Back to Journals » Vascular Health and Risk Management » Volume 15
Biomarkers in acute myocardial infarction: current perspectives
Authors Aydin S , Ugur K, Aydin S , Sahin İ , Yardim M
Received 2 November 2018
Accepted for publication 14 December 2018
Published 17 January 2019 Volume 2019:15 Pages 1—10
DOI https://doi.org/10.2147/VHRM.S166157
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Magnus Bäck
Suleyman Aydin,1 Kader Ugur,2 Suna Aydin,3 İbrahim Sahin,1,4 Meltem Yardim1
1Department of Medical Biochemistry and Clinical Biochemistry (Firat Hormones Research Group), Medical School, Firat University, Elazig 23119, Turkey; 2Department of Internal Medicine (Endocrinology and Metabolism Diseases), School of Medicine, Firat University, Elazig 23119, Turkey; 3Cardiovascular Surgery Department, Elazig Research and Education Hospital, Health Science University, Elazig 23119, Turkey; 4Department of Medical Biology, Medical School, Erzincan Binali Yildirim University, Erzincan 24100, Turkey
Purpose: Acute myocardial infarction (AMI) is the most common cause of death in the world. Comprehensive risk assessment of patients presenting with chest pain and eliminating undesirable results should decrease morbidity and mortality rates, increase the quality of life of patients, and decrease health expenditure in many countries. In this study, the advantages and disadvantages of the enzymatic and nonenzymatic biomarkers used in the diagnosis of patients with AMI are given in historical sequence, and some candidate biomarkers – hFABP, GPBB, S100, PAPP-A, RP, TNF, IL6, IL18, CD40 ligand, MPO, MMP9, cell-adhesion molecules, oxidized LDL, glutathione, homocysteine, fibrinogen, and D-dimer procalcitonin – with a possible role in the diagnosis of AMI are discussed.
Methods: The present study was carried out using meta-analyses, reviews of clinical trials, evidence-based medicine, and guidelines indexed in PubMed and Web of Science.
Results: These numerous AMI biomarkers guide clinical applications (diagnostic methods, risk stratification, and treatment). Today, however, TnI remains the gold standard for the diagnosis of AMI. Details in the text will be given of many biomarkers for the diagnosis of AMI.
Conclusion: We evaluated the advantages and disadvantages of routine enzymatic and nonenzymatic biomarkers and the literature evidence of other candidate biomarkers in the diagnosis of AMI, and discuss challenges and constraints that limit translational use from bench to bedside.
Keywords: acute myocardial infarction, cardiac protein, cardiac peptide
Introduction
Acute myocardial infarction (AMI) is one of the major causes of mortality and morbidity worldwide.1 About 10% of patients who are admitted to emergency departments with chest pain every year are diagnosed with heart attack.2 AMI is a condition that can be due to ischemic heart disease or coronary artery disease in conjunction, and it becomes manifest when an atherosclerotic plate ruptures and a developing thrombus occludes the coronary artery totally or partially, restricting blood access to the heart (Figure 1).3,4 In this case, the opening of the occluded coronary artery is usually provided by inserting a stent. However, when stents are insufficient, coronary bypass is performed by cardiac pulmonary bypass surgery using the left internal mammary artery or saphenous vein to maintain regular nourishment of the heart.5
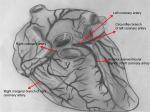  | Figure 1 Gross anatomy of heart. |
AMI has found its place in the concept of acute coronary syndrome (ACS). ACS includes a group of clinical syndromes ranging from unstable angina pectoris, AMI with non-S (downward deflection immediately after ventricular contraction)-segment elevation, and T (recovery of ventricles)-segment elevation to AMI, with ST-segment elevation and sudden death.6 The sensitivity and specificity of electrocardiography (ECG) are low in ACS.7 In the majority of cases with ST-segment elevation in ECG and typical ischemic chest pain, AMI with a Q wave (downward deflection immediately preceding ventricular contraction) develops in most cases and AMI without a Q wave develops in a few. However, the majority of cases without ST-segment elevation develop unstable angina pectoris or AMI without a Q wave, with a few developing AMI with a Q wave. ST-segment elevation changes into ST-segment depression when an oxygen-free environment persists.8
As the sensitivity and specificity of ECG are low in diagnosing AMI, the criteria for AMI were decided by the European Society of Cardiology (ESC) and the American College of Cardiology (ACC).9,10 Accordingly, a patient has to have at least two of the following: typical symptoms, a characteristic elevation or decrease pattern in cardiac markers (eg, CK-MB izoenzymes), preferably serum troponins (cTnI or cTnT), or a typical ECG trace with Q waves that indicate a diagnosis of AMI.4,11
Ischemia due to decreased coronary artery flow causes deterioration of ventricular function and myocardial necrosis.12 Therefore, such enzymes as ALT, AST, LDH, CK, and troponins have been indicators for years us a diagnosis of AMI.13,14 Therefore, in our study, cardiac markers (cTnI and cTnT), CK-MB, and myoglobin, frequently used in diagnosing AMI and determining prognosis, are mentioned in review, and our intention is to discuss some other important candidate biomarkers, such as copeptin15 and irisin,16 which may be relevant in diagnosing AMI and determining prognosis (Figure 2).
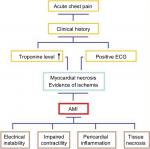  | Figure 2 Diagnosis chart of AMI and possible physiological effects. Abbreviations: ECG, electrocardiography; AMI, acute myocardial infarction. |
Methods
This systematic review was done through meta-analyses, reviews of clinical trials, evidence-based medicine, consensus development conferences, and guidelines in PubMed and Web of Science. Citations from journals specializing in clinical review studies are also included.
Search terms were: acute coronary syndromes, acute myocardial infarction, creatine kinase (CK), cardiac proteins, adropin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), Brain natriuretic peptide (BNP), chronic renal failure (CRF), copeptin, C-reactive protein (CRP), D-dimer procalcitonin, fatty-acid-binding protein (FABP), fibrinogen, glutathione, glycogen phosphorylase (GP), glycogen phosphorylase isoenzyme BB (GPBB), heart-type fatty acid binding protein (hFABP), homocysteine, intracellular calcium binding protein dimer (S100), irisin, ischemia-modified albumin (IMA), lactate dehydrogenase (LDH), macroglobulin complex, metalloproteinase 9 (MMP9), myeloperoxidase (MPO), myoglobin, oxidized LDL, phosphatidic acid (PA), phosphatidylcholine (PC), phospholipase D (PLD), plasma choline (PlCho), pregnancy associated plasma protein A (PAPP-A), tropomyosin, troponins (cTn, TnC, TnI, TnT), tumor necrosis factor (TNF), and whole-blood choline (WBCho).
What should make an ideal cardiovascular biomarker?
There are no “ideal” biochemical markers for the diagnosis of almost all diseases in medicine. This fact increases the health costs of countries and morbidity and mortality in its inadequacy. Despite recent advances in medicine, mortality and morbidity due to cardiovascular diseases remain the foremost problem in world health. The presence of an ideal biochemical marker of the cardiac system would reduce the morbidity and mortality rate associated with AMI.
An ideal cardiac marker: 1) must be sensitive enough to detect a small degree of damage to the heart, 2) should be specific to the heart muscle (it must exclude damage to other [skeletal] muscles), 3) should give information regarding the severity of the infarct and the prognosis of the disease, 4) should also show the result of reperfusion therapy in AMI, 5) needs to distinguish between reversible and irreversible damage, 6) ought not to be detected in patients showing no myocardial damage, 7) should help in early and late diagnosis, 8) should be easy to measure, fast, cheap, and quantitative, and finally, 9) should have long-term storage conditions and be stable under them.4,17,18 In the following sections, we try to formulate a scientific approach to find an ideal cardiac marker and the effort required to achieve this end.
Results
Cardiac enzymes previously used in diagnosis of AMI
Historically, AST and LDH enzymes were first used in the diagnosis of AMI. However, since these enzymes do not have the characteristics of ideal cardiac markers, they were duly abandoned. However, since the measurements of at any hospital are limited and easy, they have the capacity to give an idea to physicians when they are measured, combined with the patient’s history. It should be remembered that CK and CK-MB enzyme measurements remain as valuable parameters in the diagnosis of AMI.13,14
Role of aspartate aminotransferase in diagnosis of AMI
Ladue et al suggested in 1954 that AST (formerly called glutamate oxaloacetate transaminase) released from the necrotic cardiac myocytes to the circulation could be helpful in diagnosing AMI. This was the first biomarker used in the diagnosis of AMI, but is no longer used today because it is not specific to the heart. However, the age of the enzyme in the diagnosis of AMI in cardiology had been initiated. Just 1 year after the assertion that AST could help to diagnose AMI, LDH was seen to be a useful marker in the diagnosis of AMI.19,20
Lactate dehydrogenase in diagnosis of AMI
LDH is expressed in many organs, including skeletal muscle (maximal), kidney, liver, heart, lung, and erythrocytes. LDH has five isoenzymes. The heart has LDH1, but it is not highly specific to the heart.21 Because it is released from erythrocytes, kidney, brain, skeletal muscle, stomach, and pancreas, it can be elevated in some tumors (seminoma/dysgerminoma).22 It increases within 6–12 hours from the onset of chest pain, peaks over 1–3 days, and returns to normal values within 8–14 days. An LDH1:LDH2 ratio >1 is reported to be specific for AMI, but currently it is not used in the diagnosis of AMI.23 Today, the only use of LDH is in distinguishing acute from subacute MI in patients who reach hospital in the late stage of the disorder, with positive troponins whose CK and CK-MB values have returned to normal levels.24
Role of creatine kinase and CK-MB in diagnosis of AMI
CK is an enzyme that catalyzes the reversible transformation of creatine and ATP to creatine phosphate and ADP.25 With the discovery of a radioimmunoassay in 1970,26 measurement of CK activity was considered a better predictor of heart-muscle damage and an indispensable parameter of laboratories in the diagnosis of AMI for 20 years.27 CK is involved in mitochondria and cytosol in muscle cells. The dimeric enzyme, consisting of two subunits, M and B, has three isoenzymes: CK-BB (CK1), CK-MB (CK2), and CK-MM (CK3). CK-MM is the dominant form found in all tissue.28 CK-BB is present in the brain, kidney, and gastrointestinal tract. CK-MB can be found in the heart, skeletal muscle, small intestine, diaphragm, uterus, tongue, and prostate.29 About 20% of total CK in the myocardium is in the MB form, giving sensitivity and specificity in the diagnosis of AMI. It has a ratio of 5% in skeletal muscle. Therefore, its increasing level during trauma and inflammation reduces its specificity. Another limitation of CK-MB is that it cannot detect minor myocardial damage, due to its high molecular weight. CK-MB reaches its highest point within 24 hours, starting to increase 4–9 hours after myocardial injury and decreasing to the normal range after 48–72 hours.30 Total CK and CK-MB levels are correlated with infarct size and are important predictors of prognosis. CK-MB activity has also been found to be more sensitive and specific than CK-MB mass measurements.
After passing into the blood, CK-MB is divided into two groups as MB1 and MB2. In the plasma, the CK subtypes are normally in equilibrium. When AMI occurs, MB2 passes into blood with a low ratio and a significant change in the MB2:MB1 ratio occurs, whereas CK and CK-MB levels remain normal. An MB2:MB1 ratio ≥1.5 is interpreted in favor of AMI. CK-MB subgroup analysis has 91% sensitivity and specificity in the diagnosis of AMI during the first 6 hours. The negative predictive value during the first 6 hours is 97%, and the negative predictive value of myoglobin 95% over the same period. CK-MB is specific in the diagnosis of myocardial damage; however, determination of the CK-MB relative index (CK-MB/total CK × 100) by measuring CK-MB and total CK is also frequently used for diagnosis of MI. If this index is 2.5% or above, CK-MB is probably of myocardial origin.31
CK-MB is also valuable in evaluating reperfusion. It begins to increase after 1–2 g myocardial damage. For definitive benefit in the diagnosis of AMI, at least 10–12 hours after the onset of symptoms should pass. Its specificity is 97% just 10–12 hours after symptoms have appeared. This sensitivity is quite good if serial follow-up covers 24–48 hours. If the ECG finding does not support the CK-MB value within 4–6 hours after the pain, it becomes meaningless.31
Conditions in which CK-MB is false positive in diagnosis of AMI
Malignancy (prostate, breast), pulmonary embolism, drugs (aspirin), myocarditis, pericarditis, hypothyroidism, chronic renal failure, chronic severe exercise, cardiac trauma, contusion, surgery, inflammation (muscular dystrophy, inflammatory muscle disease), muscle trauma, rhabdomyolysis, collagen tissue diseases (systemic lupus erythematosus), hyperthermia, Reye’s syndrome, peripartum period, alcoholism, acute cholecystitis, prolonged tachyarrhythmia, convulsions, electric shock, cardioversion (multiple shocks), and intramuscular injection all cause false-positive results in CK-MB measurements. Therefore, these conditions should be considered when using CK-MB as a biomarker in the diagnosis of AMI.32,33
Other conditions to be taken into account when using CK-MB for the diagnosis of AMI include the following.13,24
- If the decrease and increase in CK-MB is irregular and there is prolonged duration, then skeletal muscle damage should be considered.
- If <5% of total CK activity is constituted of CK-MB, skeletal muscle sources should be investigated.
- If total CK is increased 20–30 times, it should be considered that the cause may be skeletal muscle in origin. However, if the CK-MB:CK ratio is >2.5%, there is a high likelihood of cardiac origin.
- Macro CK1 (CK+ macroglobulin complex) migrates instead of CK-MB in electrophoresis and results in a false-positive result. The incidence in elderly patients is 1.6%.
- Direct current cardioversion increases total plasma CK activity, but does not increase the CK-MB level unless it is repeated.
- It should also be noted that reduced clearance in hypothyroidism increases CK-MB levels.
Approximately 10% of millions of patients who present to emergency departments with chest pain or symptoms of ACS globally are reported to have real AMI clinical presentation.4 Analysis of cardiac markers is important for early detection, risk assessment, reliability, and cost reduction. In addition, delays in the diagnosis of AMI may affect the evaluation of other underlying diseases. Therefore, cardiac enzymes have been replaced by protein-based molecules called cardiac-induced troponins (TnC, TnI, and TnT) in the diagnosis of AMI.
Useful cardiac proteins in diagnosis of AMI
There are many proteins released into the circulation by the cardiac system, such as myoglobin, BNP, TnC (binding to calcium), TnI (blocking actin–myosin interaction), and TnT (bound to tropomyosin). However, most are inadequate for the diagnosis of AMI. Therefore, in the next part of this review, cardiac proteins with high specificity and sensitivity that can help in the diagnosis of MI are discussed.
Role of myoglobin in diagnosis of AMI
Myoglobin is an iron- and oxygen-binding protein abundantly present in the heart and skeletal muscle of animals and has a molecular weight of 16.8 kDa. Max Perutz and Sir John Cowdery Kendrew in 1958 analyzed its structure with crystallography.34 Myoglobin is not found in any tissue other than muscle, but it can be present in the bloodstream as a result of muscle damage. It is a sensitive marker for AMI, but has no specificity. It is rapidly released from the myocardium during the injury and is rapidly excreted from the kidneys within 24 hours.35 Myoglobin rises in the first 30 minutes in the early period after the onset of an acute event, due to its rapid kinetics, and thus is an important biomarker for early detection and/or exclusion of cardiac damage.35 It is elevated in all AMI patients within 6–10 hours and peaks at the 12th hour. Since it has no specificity, negative values are important in the clinic, rather than positive values.36 Therefore, CK-MB, cTnT, ECG, and clinical findings should be taken into consideration in the diagnosis of AMI. However, it should be noted that it is useful in evaluating infarct size and reperfusion.
Role of troponins in diagnosis of AMI
Nowadays, the most important cardiac proteins involved in the diagnosis of AMI are TnC, TnI and TnT.37 TnT and TnI are known as cardiac troponins because they are present in heart and skeletal muscle. Cardiac proteins are synthesized and released from cardiac muscle.38 These proteins interact with tropomyosin to form the main structure of the striated heart muscle. Cardiac troponin (cTn) acts on myocardial contraction by regulating the calcium-dependent interaction of actin and myosin. cTn has many isoforms specific to tissue.39 TnC has no cardiac specificity, because it is the same as the troponin isoform found in smooth muscle. On the other hand, cTnT and I are completely different from troponins in the skeletal muscle, because they are coded by different genes.38 These proteins are present in myocytes, the cytosolic pool, and the contractile apparatus. The amount of TnC present in the cytosolic pool is similar to the amount of CK-MB,40 but there is also a significant amount of cTn in the contractile apparatus. Therefore, the amount of TnC per gram of myocardium is 13–15 times greater than the amount of CK-MB. This can thus explain the higher sensitivity of cTn compared with CK-MB in the early period and the elevated level of cTn in peripheral blood despite the normal level of CK-MB after myocardial tissue damage <1 g (due to ischemia, infarct, trauma, toxic damage, or inflammation).41 For this reason, the ACC and ESC stated that it is an important biochemical marker for the diagnosis of AMI.42 Cardiac troponins noteworthily are elevated in different clinical conditions, although their sensitivity and specificity are significantly higher in detecting coronary ischemia. Except for AMI, there are clinical conditions in which troponins may be high. These conditions (elevation of troponins) are summarized in Table 1 as cardiac and noncardiac causes.43 Therefore, elevation of troponins should not always be interpreted in favor of coronary ischemia. A healthy person has low levels of TnC in serum, but it reaches a level to be measured in the case of myocyte damage due to release from the cytosolic pool in the early period and from the contractile apparatus in the late period to the peripheral blood. Therefore, blood levels increase within 2–4 hours after acute myocardial damage and reach peak levels in 24 hours. Blood cTn levels are high for 2–3 weeks. Unlike the CK-MB level, the reason for the long-lasting elevation is the continuation of the release of cTn from the contractile apparatus in the late period. New troponin tests today can in fact detect even very low levels of troponin. For example, the Singulex troponin test is a new high-precision troponin analysis using single-molecule-counting technology: Singulex Clarity (high sensitivity [hs] TnI: 0.08 ng/L), Abbott Architect (hs-TnI: 2 ng/L), and Roche Elecsys (hs-TnT: 5 ng/L). These values indicate the high sensitivity with which troponins can be measured to tenfold-higher concentrations than old values; therefore, it is now possible to measure troponin values in healthy individuals.44 According to the ESC, for an AMI diagnosis based on troponin elevation: if troponin elevation occurs first, >20% rise is needed, or in patients with a low starting value, troponin needs to rise by 50%. In low-fidelity troponins, second testing should be no sooner than 6 hours. In hs-troponins, a second laboratory repeat can be performed within 2–3 hours. Normal hs-troponin for 3 hours has a negative predictive value of 99%.45
  | Table 1 Possible causes of troponin elevation, except in acute myocardial infarction |
Candidate cardiac proteins with potential in future diagnosis of AMI
Many tissue peptides and proteins have been discovered in recent years, due to advances in molecular biology and biochemistry. Therefore, we need to see whether these molecules that are synthesized and released from the heart tissue (synthesized and released from myocytes) have a role in the diagnosis of AMI. The main objective in the search for new markers is to make comprehensive risk assessments of patients with chest pain at the earliest time and eliminate any undesirable consequences. Therefore, in this part of this review, molecules with possible roles in the diagnosis of AMI are discussed.
Role of hFABP in diagnosis of AMI
One of the biomarker proteins that arises after tissue damage is FABP. hFABP is a biochemical biomarker that has the potential to be used to detect heart attack.46 It is one of nine specific FABP families. Each gets the name of the organ from which it was identified. FABPs are 15 kDa cytoplasmic, nonenzymatic proteins involved in intracellular buffering and transport of long fatty-acid chains. FABPs are released from the damaged cells into the blood very quickly, and their half-life is 20 minutes after release from kidneys into the circulation. The primary function of FABP is to transport intracellular long-chain fatty acids. Another function is to protect cardiac myocytes against long-chain fatty acids, which are localized at high concentrations, especially during ischemia.47 The increase starts just 3 hours after the onset of chest pain. It returns to normal after 12–24 hours and is detected in lesser amounts than myoglobin, but it is more useful in diagnosing AMI. Metabolism is primarily controlled by the kidneys. Therefore, an increase in blood hFABP levels, as in myoglobin, should be combined with other suspected criteria in favor of MI.
Role of glycogen phosphorylase isoenzyme BB in diagnosis of AMI
GPBB (α-1,4-d-glucan: orthophosphate d-glucosyltransferase; EC 2.4.1.1) is an important enzyme in the regulation of carbohydrate metabolism. It is present in significant amounts in the human heart and brain. Its physiological role is to provide fuel (glucose) in conditions of increased glucose requirement, such as hypoxia and hypoglycemia, or to create energy for muscle contraction. It is released from damaged myocardial cells in the early period.48 It causes myocardial oxygen deficiency and GPBB release. CK-MB is a better predictor of AMI in the first 4 hours compared with myoglobin and TnT. It rises in the first 1–4 hours of AMI, peaks at 6–10 hours, and returns to reference values within 1–2 days. Compared with GPBB and other cardiac markers used today, there are significant differences in sensitivity in the first 4 hours, especially after the onset of chest pain (GPBB 0.77, CK-MB mass and myoglobin 0.47, TnT 0.40, CK activity 0.20).49
Role of ischemia-modified albumin in diagnosis of AMI
A structural change in the N-terminus of albumin in patients with myocardial ischemia was discovered, and this albumin showed lower metal-binding capacity with cobalt on the albumin–cobalt binding test. IMA rise can be detected by this test 3 hours after the appearance of ACS symptoms (sensitivity 70%, specificity 80%, positive predictive value 96%).50 However, the detection of high IMA levels in patients with cancer, infection, brain ischemia, liver disease, and end-stage renal disease limits the specificity of this test in the diagnosis of AMI.
Role of S100A from calcium-binding proteins in diagnosis of AMI
S100 is an intracellular calcium-binding protein dimer in vertebrates. This protein was first isolated from the bovine brain by Moore in 1965, and was named S100 because it is dissolved in 100% saturated ammonium sulfate at neutral pH. Its molecular weight is 21 kDa. There are three main dimeric isoforms consisting of two monomeric subunits – α and β. According to this, the S100A0 isoform consists of an αα subunit and can be found in striated muscle, heart, and kidneys. The S100B isoform consists of the ββ subunit and is present in glial and Schwann cells. The S100A isoform is composed of an αβ subunit and is found only in glial cells. It has local regulatory effects on metabolism and cell division. S100A serum levels increase in trauma and acute ischemic stroke, and are highly sensitive in showing myocardial injury.51
Role of choline in diagnosis of AMI
PLD catalyzes the hydrolysis of phosphatidylcholine, the most abundant phospholipid of plasma membrane, resulting in the production of choline and phosphatidic acid. Concentrations of WBCho and PlCho increase during the early period of AMI, due to coronary plaque destabilization and stimulation of PLD by macrophages in tissue ischemia. Therefore, it has been suggested that cardiac ischemia can be detected early if choline levels of patients with ischemic symptoms are observed.52,53
Suggested other new cardiac peptides with potential roles in diagnosis of AMI
In recent animal and human studies, the irisin molecule may have an early role in the diagnosis of AMI. However, since the irisin molecule is synthesized in many other biological system tissues, including heart and skeletal muscle tissue, its availability in the diagnosis of AMI is weakened. In the case of AMI, irisin decreases, unlike other known cardiac markers. Probably, it would be appropriate to diagnose AMI in future by using ECG+ CK and CK-MB+ TnI+ irisin, due to the advantage of this feature of irisin. It has also been suggested that copeptin, another novel peptide, may be used as a diagnostic marker in combination with other biomarkers in patients with suspected AMI diagnosis. Copeptin alone cannot be a single diagnostic marker in patients with suspected AMI diagnosis. Adropin molecules have been reported to increase after AMI in 1–24 hours. Therefore, this is a new parameter that will probably contribute to the sensitivity of TnI in the diagnosis of AMI. Although a large number of peptide-based molecules have been investigated in this field (to make a clear and precise diagnosis of AMI), the three molecules just mentioned promise much more and have only been mentioned here.
Other plasma-induced cardiac biomarkers that may play roles in diagnosis of AMI
Cardiac markers in the diagnosis of AMI are only detected in the blood when myocardial necrosis develops. PAPP-A is elevated 2–30 hours after the onset of chest pain.54 CRP is useful for monitoring inflammatory process, coronary artery pathologies, and the course of coronary artery disease. Sometimes hs-CRP levels in healthy people are higher than in those patients with high revascularization and the risk of myocardial incidence.55 TNF, IL6, IL18, CD40 ligand, MPO, MMP9, cell-adhesion molecules, oxidized LDL, glutathione, homocysteine, fibrinogen, and D-dimer procalcitonin could be valuable in the diagnosis and prognosis of AMI. It has also been emphasized that lipid biomarkers, LpA, ApoA, ApoB, particle density, and particle size may have roles in the diagnosis of AMI.56 In addition, these molecules may be descriptive concerning the severity of coronary artery disease and may show increased risk of future MI.
Discussion
Advantages and disadvantages of cardiac enzymes, proteins, and some peptides in diagnosis of AMI
AST currently has no place in the diagnosis of AMI. Peptide-structured molecules have not yet found any place in the diagnosis of AMI. If CK-MB is used in the diagnosis of AMI, serial increase and decrease should not be seen in the level of CK-MB. CK-MB should be at least 10–14 U/L. In monitoring at 4-hour intervals, CK-MB should be increased by 50%. If viewed in a single time period, it should be twice the normal level. If it is analyzed after 72 hours, it is important that CK-MB is seen to be higher than troponins and LDHs. Cardiac enzymes are superior to ECG in the diagnosis of AMI. Myoglobin, FABP, and GPBB are early biomarkers in the diagnosis of AMI. TnT and TnI are late markers. CK-MB is a remarkable AMI biomarker in the first 10–12 hours. An increase in TnI is an indicator of myocardial injury if CK-MB is within normal limits. For the diagnosis of AMI, TnI is more specific. While CK-MB levels return to normal within 72 hours after MI, and as cardiac troponins are released in the troponin complex, their level can be high in the blood even 7–14 days later. In other words, the analysis of troponins can be used to diagnose an individual’s past AMI within 7–14 days. TnT is not specific to the heart. TnT shows biphasic release during AMI. The first peak occurs within 24 hours of symptoms, and the second one is on the fourth day. TnT levels are high in the blood for a few days and return to normal values after 10–14 days. TnI is specific to the heart. After 9–12 hours, the sensitivity for the diagnosis of AMI is 100% and has monophasic release kinetics. In patients with chronic renal failure, TnT may increase without myocardial damage. Therefore, TnI is a more reliable biomarker in the diagnosis of AMI in patients with chronic renal failure.4 Multiple cardiac biomarkers are recommended, as they increase specificity and sensitivity in the diagnosis of AMI. The properties of some dispensable cardiac biomarkers used in the diagnosis of AMI are summarized in Table 2.28–33
Conclusion
AMI has a high mortality rate worldwide, but fast and reliable diagnosis can reduce mortality. Biomarkers are elevated because of cell death in the myocardium. Therefore, many biochemical parameters of heart-tissue origin have been used in the diagnosis of AMI from past to present. A biomarker that meets the definition of an ideal cardiac biomarker, as we previously described, has yet to be discovered. In other words, there is no consensus on the best cardiac biomarker. There is no doubt that a better one will always exist. Analysis of a single biomarker is not recommended, since there is no ideal and specific single biomarker. It should also be noted that cardiac biomarkers do not make for a diagnosis, but do help in reaching one.
Currently, hs-cTnI and hs-cTnT, which allow measurement of even low cTn concentrations with high precision, are the gold-standard biomarkers for diagnosing AMI.57–59 It has been also reported that the hs-cTnT 0-hour/1-hour algorithm in rapid ruling out of AMI had a negative predictive value similar to that observed in the APACE pilot study.59,60 Rapid ruling out of AMI in approximately half of patients with missed AMI found late increasing hs-cTnT levels.59,61 Furthermore, the ADAPT trial pointed out that patients who meet the low-risk criteria might also be considered for discharge with close follow-up with their primary-care doctor after negative 0-hour and 2-hour troponin testing.62 On the other hand, the recent ASPECT 57 study used 0- and 2-hour biomarker testing with a point-of-care multimarker panel, ECG, and TIMI score.63
CK-MB and myoglobin are no longer used in emergency-department settings, as recommended by current guidelines. However, CK-MB may be used to estimate infarct size. Also other candidate biomarkers, such as hFABP, GPBB, S100, PAPP-A, CRP, TNF, IL6, IL18, CD40 ligand, MPO, MMP9, cell-adhesion molecules, oxidized LDL, glutathione, homocysteine, fibrinogen, and D-dimer procalcitonin may play a role in the diagnosis of AMI. Many of these candidate-markers result obtained with hFABP, S100A1 protein, fibrinogen, or others in the myocardial tissue of subjects dead from myocardial ischemia (by immunohistochemistry) were also analyzed in forensic literature to obtain a better diagnosis of early evolving MI.64–66
Salivary and urine biomarkers were also under investigation in determining the diagnosis and prognosis of AMI. Determination of salivary and urine biomarkers could represent a noninvasive alternative to serum ones that can be useful in clinical practice, but no fully reliable kits are available to detect cardiac marker in saliva and urine yet. In future, combining enzymatic and nonenzymatic biomarkers and candidate biomarkers might be more useful in the diagnosis of AMI.
Acknowledgment
We would like to extend our sincerest thanks to our former trainee Hacer Karabektas, MD, who produced charcoal drawings of the heart.
Disclosure
The authors report no conflicts of interest in this work.
References
Reindl M, Reinstadler SJ, Feistritzer HJ, et al. Acute myocardial infarction as a manifestation of systemic vasculitis. Wien Klin Wochenschr. 2016;128(21-22):841–843. | ||
Haasenritter J, Stanze D, Widera G, et al. Does the patient with chest pain have a coronary heart disease? Diagnostic value of single symptoms and signs--a meta-analysis. Croat Med J. 2012;53(5):432–441. | ||
Liakos M, Parikh PB. Gender disparities in presentation, management, and outcomes of acute myocardial infarction. Curr Cardiol Rep. 2018;20(8):64. | ||
Aydin S, Aydin S. Irisin concentrations as a myocardial biomarker. In: Patel VB, Preedy VR, editors. Biomarkers in Cardiovascular Disease. Dordrecht: Springer; 2016;489–504. | ||
Aydin S, Aydin S, Nesimi Eren M, et al. The cardiovascular system and the biochemistry of grafts used in heart surgery. Springerplus. 2013;2(1):612. | ||
Cervellin G, Rastelli G. The clinics of acute coronary syndrome. Ann Transl Med. 2016;4(10):191. | ||
Wang JJ, Pahlm O, Warren JW, Sapp JL, Horáček BM. Criteria for ECG detection of acute myocardial ischemia: sensitivity versus specificity. J Electrocardiol. 2018;51(6S):S12–S17. | ||
Dizon JM, Brener SJ, Maehara A, et al. Relationship between ST-segment resolution and anterior infarct size after primary percutaneous coronary intervention: analysis from the INFUSE-AMI trial. Eur Heart J Acute Cardiovasc Care. 2014;3(1):78–83. | ||
Nagurney JT, Huang C, Heredia O, et al. The new and old definitions of acute myocardial infarction: a data-based comparison. Am J Emerg Med. 2008;26(5):523–531. | ||
Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. Eur Heart J. In press 2018. | ||
Martin TN, Groenning BA, Murray HM, et al. ST-segment deviation analysis of the admission 12-lead electrocardiogram as an aid to early diagnosis of acute myocardial infarction with a cardiac magnetic resonance imaging gold standard. J Am Coll Cardiol. 2007;50(11):1021–1028. | ||
Feng QZ, Cheng LQ, Li YF. Progressive deterioration of left ventricular function in a patient with a normal coronary angiogram. World J Cardiol. 2012;4(4):130–134. | ||
Danese E, Montagnana M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Ann Transl Med. 2016;4(10):194. | ||
Mythili S, Malathi N. Diagnostic markers of acute myocardial infarction. Biomed Rep. 2015;3(6):743–748. | ||
Ay MO, Erenler AK, Dogan T, Yetim M. Diagnostic value of copeptin in acute myocardial infarction. Eur Rev Med Pharmacol Sci. 2017;21(7):1576–1582. | ||
Aydin S, Aydin S, Kobat MA, et al. Decreased saliva/serum irisin concentrations in the acute myocardial infarction promising for being a new candidate biomarker for diagnosis of this pathology. Peptides. 2014;56:141–145. | ||
Khan IA, Wattanasuwan N. Role of biochemical markers in diagnosis of myocardial infarction. Int J Cardiol. 2005;104(2):238–240. | ||
Wu AH, Apple FS, Gibler WB, Jesse RL, Warshaw MM, Valdes R. National academy of clinical biochemistry standards of laboratory practice: recommendations for the use of cardiac markers in coronary artery diseases. Clin Chem. 1999;45(7):1104–1121. | ||
Ladue JS, Wroblewski F, Karmen A. Serum glutamic oxaloacetic transaminase activity in human acute transmural myocardial infarction. Science. 1954;120(3117):497–499. | ||
Wroblewski F, Ladue JS. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955;90(1):210–213. | ||
Heinova D, Rosival I, Avidar Y, Bogin E. Lactate dehydrogenase isoenzyme distribution and patterns in chicken organs. Res Vet Sci. 1999;67(3):309–312. | ||
Fujii S, Konishi I, Suzuki A, Okamura H, Okazaki T, Mori T. Analysis of serum lactic dehydrogenase levels and its isoenzymes in ovarian dysgerminoma. Gynecol Oncol. 1985;22(1):65–72. | ||
Schmiechen NJ, Han C, Milzman DP. ED use of rapid lactate to evaluate patients with acute chest pain. Ann Emerg Med. 1997;30(5):571–577. | ||
Yun DD, Alpert JS. Acute coronary syndromes. Cardiology. 1997;88(3):223–237. | ||
McLeish MJ, Kenyon GL. Relating structure to mechanism in creatine kinase. Crit Rev Biochem Mol Biol. 2005;40(1):1–20. | ||
Walsh JH, Yalow R, Berson SA. Detection of Australia antigen and antibody by means of radioimmunoassay techniques. J Infect Dis. 1970;121(5):550–554. | ||
Knudsen J, Steenstrup B, Byrjalsen I, Hildebrandt P, Sørensen S. At what level of serum total creatine kinase activity can measurement of serum creatine kinase MB isoenzyme activity be omitted in suspected myocardial infarction? Scand J Clin Lab Invest. 1989;49(7):661–665. | ||
Schlattner U, Tokarska-Schlattner M, Wallimann T. Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta. 2006;1762(2):164–180. | ||
Ingwall JS, Kramer MF, Fifer MA, et al. The creatine kinase system in normal and diseased human myocardium. N Engl J Med. 1985;313(17):1050–1054. | ||
Hawkins RC, Tan HL. Comparison of the diagnostic utility of CK, CK-MB (activity and mass), troponin T and troponin I in patients with suspected acute myocardial infarction. Singapore Med J. 1999;40(11):680–684. | ||
Keffer JH. Myocardial markers of injury. Evolution and insights. Am J Clin Pathol. 1996;105(3):305–320. | ||
Kim S, Um TH, Cho CR, Jeon JS. False-positive elevation of creatine kinase MB mass concentrations caused by macromolecules in a patient who underwent nephrectomy for renal cell carcinoma. Ann Lab Med. 2014;34(5):405–407. | ||
Murthy VV. Identification of false-positive CK-MB activity in an elderly patient. Am J Clin Pathol. 1993;99(1):97–100. | ||
Strandberg B. Chapter 1: building the ground for the first two protein structures: myoglobin and haemoglobin. J Mol Biol. 2009;392(1):2–10. | ||
Klocke FJ, Copley DP, Krawczyk JA, Reichlin M. Rapid renal clearance of immunoreactive canine plasma myoglobin. Circulation. 1982;65(7):1522–1528. | ||
Mair J, Artner-Dworzak E, Lechleitner P, et al. Early diagnosis of acute myocardial infarction by a newly developed rapid immunoturbidimetric assay for myoglobin. Heart. 1992;68(11):462–468. | ||
Park KC, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res. 2017;113(14):1708–1718. | ||
Wei B, Jin JP. Troponin T isoforms and posttranscriptional modifications: evolution, regulation and function. Arch Biochem Biophys. 2011;505(2):144–154. | ||
Anderson PA, Malouf NN, Oakeley AE, Pagani ED, Allen PD. Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ Res. 1991;69(5):1226–1233. | ||
Al-Hadi HA, Fox KA. Cardiac markers in the early diagnosis and management of patients with acute coronary syndrome. Sultan Qaboos Univ Med J. 2009;9(3):231–246. | ||
Bodor GS. Biochemical markers of myocardial damage. EJIFCC. 2016;27(2):95–111. | ||
Wessler JD, Stant J, Duru S, Rabbani L, Kirtane AJ. Updates to the ACCF/AHA and ESC STEMI and NSTEMI guidelines: putting guidelines into clinical practice. Am J Cardiol. 2015;115(5):23A–28. | ||
Korff S, Katus HA, Giannitsis E. Differential diagnosis of elevated troponins. Heart. 2006;92(7):987–993. | ||
Shah AS, McAllister DA, Mills R, et al. Sensitive troponin assay and the classification of myocardial infarction. Am J Med. 2015;128(5):493–501. | ||
Neumann JT, Sörensen NA, Ojeda F, et al. Early diagnosis of acute myocardial infarction using high-sensitivity troponin I. PLoS One. 2017;12(3):e0174288. | ||
Sotoudeh Anvari M, Karimi M, Shafiee A, et al. Complementary diagnostic value of heart type fatty acid-binding protein in early detection of acute myocardial infarction. Crit Pathw Cardiol. 2018;17(1):43–46. | ||
Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7(6):489–503. | ||
Rabitzsch G, Mair J, Lechleitner P, et al. Immunoenzymometric assay of human glycogen phosphorylase isoenzyme BB in diagnosis of ischemic myocardial injury. Clin Chem. 1995;41(7):966–978. | ||
Mair J, Apple F. Progress in myocardial damage detection: new biochemical markers for clinicians. Crit Rev Clin Lab Sci. 1997; 34(1):1–66. | ||
Gurumurthy P, Borra SK, Yeruva RK, Victor D, Babu S, Cherian KM. Estimation of ischemia modified albumin (IMA) levels in patients with acute coronary syndrome. Indian J Clin Biochem. 2014;29(3):367–371. | ||
Gong XJ, Song XY, Wei H, Wang J, Niu M. Serum S100A4 levels as a novel biomarker for detection of acute myocardial infarction. Eur Rev Med Pharmacol Sci. 2015;19(12):2221–2225. | ||
Ohkawa R, Kurano M, Sakai N, et al. Measurement of plasma choline in acute coronary syndrome: importance of suitable sampling conditions for this assay. Sci Rep. 2018;8(1):4725. | ||
Body R, Griffith CA, Keevil B, et al. Choline for diagnosis and prognostication of acute coronary syndromes in the Emergency Department. Clin Chim Acta. 2009;404(2):89–94. | ||
Lippi G, Mattiuzzi C, Cervellin G. Pregnancy-associated plasma protein A (PAPP-A) for the early diagnosis of myocardial infarction: more doubts than certainties. Indian Heart J. 2012;64(6):625–626. | ||
Berton G, Cordiano R, Palmieri R, Pianca S, Pagliara V, Palatini P. C-reactive protein in acute myocardial infarction: association with heart failure. Am Heart J. 2003;145(6):1094–1101. | ||
Martín-Ventura JL, Blanco-Colio LM, Tuñón J, et al. Biomarkers in cardiovascular medicine. Rev Esp Cardiol. 2009;62(6):677–688. | ||
Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172(16):1211–1218. | ||
Reichlin T, Irfan A, Twerenbold R, et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation. 2011;124(2):136–145. | ||
Mueller C, Giannitsis E, Christ M, et al. Multicenter evaluation of a 0-hour/1-hour algorithm in the diagnosis of myocardial infarction with high-sensitivity cardiac troponin T. Ann Emerg Med. 2016;68(1):76–87. | ||
Reichlin T, Twerenbold R, Wildi K, et al. Prospective validation of a 1-hour algorithm to rule-out and rule-in acute myocardial infarction using a high-sensitivity cardiac troponin T assay. CMAJ. 2015;187(8):E243–E252. | ||
Hammarsten O, Fu ML, Sigurjonsdottir R, et al. Troponin T percentiles from a random population sample, emergency room patients and patients with myocardial infarction. Clin Chem. 2012;58(3):628–637. | ||
Than M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol. 2012;59(23):2091–2098. | ||
Than M, Cullen L, Reid CM, et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. Lancet. 2011;377(9771):1077–1084. | ||
Beausire T, Faouzi M, Palmiere C, Fracasso T, Michaud K. High-sensitive cardiac troponin hs-TnT levels in sudden deaths related to atherosclerotic coronary artery disease. Forensic Sci Int. 2018;289:238–243. | ||
Mondello C, Cardia L, Ventura-Spagnolo E. Immunohistochemical detection of early myocardial infarction: a systematic review. Int J Legal Med. 2017;131(2):411–421. | ||
Aljakna A, Fracasso T, Sabatasso S. Molecular tissue changes in early myocardial ischemia: from pathophysiology to the identification of new diagnostic markers. Int J Legal Med. 2018;132(2):425–438. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

