical characteristics were collected using a structured interview and clinical evaluations. Data were analyzed by descriptive statistics using SPSS software version 20. Various clinical samples collected from participants were processed and bacteria were isolated by using standard microbiological methods recommended by the World Health Organization.
Result: The total incidence rate of HCAI was 9.7 [95% CI: 7.1– 12.9] cases per 1000 persons-days. Specific incidence rates were as follows: 8 cases per 1000 person-days [95% CI: 08.74, 20.66] for surgical site infections; 60.2 cases per 1000 device-days [95% CI: 33.47, 100.3] for catheter-associated urinary tract infections; 1.4 cases per 1000 device-days [95% CI: 0.06752, 6.656] for catheter-associated bloodstream infections; 14.1 cases per 1000 device-days [95% CI: 0.7047, 69.46] for ventilator-associated pneumonia; 73.5 cases per 1000 person-days [95% CI: 26.94, 163] for non-surgical skin break infections and 0.6 cases per 1000 person-days [95% CI: 0.02906, 2.864] for antibiotic-associated diarrhea. Most of the infections were caused by Gram-negative bacteria. Renal disease and type 2 diabetes mellitus were significantly associated with HCAI (P< 0.05).
Conclusion: HCAI was predominant in this study. The major contributing factors for HCAI at AHMC were renal disease and type 2diabetes mellitus.
Keywords: healthcare-associated infection, risk factors, Adama Hospital Medical College, Ethiopia
Introduction
In developed countries, 5–15% of hospitalized patients and more than 50% of patients in intensive care units (ICUs) develop healthcare-associated infections (HCAIs).1–3 In resource-limited countries, the magnitude of HCAIs is underestimated or unknown due to the absence of well-established surveillance system.4 The burden of HCAIs is assumed to be high in less developed countries because of health-care system deficiencies such as overcrowding in healthcare settings, understaffing, inadequate infection control practices, and lack of infection control policies.5
HCAI can be caused by normal flora which are transferred to sterile sites of the human body and by use of contaminated medical devices.6 In addition, healthcare workers can transmit potential pathogens from one patient to the other or from themselves to the other patients while providing healthcare services.6
HCAI causes about 100,000 deaths and loss of 20 billion United States (US) dollars each year.7 HCAIs can lead to prolonged hospital stays, long-term disabilities; and adds additional costs to patients and government.2,8,9 Central line associated blood stream infection (CLABSI) occurs up to 80,000 times per year resulting in 28,000 deaths among patients in the ICU.9
Even though there is an improvement in the treatment and control, infectious diseases remain a significant cause of morbidity and mortality in low and middle-income countries when compared to high-income countries.2,8 Approximately, 10% of patients admitted to hospitals in developed countries acquire HCAI but the risk may reach up to 20 times in developing countries.1 HCAI represents a major threat to patient safety and quality of healthcare in developing countries.10 There are limited data on HCAIs from Ethiopia.8 In this study, we sought to determine the incidence of healthcare-associated infections and associated risk factors at Adama Hospital Medical College (AHMC). The finding of this study will contribute in strengthening infection prevention strategy in the study area.
Methods
Study Setting
AHMC is located in Adama city which is 98 kilometer far away to the east of Addis Ababa, the capital of Ethiopia. AHMC has a total of 184 beds. At AHMC, the average outpatient flow per week and the admission rate per week during the study period were 856 and 173, respectively. In the AHMC hospital, there was an infection prevention and control protocol; however, there was no routine surveillance for HCAIs during the study period.
Study Design and Study Population
A hospital-based longitudinal study was conducted from February to April 2017. All patients without evidence of bacterial infections during admission to AHMC were considered for the study. A total of 300 study participants were included and followed for a maximum of three months until they leave the hospital.
Inclusion Exclusion Criteria
Patients admitted to and stayed at AHMC for a minimum of 48 hours were included in the study. Patients who stayed in the hospital for less than 48 hours; patient admitted due to bacterial infection and those aged less than 18 years were excluded from the study.
Study Variables
Dependent variables: Healthcare-associated infection.
Independent variables: Factors that could be associated with healthcare-associated infection.
Operational Definitions
Incidence of healthcare-associated infections: Incidence rate or person-time rate is a measure of incidence that incorporates time directly into the denominator. A person-time rate is generally calculated from a long-term cohort follow-up study, wherein enrollees are followed over time and the occurrence of new cases of disease is documented. Typically, each person is observed from an established starting time until one of end points is reached. The length of stay of the study participants are the sum of the time each person was observed, added for all persons.
(http://www.openepi.com/PersonTime1/PersonTime1.htm)
Surgical site infection (SSI): Infection that occurs within 30 days after an operative procedure.
Cather associated Urinary tract infection (CAUTI): Infection of urinary tract after the patient had an indwelling urinary catheter that had been in place for >2 days on the date of event.
Central line-associated bloodstream infection (CABI): Is a laboratory-confirmed bloodstream infection in a patient where the central line was in place for >2 calendar days (48 hours).
Ventilator-associated pneumonia (VAP): Is a type of pneumonia that occurs within 48–72 hours or thereafter following endotracheal intubation or ventilator use.
Antibiotic associated diarrhea (AAD): Is a diarrhea that occurs usually four to ten days after taking an antibiotic.
Non-surgical skin-break infections (NSSI): Is an infection that occurs after a skin break occurs due to pressure sore, diabetic ulcer, abscess, etc., during hospital admission.
Data Collection
Socio-demographic and clinical data were collected using a structured questionnaire. Study participants were followed until they develop HCAI, recover, referred to other hospital, or death occurred. Specimens such as swabs, clean-catch midstream urine, blood, sputum, and stool were collected from participants who were suspected of HCAIs. The specimens were processed and bacteria were isolated by using standard microbiological techniques according to the World Health Organization (WHO) manual.11 Briefly the specimen was assessed first by using Gram staining then cultured on different medias (blood agar, chocolate agar, MacConky agar) depending on the type of specimen. Identity of bacteria was confirmed by using various biochemical tests such as catalase tests, coagulase tests, and biochemical test panel for Gram-negative bacteria.
Data Management and Analysis
Data were checked for completeness before entry and then entered, cleaned and analyzed using statistical software –SPSS version 20. The incidence of HCAI was determined with 95% CI. A Chi-square test was used to identify factors that could be associated with HCAIs. Bivariate and multivariable logistic regressions were used to determine the crude relative risk (RR) and adjusted relative risk (ARR). A P-value was used as a level of statistical significance at the 0.05.
Data Quality Control
The quality of socio-demographic and clinical data was maintained by using a pre-tested and structured questionnaire. For laboratory analysis, the sterility of the culture media was checked by incubating 5% of them inappropriate conditions. Quality control strains were used throughout the laboratory procedures for checking the performance of culture methods.
Results
Socio-Demographic Data
In this study, 300 patients out of 400 were included; 100 were excluded because they developed infections within 48 hours of admission. From the total of 300 study participants recruited, 296 (98.7%) were from Oromia, 185 (61.7%) were females, 108 (36%) belong to the 25–34 age group. The average age of the study participants were 37 years. Majority of the admissions were emergency type (Table 1).
 |
Table 1 Socio-Demographic Data of the Study Participants Who Were Admitted at Adama Hospital Medical College, Adama Ethiopia, 2017 (N=300) |
Magnitude of HCAIs and Discharge Outcome
Out of the 300 participants included in the current study, 42 (14%) developed HCAIs (culture-confirmed infections). Proportions of specific culture-confirmed HCAIs were as follows: SSI (n=21; 7%), CAUTI (n=12; 4%), CABSI (n=1; 0.3%), VAP (n=1; 0.3%), AAD (n=1; 0.3%), and non-surgical skin-break infections (NSSBI) (n=5; 1.7%). The total incidence of HCAI was 9.7 cases/1000 persons-days, 95% CI [7.1, 12.9]. In the current study the incidence of various HCAIs is presented as follows: 13.8 per 1000 person-days CI [8.7, 20.7] for SSIs; 60.2 per 1000 device-days CI [33.5, 100.3] for CAUTI; 1.4 per 1000 device-days CI [0.1, 6.7] for CABSI; 14.1 per 1000 device-days CI [0.7, 69.5] for VAP; 73.5 per 1000 person-days CI [26.9, 163] for NSSBI, and (0.6 per 1000 person-days CI [0.03, 2.9]) for AAD. Most culture-confirmed HCAIs were from orthopedic ward and from participants within the 25–34 age group (Table 2). One percent of study participants died (all of them were from those who developed HCAIs), 94% recovered, 3% were referred to other hospitals for further management, 2% were self-discharged (Table 3).
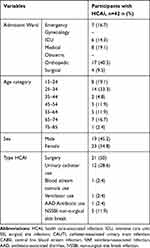 |
Table 2 Magnitude of HCAI Among Patients Admitted in Different Ward and Type of Services Service at Adama Hospital Medical Collages, Adama, Ethiopia |
 |
Table 3 Reason for Discharge of Study Participants at Adama Hospital Medical College, Adama Ethiopia, 2017 (N=300) |
The total patient lengths of stays at AHMC were 3196 days and the average lengths of stay were 10.65 (95% CI: 9.55, 11.76) days. The highest and the least length of stay were 804 and 85 days which was observed at the orthopedic and emergency ward, respectively. The total length of patient stay with and without HCAI in each ward were 737 and 2459 days and the average length of stays were 17.5 (95% CI: 13.9, 20.7) and 8.28 (7.44, 9.32) days, respectively.
Risk Factors Associated with HCAIs
Among factors assessed in this study, renal disease and type 2 diabetic mellitus were significantly associated with HCAIs (P<0.05) (Tables 4 and 5).
 |
Table 4 Calculated Value of HCAI Relative Risk, Risk in Exposed and Incident Rate at Adama Hospital, Adama Ethiopia 2017 (N=300) |
 |
Table 5 Risk Factors Associated with HCAIs at Adama Hospital, Adama Ethiopia 2017 |
Bacteria Isolated from Study Participants with HCAIs
In the present study, majority of bacteria isolated were Gram-negative: E. coli (30.9%) and Citrobacter freundii (14.3%). Most bacteria were isolated from patients admitted to the orthopedic and medical ward (Table 6).
 |
Table 6 Distribution of Bacterial Isolates from Various Wards at Adama Hospital Medical College, Adama, Ethiopia, 2017 (N=42) |
Discussion
In the current study, 14% of patients admitted to AHMC developed culture-confirmed HCAIs. This finding is similar to the study conducted in Amhara regional state, Ethiopia (14.9%); however, it is low compared to a report from Jimma, Ethiopia (19.4%).12 The finding is within the range of a report from a systematic review conducted in Africa (2.5–14.8%).5 The proportion identified in the present study is high compared to proportions of HCAIs reported from Ireland (5.3%),13 United States (2.8%),14 Gabon (1.6%),2 and other developed countries (5–10%);1 however, with respect to low-middle income countries it is low compared Brazil (51.2%)15 and it is high compared to study from Slovenia (4.4%).16 This variability could be attributed to the demographic characteristics of the study participants, quality of services provided, poor hygiene, the underlying medical conditions, type of ward, and laboratory method used.
The cumulative incidence of HCAI in our study area, 9.7 cases/1000 persons-days, is lower than the incidence of HCAI recently reported from Jimma, Ethiopia (28.15/1000 patient days)17 and other countries.18 The proportion of SSIs (7%) we found in this study is not in line with finding reported from Bahirdar, Ethiopia (10.2%)19 but it is higher than a report from Slovenia (1.5%).16 The incidence of SSI in our study, 13.8 per 1000 person-days, is comparable with findings from low and middle-income countries; however, it is high compared to developed countries.8
The incidence of CAUTI in this study, 60.2 cases per 1000 device-days, is higher than the incidence reported from India.20,21 This finding is not in line with studies conducted in other Asian countries.17 This variability is maybe due to the type of urinary catheters used in respective hospitals, the experience of the nurse to insert the catheter, underlying medical condition.20 The incidence (14.1 cases/1000 device-days) and the proportion (0.3%) of VAP identified in the current study is not comparable with a report from low-middle income countries.17,20,22,23
Among patients who used an intravenous cannula, the proportion of CABSI was 0.3% which is lower than the study conducted in Bahirdar, Ethiopia (2.4%).19 The incidence of CABSI (1.4 cases/1000 device-days) found in the current study is low compared to a incidence reported Scotland24 and India.20,21 The reason for this can be due to the aseptic usage of intravenous catheters in our study area and the majority of the patients were given antibiotics during admission as prophylaxis before surgery and other medical procedure.
The proportion of HCAIs among VAP was 0.3% and the incidence was 14.1 cases per 1000 device-days which are in agreement with a systematic literature review and a meta-analysis conducted in Southeast Asia.17 A study conducted by the American Thoracic Society and Infectious Disease Society of America (IDSA) indicated that VAP occurs at a rate between 5 and 15 cases per 1,000 hospital admissions and accounted for approximately 15% of all hospital-acquired infections.20,23 Our finding is higher than studies conducted in India (5.42/1000 ventilator days)20 and Scotland (5.2 per 1000 invasive respiratory device days).24 This variation is due to the type of wards where the study was conducted, in both studies the setting was ICU but in our study, all patients were from general ward. In this study, five patients developed an infection from eight study participants with non-surgical skin breaks due to diabetes, pressure sore and other reasons during admission.
The most prevalent bacteria that caused HCAIs in our study were E. coli (30.9%), C. freundii (14.3), Pseudomonas aeruginosa (9.5%) and Klebsiella pneumoniae (9.5%) and the least prevalent were Candida albicans (2.4%). The majority of the bacteria were Gram-negative rod bacteria. E. coli was the most predominant bacteria in all wards. This finding is in agreement with studies conducted in Tigray, Ethiopia25 and Brazil;15 However, another study from Ethiopia indicated Klebsiella species (22.44%) and Staphylococcus aureus (20.4%) to be the predominant bacteria among patients with HCAIs.18 According to a study from Bahirdar, Ethiopia, S. aureus (26.2%), E. coli (21.4%) and Coagulase-negative Staphylococcus species (21.4%) were the predominant bacteria.19 Unlike the current study, the most common bacteria associated with HCAI in Northern India were P. aeruginosa and Acinetobacter species.21 The most common bacteria isolated from urine in India were P. aeruginosa (34.48%), Enterococcus species (13.79%), K. pneumoniae (13.79%), and Candida species (13.79%).20 This indicates the type of bacteria isolated from HCAIs may vary with geographical locations, nature of the patient immunity, and the type of services obtained in the respective health settings. Among factors assessed in this study, renal disease (P=0.02) and type 2 diabetes mellitus (P=0.02) were significantly associated with HCAIs. The immune status of patients with type 2 diabetes mellitus and frequent use of the external device could have contributed to the development of HCAIs.26,27
Limitation of the Study
The first limitation is this study was a short-term and single-centered study without follow-up. The other limitation is HCAI that could develop after discharge and those which could occur among individuals aged less than 18 years was not determined.
Conclusion
In this study, out of 300 patients admitted at AHMC, 14% of them developed HCAIs. The proportion of SSI, CAUTI, CABSI, VAP, AAD, and NSSBI were 7%, 4%, 0.3%, 0.3%, 0.3%, and 1.7, respectively. The total incidence of HCAI was 9.7 cases/1000 persons-days. The predominant bacteria were E. coli. Diabetes mellitus type 2 and renal disease were significantly associated with healthcare-associated infections.
Abbreviations
HCAI, healthcare-associated infection; AAD, antibiotic-associated diarrhea; CABSI, central line-associated bloodstream infection; VAP, ventilator-associated pneumonia; SSI, surgical site infection; ICU, intensive care unit; AHMC, Adama hospital Medical College; WHO, World Health Organization.
Ethical Approval and Consent to Participate
The study was approved by the Institutional Review Board of the College of Health Sciences, Addis Ababa University (Ref No: DRERC/231/16/MLS). Written informed consent was obtained from all study participants. The study was conducted in accordance with the Declaration of Helsinki.
Acknowledgment
We would like to acknowledge staffs of AHMC and Oromia public health laboratory for facilitating the study during sample collection and processing. We also acknowledge study participants for their willingness to participate in the study.
Author Contributions
AZC Conceived designed the experiments, writing the research proposal, laboratory work, data analysis and interpretation and manuscript preparation. KD Writing research proposal, data analysis and manuscript preparation. MM writing the research proposal, data analysis, supervision and Manuscript preparation. FB Facilitated laboratory work, writing research proposal, supervision and manuscript preparation. BZ Proposal write up, Supervision of work, Execution of activity, acquisition of data, report writing, and manuscript preparation MMA Proposal write up, data management, data analysis and interpretation and manuscript preparation. All authors made significant contributions to study design, study execution, acquisition of data or analysis of data. All authors drafted, or substantially revised the manuscript, agreed on the journal for submission, and reviewed and approved all versions of the article before submission, and during the revision stage. All authors agree to take responsibility and be accountable for the contents of the article.
Funding
This study was supported by Addis Ababa University and Oromia public health laboratory. Addis Ababa University provided supplies required for the study. Oromia public health laboratory provided supplies and space required for the study. It also covered payment for data collectors.
Disclosure
The authors have declared that no competing interests exist.
References
1. Khan HA, Ahmad A, Mehboob R. Nosocomial infections and their control strategies. Asian Pac J Trop Biomed. 2015;5(7):509–514. doi:10.1016/j.apjtb.2015.05.001
2. Pittet D, Allegranzi B, Boyce J, World Health Organization World Alliance for Patient Safety First Global Patient Safety Challenge Core Group of Experts. The World Health Organization guidelines on hand hygiene in health care and their consensus recommendations. Infect Control Hosp Epidemiol. 2009;30(7):611–622. doi:10.1086/600379
3. Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. doi:doi:10.1001/jama.2009.1754
4. Allegranzi B, Pittet D. Preventing infections acquired during health-care delivery. The Lancet. 2008;372(9651):1719–1720. doi:10.1016/S0140-6736(08)61715-8
5. Nejad SB, Allegranzi B, Syed SM, Ellis B, Pittet D. Health-care –associated infection in Africa: a systematic review. Bull World Health Organ. 2011;89:757–765. doi:doi:10.2471/BLT.11.088179.
6. Kolmos HJ. Health care associated infections: sources and routes of transmission. In: Sudhakar C, editor. Infection Control–Updates. Vol. 22. Rijeka, Croatia: Intech; 2012:21–38.
7. Sprague L. Health Care-Associated Infections. Washington, D.C.: National Health Policy Forum 2009; 1–14. Available from: www.nhpf.org.
8. World Health Organization. Report on the burden of endemic health care-associated infection worldwide. Available from: https://apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng.pdf.
9. Tripathi S. Health care quality and hospital acquired infection in Intensive care. Br J Med Pract. 2014;7(2):7–9.
10. Raka L, Mulliqi-Osmani G. Infection control in developing world. Infection Control e Updates InTech. 2012;22:65e78.
11. Vandepitte J, Verhaegen J, Engbaek K, et al. Basic Laboratory Procedures in Clinical Bacteriology. World Health Organization; 2003:31.
12. Ali S, Birhane M, Bekele S, et al. Healthcare associated infection and its risk factors among patients admitted to a tertiary hospital in Ethiopia: longitudinal study. Antimicrob Resist Infect Control. 2018;7(1):2. doi:10.1186/s13756-017-0298-5.
13. Roche FM, Donlon S, Burns K. Point prevalence survey of healthcare-associated infections and use of antimicrobials in Irish intellectual disability long-term care facilities: 2013. J Hosp Infect. 2016;93(4):410–417. doi:10.1016/j.jhin.2016.03.006
14. Greco G, Shi W, Michler RE, et al. Costs associated with health care–associated infections in cardiac surgery. J Am Coll Cardiol. 2015;65(1):15–23. doi:10.1016/j.jacc.2014.09.079
15. Braga IA, Campos PA, Gontijo-Filho PP, Ribas RM. Multi-hospital point prevalence study of healthcare-associated infections in 28 adult intensive care units in Brazil. J Hosp Infect. 2018;99(3):318–324. doi:10.1016/j.jhin.2018.03.003
16. Klavs I, Serdt M, Korošec A, Zupanc TL, Pečavar B. Prevalence of and factors associated with healthcare-associated infections in Slovenian acute care hospitals: results of the third national survey. Slovenian J Public Health. 2019;58(2):62. doi:10.2478/sjph-2019-0008
17. Ling ML, Apisarnthanarak A, Madriaga G. The burden of healthcare-associated infections in Southeast Asia: a systematic literature review and meta-analysis. Clin Infect Dis. 2015;60(11):1690–1699. doi:10.1093/cid/civ095
18. Yallew WW, Kumie A, Yehuala FM. Point prevalence of hospital-acquired infections in two teaching hospitals of Amhara region in Ethiopia. Drug Healthc Patient Saf. 2016;8:71. doi:10.2147/DHPS.S107344
19. Mulu W, Kibru G, Beyene G, Damtie M. Postoperative nosocomial infections and antimicrobial resistance pattern of bacteria isolates among patients admitted at FelegeHiwot Referral Hospital, Bahirdar, Ethiopia. Ethiop J Health Sci. 2012;22(1):7–18.
20. Masih SM, Goel S, Singh A, Khichi SK. Epidemiology and risk factors of healthcare associated infections from intensive care unit of a tertiary care hospital. Int J Res Med Sci. 2016;4(5):1706. doi:10.18203/2320-6012.ijrms20161254
21. Datta P, Rani H, Chauhan R, Gombar S, Chander J. Health-care-associated infections: risk factors and epidemiology from an intensive care unit in Northern India. Indian J Anaesth. 2014;58(1):30. doi:10.4103/0019-5049.126785
22. Mello MJ, Albuquerque MD, Lacerda HR, Souza WV, Correia JB, Britto MC. Risk factors for healthcare-associated infection in pediatric intensive care units: a systematic review. Cadernos De Saude Publica. 2009;25:S373–91.
23. Tedja R Hospital – acquired, health care – associated, and ventilator – associated pneumonia. Cleveland Clinic. 2013. Available from: ww.cdc.gov/nhsn/PDFs/vae/CDC_VAE_CommunicationsSummary-for-compliance_20120313.pdf.
24. Surveillance of Healthcare Associated Infections in Scottish Intensive Care Units. National Services Scotland. 2012. Available from: https://www.hps.scot.nhs.uk/web-resources-container/surveillance-of-healthcare-associated-infections-in-scottish-intensive-care-units-annual-report-of-data-from-january-2011-to-december-2011/.
25. Tesfahunegn Z, Asrat D, Woldeamanuel Y, Estifanos K. Bacteriology of surgical site and catheter related urinary tract infections among patients admitted in Mekelle Hospital, Mekelle, Tigray, Ethiopia. Ethiop Med J. 2009;47(2):117–127.
26. Wright S, Doron S, Sarnak MJ. Kidney function and hospital-acquired infections: worth a deeper look. Am J Kidney Dis. 2019;73(1):1–3. doi:10.1053/j.ajkd.2018.08.019
27. Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88(11):1254–1264. doi:10.2522/ptj.20080020
28. http://etd.aau.edu.et/bitstream/handle/123456789/3016/Adinew%20Zewdu.pdf?sequence=1&isAllowed=y.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

