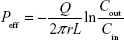Back to Journals » International Journal of Nanomedicine » Volume 12
Biocompatible nanoemulsions based on hemp oil and less surfactants for oral delivery of baicalein with enhanced bioavailability
Authors Yin J, Xiang C, Wang P, Yin Y, Hou Y
Received 27 December 2016
Accepted for publication 1 March 2017
Published 10 April 2017 Volume 2017:12 Pages 2923—2931
DOI https://doi.org/10.2147/IJN.S131167
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Juntao Yin,1,* Cuiyu Xiang,1,* Peiqing Wang,1 Yuyun Yin,2 Yantao Hou3
1Department of Pharmaceutics, Huaihe Hospital Affiliated to Henan University, Kaifeng, 2Department of Physiochemical Analysis, Henan Provincial Institute for Food and Drug Control, Zhengzhou, 3Department of Pharmaceutical Engineering, Henan Vocational College of Applied Technology, Kaifeng, People’s Republic of China
*These authors contributed equally to this work
Abstract: Baicalein (BCL) possesses high pharmacological activities but low solubility and stability in the intestinal tract. This study aimed to probe the potential of nanoemulsions (NEs) consisting of hemp oil and less surfactants in ameliorating the oral bioavailability of BCL. BCL-loaded NEs (BCL-NEs) were prepared by high-pressure homogenization technique to reduce the amount of surfactants. BCL-NEs were characterized by particle size, entrapment efficiency (EE), in vitro drug release, and morphology. Bioavailability was studied in Sprague-Dawley rats following oral administration of BCL suspensions, BCL conventional emulsions, and BCL-NEs. The obtained NEs were ~90 nm in particle size with an EE of 99.31%. BCL-NEs significantly enhanced the oral bioavailability of BCL, up to 524.7% and 242.1% relative to the suspensions and conventional emulsions, respectively. BCL-NEs exhibited excellent intestinal permeability and transcellular transport ability. The cytotoxicity of BCL-NEs was documented to be low and acceptable for oral purpose. Our findings suggest that such novel NEs and preparative process provide a promising alternative to current formulation technologies and suitable for oral delivery of drugs with bioavailability issues.
Keywords: baicalein, nanoemulsions, biocompatibility, high-pressure homogenization, hemp oil, bioavailability
Introduction
Baicalein (BCL), a flavonoid rich in the root of Scutellaria baicalensis, has been reported to have various pharmacological activities, such as anticancer, antioxidation, antiallergy, antivirus, and anti-inflammatory activities.1 BCL is also one of the active ingredients of Sho-Saiko-To, a Japanese herbal supplement believed to be able to improve the liver health. It is highly insoluble in acidic medium and soluble in alkali medium but unstable. Poor solubility in the gastric fluid and instability in the intestinal fluid greatly limit its oral bioavailability and clinical efficacy. To improve the oral absorption of BCL, a number of novel formulation approaches have been explored, including solid dispersion,2 cyclodextrin inclusion,2 nanocrystal,3 self-microemulsifying,1 phospholipid complex,4 and liposomes.5 Nevertheless, these approaches still have some shortcomings to be used as oral delivery vehicles due to inadequate drug protection, poor biocompatibility, and larger particle size. It is urgently necessary to develop a low-toxic and high-effective drug delivery system for oral delivery of BCL.
Nanoemulsions (NEs) are oil-in-water or water-in-oil droplets emulsified by surfactant, cosurfactant, oil, and water with appropriate ratios.6 Generally, NEs can spontaneously form owing to the use of a large quantity of surfactants (wt, ~20% of the oil phase). The smaller particle size (10–100 nm) imparts NEs excellent drug delivery efficacy, though the high surfactant content raises the safety risk of delivery system. The formulation properties of NEs largely depend on the formulation components and preparative technique.6 Suitable process and carrier materials can jointly reduce the use amount of surfactants and hence the toxicity of NEs. Aside from spontaneous formation, NEs can be manufactured through exerting strong mechanical power, by which the amount of surfactants used can be reduced to a large extent. High-pressure homogenization (HPH) technique is widely used to produce submicron lipid emulsions.7 It should be an effective means using HPH to prepare NEs to maximally reduce the surfactant level in NEs. Hemp oil extracted from hemp seeds has become an important source of edible oil for human nutrition. Besides some health effections,8,9 it can be more easily emulsified by emulsifiers than other frequently used oils due to high intersolubility with water. This will be favorable to further the reduction of surfactants in NEs. However, hemp oil-based NEs prepared by HPH technique have not been reported and applied for oral delivery of BCL.
In this study, NEs composed of hemp oil and reduced surfactants were developed to improve the intestinal stability and permeability of BCL and hence the oral bioavailability (as illustrated in Scheme 1). We took advantage of HPH technique to prepare BCL-loaded NEs (BCL-NEs) and characterized them by particle size, entrapment efficiency (EE), morphology, and in vitro release. The capability of NEs to enhance the bioavailability of BCL was investigated in Sprague-Dawley (SD) rats following oral administration and compared with suspensions and conventional emulsions. To evaluate the formulation performance, intestinal permeability, transcellular transport, and cytotoxicity of BCL-NEs were examined.
Materials and methods
Materials
BCL was purchased from Zhengzhou Agricultural Technology Co, Ltd (Zhengzhou, People’s Republic of China). Hemp oil was obtained from Impressions of Life Experience Industry Co, Ltd (Bama, People’s Republic of China). Poly(ethylene glycol) monooleate (PM; Mw~1,400) was from obtained Aoke Chemicals (Shanghai, People’s Republic of China). Sodium oleate, Hoechst 33258, and 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Deionized water was prepared by a Milli-Q water purifier (Molsheim, Millipore, France). High performance liquid chromatography (HPLC)-grade methanol was provided by Merck (Darmstadt, Germany). All other chemicals were of analytical grade and used as received.
Solubility determination
A slight excess of BCL was added into 2 mL of each tested vehicle (soybean oil, medium-chain triglycerides [MCT], Capryol™ 90, hemp oil, PM, and hemp oil/PM), and the mixture was stirred for 48 h at 25°C under 800 rpm. After equilibrium, the mixtures were centrifuged at 12,000 rpm for 10 min. BCL in the supernatant was quantified by HPLC. Determination was performed on Agilent 1100 Series HPLC System (Santa Clara, CA, USA). Samples were eluted by a Hypersil™ C18 Column (5 μm, 4.6×200 mm; Elite, Dalian, People’s Republic of China) at 35°C with an injection volume of 20 μL. Chromatographic signals were collected at 276 nm. The mobile phase consisted of 46% methanol and 54% phosphoric acid solution (0.1%) pumped at a flow rate of 1.0 mL/min.
Preparation of BCL-NEs
BCL-NEs were prepared by the emulsifying/HPH process. Briefly, BCL, hemp oil, PM, and appropriate amount of ethanol were mixed and heated (50°C) to melting, and sodium oleate was dissolved in deionized water. Then, the water phase was transferred into the oil phase followed by high shearing to manufacture coarse emulsions using a high-speed disperser (T25; IKA, Staufen, Germany) at 8,000 rpm for 5 min. The obtained coarse emulsions were further homogenized with Microfluidizer (Nano DeBEE, Westwood, MA, USA) to produce the final NEs. Factors affecting the properties of NEs were screened, including the ratios of drug/excipients, surfactants/oil, and the homogenizing condition. Surfactants were composed of PM and sodium oleate at a fixed ratio of 1:1. BCL suspensions, and conventional emulsions were prepared by grinding the drug with sodium carboxymethyl cellulose (0.5%) or excipients used in BCL-NEs as control formulations.
Characterization of BCL-NEs
The particle size of BCL-NEs was determined by Zetasizer Nano ZS (Malvern, Worcestershire, UK) at 25°C. To measure the particle size, BCL-NEs were diluted appropriately and placed in a disposable cuvette. After equilibrium for 120 s, the sample was subjected to laser diffraction for particle size analysis based on dynamic light scattering.
The morphology of BCL-NEs was inspected with transmission electron microscopy (TEM). BCL-NEs diluted to 25-fold were dropped onto a carbon-coated copper grid and attached by air drying. Then, the fixed particles were forwarded to TEM (Tecnai 10; Philips, Amsterdam, Netherlands) for observation. Morphological micrographs were gathered at the acceleration voltage of 100 kV.
The percentage of EE of BCL-NEs was determined by separating free BCL from the emulsion system. In brief, freshly prepared BCL-NEs were centrifuged in a centrifugal filter device (Amicon® Ultra-0.5, MWCO 10K; Millipore) to remove the nonentrapped BCL. BCL in the filtrate was determined by HPLC mentioned earlier. The EE of BCL-NEs was calculated according to the following equation: EE (%) = (1− Mfre/Mtot) ×100%, where Mfre and Mtot represent the amount of free and total BCL in the system, respectively.
In vitro release study
The release of BCL from BCL-NEs was studied using the dialysis bag method.10 Briefly, aliquots of BCL-NEs equivalent to 25 mg BCL were put into dialysis bags and then placed in 900 mL of water, 0.1 M HCl solution, or phosphate-buffered saline (PBS), pH 6.8, in which 0.25% (w/v) sodium dodecyl sulfate was introduced as solubilizer. At the time of 0.5, 1, 2, 4, 8, and 12 h, 5 mL of solution was withdrawn and immediately replenished with the same volume of fresh medium. Subsequently, the release solution was subjected to HPLC analysis for BCL quantification, and the accumulative release percentage of BCL from BCL-NEs was calculated from the ratio of released drug to total drug.
Bioavailability study
SD rats (250±20 g) were fasted overnight before administration but accessible to water ad lib. Rats were randomly divided into three groups with six rats in each group. The first group was administered BCL suspensions, while the second and the third groups were given BCL conventional emulsions and BCL-NEs, respectively. Rats were given the three formulations by gavage at the dose of 25 mg/kg. Blood was sampled into the heparinized tubes via the jugular vein at 0.5, 1, 2, 4, 6, 8, and 12 h after administration. The blood samples were centrifuged at 4,500 rpm for 5 min to prepare the plasma. All animal experiments were conducted according to protocols issued by the Experimental Animal Ethical Committee of Huaihai Hospital Affiliated to Henan University, and all animal experiments were reviewed and approved by the Ethical Committee.
To quantify the plasma BCL as well as bailcalin, its glucuronide metabolite, a deproteinization procedure was performed by adding four aliquots of methanol into an aliquot of plasma. After multiple vortex and sonication, the samples were centrifuged at 10,000 rpm for 10 min to separate the supernatants. Then, the supernatants were evaporated to dry at 37°C for 2 h under vacuum using Concentrator plus (Eppendorf, Hamburg, Germany). The residue was reconstituted in 100 mL of 50% acetonitrile in water. After centrifugation, the resulting solution was subjected to ultra-performance liquid chromatography quadrupole time of flight mass spectrometry ( UPLC–qTOF/MS) analysis (Xevo G2 QTof; Waters, Milford, CT, USA). The instrument configuration and parameters setting referred to the literature.11 The whole BCL in the blood was quantified based on the extracted ion chromatograms using MassLynx. Pharmacokinetic parameters were processed using the PKSolver 2.0 program.
Intestinal permeability study
To evaluate the intestinal permeability of BCL-NEs, in situ single-pass intestinal perfusion was carried out following the reported procedure.12 SD rats were fasted for 12 h before experiment. The rats were then rendered unconscious with 20% urethane injection. The intestines were exposed by making a midline abdominal incision. The segments of duodenum, jejunum, and ileum were identified and cannulated with silicone tubes. The intestinal contents were fully washed with Krebs Ringer’s buffer, pH 7.4 (Seebio Biotech, Inc, Shanghai, People’s Republic of China). Perfusion samples were prepared by diluting BCL (dissolved in ethanol) or BCL-NEs into Krebs Ringer’s buffer, resulting in 25 μg/mL of BCL concentration. After preperfusion for 30 min, the perfusates were collected per 15 min up to 120 min. Net water flux was calibrated by weight with the sham-operated group. The effective permeability coefficient (Peff) was calculated according to the equation below:
|
|
where Q denotes the flow rate (0.2 mL/min), r and L are the radius and length of each intestinal segment (cm), and Cin and Cout represent the inlet and outlet concentrations of BCL, respectively.
Cell uptake and internalization
Caco-2 cells, which were provided by the Cell Bank of Chinese Academy of Sciences (Shanghai, People’s Republic of China) from the American Type Culture Collection (ATCC), are used to investigate the cell uptake and internalization of BCL-NEs, and the culture refers to the reported protocol.13 The well-cultured cells were washed twice with PBS, pH 7.4, and seeded in six-well plate at a density of 1×106 cells/well. When the cell confluence approached to 80%–90%, they were used for the cellular uptake experiment. BCL solution and BCL-NEs, diluted to 10 μg/mL with the culture medium, were introduced into the wells with Caco-2 cells. At the time of 0.5, 1, 2, and 4 h, the incubation media were removed and the cells were washed with cold PBS. Then, the treated cells were lysed with radio immunoprecipitation assay lysis buffer (0.1% phenylmethanesulfonyl fluoride) (Sigma-Aldrich). The supernatant was obtained by centrifugation with protein content quantified using BCA protein assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, People’s Republic of China). BCL concentration in the supernatant was determined by UPLC–qTOF/MS mentioned earlier and corrected by the cell protein level of each well.
To observe the cellular internalization of BCL-NEs, DiO-labeled BCL-NEs were prepared by loading DiO into BCL-NEs upon preparation. Caco-2 cells incubated with DiO-labeled BCL-NEs for 0.5 h at 37°C. The medium was removed, and the cells were rinsed with PBS thrice. The cells were immobilized by 4% paraformaldehyde and proceeded to inspect for confocal laser scanning microscopy (CLSM) after staining with Hoechst 33258.
Cytotoxicity evaluation
The cytotoxicity of BCL-NEs was checked by assessing the effect on the viability of Caco-2 cells based on 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay. Caco-2 cells were cultured for 48 h and washed thrice with PBS. Then, BCL-NEs with various levels were added into the cells to incubate for 24 h at 37°C. After that, MTT solution (20 μL, 5 mg/mL; Sigma-Aldrich) was introduced into each well and incubated for another 4 h. To dissolve resultant formazan, 200 μL of dimethylsulfoxide was subsequently added. The ultraviolet absorbance of each well was measured at 570 nm. The cell viability is calculated using the following equation: cell viability (%) = (Atri/Acon) ×100%, where Atri and Acon represent the absorbance of living cell treated with BCL-NEs and blank culture medium, respectively.
Results and discussion
Solubility of BCL in various vehicles
The primary purpose of developing NEs is to solubilize poorly water-soluble drugs. The solubility of drug in the oil intended for NEs determines the solubilizing power and stability of formulation. Table 1 presents the solubility of BCL in various vehicles. Among four tested oils, hemp oil displayed the highest solubilization toward BCL, in which the solubility of BCL was up to 8.592 mg/g. Generally, poorly water-soluble drugs have the solubility following the rank of long-chain oil < medium-chain oil < short-chain oil.14 Soybean oil is the representation of long-chain oil, and Capryol™ 90 belongs to short-chain oil. Interestingly, BCL exhibited superior solubility in hemp oil, much higher than in Capryol™ 90. This may be attributed to the hydrophilic nature of hemp oil. Hemp oil is famously known as aqueous edible oil in China that can be well miscible with water. PM is an amphiphilic material containing the moiety of polyethyleneglycol. BCL belongs to the polyphenolic compound (Figure 1) and has an inadequate hydrophile–lipophile balance, resulting in poor solubility both in water and oil. Therefore, PM, depending on its excellent solubilizing power, makes BCL more easily soluble in it. A combined use of hemp oil and PM further increases the solubility of BCL. Our finding suggests that hemp oil as well as its combination with PM is more suitable for the preparation of NEs for loading BCL.
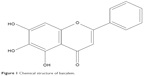  | Figure 1 Chemical structure of baicalein. |
Preparation and characterization of BCL-NEs
As a rule, NEs can be formed via self-emulsifying with agitation or vortexing in the presence of several emulsifiers. For instance, to achieve a good self-nanoemulsifying drug delivery system of econazole nitrate, 60% (w/w) Cremophor® RH 40 and high ratio of cosurfactant (Transcutol® HP) were used in the formulation.15 However, the use of massive emulsifiers or surfactants will give rise to high physiological toxicity. To minimize the use of surfactants and achieve nanoscale emulsions, HPH was adopted in the processing of BCL-NEs to decrease the particle size by providing strong mechanical energy. This preparative technique resembles the one recruited in the preparation of solid lipid nanoparticles or nanostructured lipid carriers.16,17 The particle size of nanocarriers influences the intestinal absorption of payload. In the preliminary experiment, we found that factors affecting the particle size of BCL-NEs mainly included the ratios of drug/excipients, surfactants/oil, and the homogenizing condition. The effects of formulation variables on the particle size are shown in Figure 2. The ratio of drug/oil had a significant effect on the particle size of BCL-NEs. High drug involvement resulted in larger emulsion droplets due to impaired emulsifiability of inner oil phase. Conversely, high ratio of surfactants exerted a positive effect on the reduction of particle size. Of note, NEs <100 nm were still able to be produced from the formulation with less surfactants (10% relative to the oil). In the case of homogenizing condition, the particle size of BCL-NEs decreased with an increase in homogenization cycle at 20,000 psi. But there was no significant difference between 8 and 12 cycles in terms of the particle size.
Considering the merits of smaller particle size and less surfactants in oral drug delivery, the formulation was finally decided as 40 mg of BCL, 1,000 mg of hemp oil, 50 mg of PM, and 50 mg of sodium oleate emulsified with 20 mL of water. The resulting BCL-NEs possessed a particle size of 90.6 nm with a polydispersity index of 0.225 (Figure 3A). The ζ potential was determined to be −45.2 mV, showing them to be stable as colloidal dispersion system. BCL-NEs exhibited a high EE that was up to 99.31%. BCL-NEs were opalescent in appearance and took on a blue light upon dilution (Figure 3B). BCL-NEs presented a near-spherical morphology as observed by TEM (Figure 3C). The particle size judged from the scale bar (200 nm) accorded well with the hydrodynamic size measured based on dynamic light scattering.
  | Figure 3 Physical characterization of BCL-NEs: (A) size distribution, (B) appearance, and (C) TEM micrograph. |
In vitro release study
The release profiles of BCL from NEs in various media are presented in Figure 4. BCL-NEs exhibited exceedingly slow drug release in water, 0.1 M HCl, and PBS, pH 6.8. The release increased with the time in three different kinds of media, indicating that BCL could be continuously released from BCL-NEs. Nevertheless, the release amount was fairly low due to poor solubility of BCL, which were <7% within 12 h in three media. It was noted that the release of BCL-NEs in 0.1 M HCl was somewhat different in water and PBS, pH 6.8. In 0.1 M HCl, BCL-NEs showed lower drug release, which may be associated with the poorer solubility of BCL in the acidic medium. Overall, BCL release from BCL-NEs was rather limited, and most parts of the BCL were retained in the NEs. The release feature rendered BCL able to be transported via the vehicle of NEs rather than the free form. Negligible drug release for NEs has also been investigated by other groups.18,19
Enhanced bioavailability
The pharmacokinetic profiles of BCL following oral administration of various formulations are shown in Figure 5, and the main pharmacokinetic parameters are listed in Table 2. The formulation of suspensions brought about poor absorption of BCL both in the rate and the extent. The maximum plasma concentration (Cmax) and area under the plasma concentration–time curve (AUC0–t) were just 1.43 μg/mL and 7.96 μg h/mL, respectively. Compared with the formulation of BCL suspensions, the conventional emulsions, to a certain extent, promoted the oral absorption of BCL, resulting in an apparent improvement in the blood drug concentration. The finding suggests that lipid-based formulation can enhance the oral bioavailability of BCL. However, in the case of BCL-NEs, a dramatic enhancement in the oral absorption of BCL was achieved. BCL-NEs yielded higher blood BCL concentration at each time point. The Cmax and AUC0–t were separately up to 10.96 μg/mL and 41.77 μg h/mL, respectively. The oral bioavailability of BCL-NEs was calculated to be 524.7 and 242.1% relative to the suspensions and conventional emulsions, respectively. In terms of the time to maximum plasma concentration (Tmax), two kinds of emulsions were similar (~2 h) but different from the formulation of suspensions. In addition, the terminal half-life (T1/2) among three formulations was significantly distinct. These results indicate that there are differences in the absorption rate of BCL as formulated in different modalities. In contrast, NEs possessed a greater advantage in enhancing the oral absorption of BCL.
NEs are nanoscale colloidal particles, which represent one of the most advanced nanoparticle systems for oral drug delivery.20 NEs are thermodynamically and kinetically stable, therefore flocculation, aggregation, creaming, and coalescence rarely take place. High surface area can increase the absorption rate and reduce the absorption variability, thus enhancing the bioavailability of the drug. Furthermore, they can protect the payload from degradation and metabolism due to encapsulation in the inner oil phase,21 which is similar to the micellar system.22 In stabilizing the labile drug, NEs have advantages over solid dispersions and cyclodextrin inclusion complexes. However, the main disadvantage of NEs involves large concentration of surfactant/cosurfactant that is required for stabilization. In comparison with the self-microemulsifying drug delivery system,1 our developed NEs contain less surfactants and cosurfactants with the assistance of HPH upon preparation. The novel NEs with hemp oil as vehicle exhibit a high oral delivery efficacy, which are more suitable for oral delivery of BCL.
Improved intestinal permeability
The permeability of a drug can be assessed by either Caco-2 cell monolayer model or in situ single-pass intestinal perfusion.23,24 In this study, the in situ single-pass intestinal perfusion was adopted to measure the Peff. The Peff values of free BCL and encapsulated BCL in NEs are listed in Table 3. BCL itself possesses poor permeability in various intestinal segments with Peff<5×10−6 cm/s. It seems that BCL has higher permeability in the lower section of the small intestine. As encapsulated into NEs, the Peff of BCL in the main absorption intestinal parts significantly increased, especially in the ileum. Relatively, the ileum possesses larger absorption area and transport activity. We assumed that enlarged surface area and particle-associated membrane mobile transport should be responsible for the improved permeability.25 The in situ single-pass intestinal perfusion experiment demonstrated the positive role of NEs in enhancing the oral absorption of BCL.
Transcellular transport betterment
Figure 6A shows the cellular uptake of free BCL and BCL-NEs with the incubation time. There existed a significant difference in the cellular uptake between free BCL and encapsulated BCL. In the first time point (0.5 h), free BCL was taken up by Caco-2 cells, a little quicker than BCL-NEs. This may be connected with the high concentration of BCL surrounding the cells due to complete exposure. However, after that, the cellular uptake rate of BCL was accelerated by NEs compared with free BCL. The total uptake quantity of BCL-NEs was 1.86-fold as much as that of free BCL at 4 h. BCL-NEs resulted in higher BCL uptake, showing them to be promising in facilitating the transcellular transport of BCL.
The facilitative effect of BCL transport with NEs can also be extrapolated from the internalization of BCL-NEs. Intense cellular internalization took place on BCL-NEs (Figure 6B). There was considerable NE-associated fluorescence distributing within the cytoplasm, even internalizing into the nucleus. Permeability enhancement via NEs has been investigated in other tested drug.26 Excellent affinity and permeability of NEs to enterocytes enable them to be qualified for oral delivery of poorly absorbed drugs.
Acceptable biocompatibility
For the application of NEs, a great concern is the safety of formulation. The toxicity of NEs generally comes from the use of large surfactants and cosurfactants. Figure 7 shows the viability of Caco-2 cells after treatment with BCL-NEs. There was no obvious cytotoxicity observable for BCL-NEs at various drug concentrations. All the cell survival rates remained >90% after 24 h incubation, manifesting the low cytotoxicity of BCL-NEs. In our developed NE system, PM and sodium oleate are the preferred surfactants. They are both low-toxic and in vivo degradable, thus not inducing apparent cytotoxicity. Emulsions represent one of the welcome dosage forms that patients would like to take. The low toxicity and compliance confer BCL-NEs a vitality as potential oral delivery carrier.
  | Figure 7 Relative cell viability of Caco-2 incubated with nanoemulsions with different BCL levels (n=3, mean ± StD). |
Conclusion
In this article, a novel NE formulation based on hemp oil and less surfactant was developed for oral delivery of BCL. The suitability of NEs as oral delivery carrier of BCL was evaluated. The NEs were readily produced with the HPH technique and possessed smaller particle size (~90 nm). High BCL entrapment and low BCL release were accomplished by the way of NEs. The in vivo pharmacokinetics showed that NEs significantly enhanced the oral bioavailability of BCL. Improved oral absorption of BCL could be attributed to betterment in transcellular transport because of encapsulation into NEs. In addition, our developed NEs were perfectly biocompatible, thanks to the involvement of less surfactants. This study provides valuable information to design innovative NEs to more efficiently deliver poorly absorbed drugs via the oral route.
Acknowledgments
This study is supported by the Key Research Project of Henan Provincial Science and Technology Department (no. 112102310306), and the authors are thankful to the financial assistance from the Department of Pharmaceutics of Huaihe Hospital Affiliated to Henan University.
Disclosure
The authors report no conflicts of interest in this work.
References
Liu W, Tian R, Hu W, et al. Preparation and evaluation of self-microemulsifying drug delivery system of baicalein. Fitoterapia. 2012;83(8):1532–1539. | ||
He X, Pei L, Tong HH, Zheng Y. Comparison of spray freeze drying and the solvent evaporation method for preparing solid dispersions of baicalein with Pluronic F68 to improve dissolution and oral bioavailability. AAPS PharmSciTech. 2011;12(1):104–113. | ||
Zhang J, Lv H, Jiang K, Gao Y. Enhanced bioavailability after oral and pulmonary administration of baicalein nanocrystal. Int J Pharm. 2011;420(1):180–188. | ||
Rawat DS, Thakur BK, Semalty M, Semalty A, Badoni P, Rawat MS. Baicalein-phospholipid complex: a novel drug delivery technology for phytotherapeutics. Curr Drug Discov Technol. 2013;10(3):224–232. | ||
Liang J, Wu W, Liu Q, Chen S. Long-circulating nanoliposomes (LCNs) sustained delivery of baicalein (BAI) with desired oral bioavailability in vivo. Drug Deliv. 2013;20(8):319–323. | ||
Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12(11):2826–2841. | ||
Zhang X, Wu B. Submicron lipid emulsions: a versatile platform for drug delivery. Curr Drug Metab. 2015;16(3):211–220. | ||
Kaul N, Kreml R, Austria JA, et al. A comparison of fish oil, flaxseed oil and hempseed oil supplementation on selected parameters of cardiovascular health in healthy volunteers. J Am Coll Nutr. 2008;27(1):51–58. | ||
Jeong M, Cho J, Shin JI, et al. Hempseed oil induces reactive oxygen species- and C/EBP homologous protein-mediated apoptosis in MH7A human rheumatoid arthritis fibroblast-like synovial cells. J Ethnopharmacol. 2014;154(3):745–752. | ||
Zhou X, Zhang X, Ye Y, et al. Nanostructured lipid carriers used for oral delivery of oridonin: an effect of ligand modification on absorption. Int J Pharm. 2015;479(2):391–398. | ||
Liu W, Liu H, Sun H, et al. Metabolite elucidation of the Hsp90 inhibitor SNX-2112 using ultraperformance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC–QTOF/MS). Xenobiotica. 2014;44(5):455–464. | ||
Li W, Zhang T, Ye Y, Zhang X, Wu B. Enhanced bioavailability of tripterine through lipid nanoparticles using broccoli-derived lipids as a carrier material. Int J Pharm. 2015;495(2):948–955. | ||
Natoli M, Leoni BD, D’Agnano I, Zucco F, Felsani A. Good Caco-2 cell culture practices. Toxicol In Vitro. 2012;26(8):1243–1246. | ||
Sapra B, Thatai P, Bhandari S, Sood J, Jindal M, Tiwary A. A critical appraisal of microemulsions for drug delivery: part I. Ther Deliv. 2013;4(12):1547–1564. | ||
Elkasabgy NA. Ocular supersaturated self-nanoemulsifying drug delivery systems (S-SNEDDS) to enhance econazole nitrate bioavailability. Int J Pharm. 2014;460(1–2):33–44. | ||
Silva AC, Gonzalez-Mira E, Garcia ML, et al. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): high pressure homogenization versus ultrasound. Colloids Surf B Biointerfaces. 2011;86(1):158–165. | ||
Yu Q, Hu X, Ma Y, et al. Lipids-based nanostructured lipid carriers (NLCs) for improved oral bioavailability of sirolimus. Drug Deliv. 2016;23(4):1469–1475. | ||
Sun D, Wei X, Xue X, et al. Enhanced oral absorption and therapeutic effect of acetylpuerarin based on D-alpha-tocopheryl polyethylene glycol 1000 succinate nanoemulsions. Int J Nanomedicine. 2014;9:3413–3423. | ||
Lu R, Liu S, Wang Q, Li X. Nanoemulsions as novel oral carriers of stiripentol: insights into the protective effect and absorption enhancement. Int J Nanomedicine. 2015;10:4937–4946. | ||
Gibaud S, Attivi D. Microemulsions for oral administration and their therapeutic applications. Expert Opin Drug Deliv. 2012;9(8):937–951. | ||
Lee EH, Kim JK, Lim JS, Lim SJ. Enhancement of indocyanine green stability and cellular uptake by incorporating cationic lipid into indocyanine green-loaded nanoemulsions. Colloids Surf B Biointerfaces. 2015;136:305–313. | ||
Zhang X, Wang H, Zhang T, Zhou X, Wu B. Exploring the potential of self-assembled mixed micelles in enhancing the stability and oral bioavailability of an acid-labile drug. Eur J Pharm Sci. 2014;62:301–308. | ||
Varma MV, Gardner I, Steyn SJ, et al. pH-dependent solubility and permeability criteria for provisional biopharmaceutics classification (BCS and BDDCS) in early drug discovery. Mol Pharm. 2012;9(5):1199–1212. | ||
Zakeri-Milani P, Valizadeh H, Tajerzadeh H, et al. Predicting human intestinal permeability using single-pass intestinal perfusion in rat. J Pharm Pharm Sci. 2007;10(3):368–379. | ||
Zhang J, Li J, Ju Y, Fu Y, Gong T, Zhang Z. Mechanism of enhanced oral absorption of morin by phospholipid complex based self-nanoemulsifying drug delivery system. Mol Pharm. 2015;12(2):504–513. | ||
Fofaria NM, Qhattal HS, Liu X, Srivastava SK. Nanoemulsion formulations for anti-cancer agent piplartine – characterization, toxicological, pharmacokinetics and efficacy studies. Int J Pharm. 2016;498(1–2):12–22. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.