Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 8
Bioavailability study of dronabinol oral solution versus dronabinol capsules in healthy volunteers
Authors Parikh N, Kramer WG, Khurana V, Cognata Smith C, Vetticaden S
Received 24 June 2016
Accepted for publication 16 August 2016
Published 12 October 2016 Volume 2016:8 Pages 155—162
DOI https://doi.org/10.2147/CPAA.S115679
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Neha Parikh,1 William G Kramer,2 Varun Khurana,1 Christina Cognata Smith,1 Santosh Vetticaden,1
1INSYS Therapeutics, Inc., Chandler, AZ, USA; 2Kramer Consulting LLC, North Potomac, MD, USA
Background: Dronabinol, a pharmaceutical Δ-9-tetrahydrocannabinol, was originally developed as an oral capsule. This study evaluated the bioavailability of a new formulation, dronabinol oral solution, versus a dronabinol capsule formulation.
Methods: In an open-label, four-period, single-dose, crossover study, healthy volunteers were randomly assigned to one of two treatment sequences (T-R-T-R and R-T-R-T; T = dronabinol 4.25 mg oral solution and R = dronabinol 5 mg capsule) under fasted conditions, with a minimum 7-day washout period between doses. Analyses were performed on venous blood samples drawn 15 minutes to 48 hours postdose, and dronabinol concentrations were assayed by liquid chromatography–tandem mass spectrometry.
Results: Fifty-one of 52 individuals had pharmacokinetic data for analysis. The 90% confidence interval of the geometric mean ratio (oral solution/capsule) for dronabinol was within the 80%–125% bioequivalence range for area under the plasma concentration–time curve (AUC) from time zero to last measurable concentration (AUC0–t) and AUC from time zero to infinity (AUC0–∞). Maximum plasma concentration was also bioequivalent for the two dronabinol formulations. Intraindividual variability in AUC0–∞ was >60% lower for dronabinol oral solution 4.25 mg versus dronabinol capsule 5 mg. Plasma dronabinol concentrations were detected within 15 minutes postdose in 100% of patients when receiving oral solution and in <25% of patients when receiving capsules.
Conclusion: Single-dose dronabinol oral solution 4.25 mg was bioequivalent to dronabinol capsule 5 mg under fasted conditions. Dronabinol oral solution formulation may provide an easy-to-swallow administration option with lower intraindividual variability as well as more rapid absorption versus dronabinol capsules.
Keywords: pharmacokinetics, Δ-9-tetrahydrocannabinol, safety, variability
Introduction
Dronabinol, a pharmaceutical formulation of Δ-9-tetrahydrocannabinol (Δ9-THC), is orally active (ie, oral administration results in adequate bioavailability to produce physiologic effects) and, similar to other cannabinoids, has complex effects on the central nervous system.1,2 Dronabinol has demonstrated efficacy in the treatment of anorexia associated with weight loss in patients with AIDS3,4 and nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments5–8 and has been approved by the US Food and Drug Administration (FDA) for these indications.
Dronabinol was originally developed as a soft gelatin capsule. In this capsule formulation, oral dronabinol is almost completely absorbed (90%–95%) after single dosing.9 However, only 10%–20% of oral dronabinol reaches the systemic circulation due to first-pass hepatic metabolism and high lipid solubility. In multiple-dose pharmacokinetic studies using healthy adults, the mean (standard deviation [SD]) maximum plasma concentration (Cmax) of oral dronabinol capsule 5 mg twice daily was 3.0 (1.8) ng/mL, the median time to Cmax (Tmax) was 2.5 hours (range, 0.5–4.0 hours), and the mean (SD) area under the plasma concentration–time curve (AUC) from time zero to 12 hours (AUC0–12) was 6.2 (1.8) h×ng/mL.9 However, high variability in the pharmacokinetic parameters of oral THC, including dronabinol capsules, has been demonstrated in studies of healthy adults.10–12 Dronabinol has been shown to undergo extensive first-pass hepatic metabolism yielding the major metabolite 11-OH-Δ9-THC that is pharmacologically active and has an AUC similar to that of the parent drug.9
The oral delivery of dronabinol via a capsule may be less than ideal, such as in those with nausea and vomiting associated with cancer chemotherapy or swallowing difficulties. Thus, an easy-to-swallow oral solution formulation of dronabinol has been developed to provide an alternative delivery method. This study was designed to evaluate the bioequivalence of dronabinol oral solution 4.25 mg versus dronabinol capsule 5 mg.
Methods
Participants
Males and females aged 18–55 years were eligible for the study if they were in good health as assessed by medical history, physical examination, and clinical laboratory investigations; had a body weight ≥50 kg and a body mass index of 19.0–29.9 kg/m2; and females were neither pregnant nor lactating. Individuals were excluded if they had used any prescription medicines (other than hormonal contraceptives) within 14 days, any over-the-counter medication within 7 days, or any vitamins or herbal supplements within 3 days before the first dose of study medication. Other exclusion criteria included marijuana use in the previous 90 days; smoking or other tobacco use in the previous 6 months; a history of mental illness, alcohol abuse, or physical dependence on any opioid, barbiturate, amphetamine, cocaine, or benzodiazepine in the past 10 years; or a positive screening test result for drugs of abuse. The study was conducted in accordance with the International Conference on Harmonisation principles of Good Clinical Practice and the Declaration of Helsinki. The study received institutional review board approval (IntegReview IRB, Austin, TX), and all patients provided written informed consent.
Study design and treatment
This randomized, open-label, two-treatment, four-period, two-sequence, single-dose, crossover study was conducted between September 2012 and October 2012 (ClinicalTrials.gov identifier: NCT01448772). Participants were randomly assigned to one of two treatment sequences: sequence 1 (T-R-T-R) and sequence 2 (R-T-R-T), where T was the test product (single oral dose of dronabinol oral solution 4.25 mg, lot number 100310 [Syndros]; INSYS Therapeutics, Inc., Phoenix, AZ, USA) and R was the reference product (single oral dose of dronabinol capsule 5 mg, lot number 277967A [Marinol®]; expiration date December 2013; AbbVie, Inc., North Chicago, IL, USA). Each dose was administered under fasted conditions (overnight; ≥10 hours) with 240 mL of water. Additional water was permitted as needed during the study except 1 hour predose through 1 hour postdose. Standardized meals were allowed starting 4 hours postdose. Each dose of study medication (test or reference product) was separated by a minimum 7-day washout period.
Assessments
Blood samples were collected by venipuncture before (predose) and at 0.25 hour, 0.5 hour, 0.75 hour, 1 hour, 1.5 hours, 2 hours, 2.5 hours, 3 hours, 3.5 hours, 4 hours, 6 hours, 8 hours, 12 hours, 16 hours, 24 hours, 36 hours, and 48 hours postdose. The plasma was harvested and frozen at –20°C until analysis. Plasma concentrations of dronabinol and the primary metabolite 11-OH-Δ9-THC were analyzed by Worldwide Clinical Trials Drug Development Solutions (Austin, TX, USA) using a validated liquid chromatography–tandem mass spectrometry method, with a range of 0.025–10.0 ng/mL for each analyte, based on the analysis of 0.500 mL of plasma. Adverse events (AEs) were monitored throughout the study.
Statistical analysis
The safety population included all patients who received ≥1 dose of study medication. The pharmacokinetic analysis population included all patients who completed the first two periods (T-R or R-T) within the sequence in which they were randomized. All statistical analyses were performed using SAS® (version 9.3; SAS Institute Inc., Cary, NC, USA).
Pharmacokinetic parameters for dronabinol and 11-OH-Δ9-THC were determined using standard noncompartmental methods. The Cmax and Tmax were determined using observed data. The terminal elimination rate constant, λz, was calculated as the negative of the slope of the terminal log-linear segment of the plasma concentration–time curve. Elimination half-life (t1/2) was calculated according to the following equation: t1/2 = 0.693/λz. AUC from time zero to the final sample with a concentration greater than or equal to the lower limits of quantitation (AUC0–t) was calculated using the linear trapezoidal method and extrapolated to infinity using the equation
AUC0–∞ = AUC0–t + Ctf/λz
in which Ctf was the final concentration that is greater than or equal to the lower limits of quantitation.
A linear mixed-effects model was used to compare dronabinol oral solution and dronabinol capsule formulations using natural log-transformed pharmacokinetic parameters (Cmax, AUC0–t, and AUC0–∞), uncorrected for dose, with sequence, treatment, period, and group as fixed effects and subject as a random effect. Geometric mean (GM) ratios (solution/capsule) were calculated for Cmax, AUC0–t, and AUC0–∞, and corresponding 90% confidence intervals (CIs) were determined using the two one-sided t-tests procedure13 on log-transformed data. The point estimates and confidence limits were back-transformed to the original scale. The two treatments were considered bioequivalent if the 90% CIs for Cmax, AUC0–t, and AUC0–∞ were within the range of 80%–125%. For products demonstrating high intraindividual variability, the bioequivalence acceptance limit is sometimes adjusted based on the intraindividual variability of the capsule (reference) formulation.14–16 In accordance with the FDA-recommended algorithm, if the intraindividual SDs (IISDs) for Cmax and/or AUC of the reference product, as observed in the bioequivalence study, is or exceeds the cutoff value of 0.294, then the bioequivalence limits for the test product are scaled based on the degree of variability of the reference product.16 A sample size of 60 individuals was determined to provide at least 80% power, a = 0.05, to determine that the 90% CI for the treatment comparison was between 80% and 125%, assuming a difference in intraindividual coefficient between formulations of ≤5% for Cmax.
A post hoc analysis evaluated intraindividual variability on pharmacokinetic parameters and time to detectable plasma dronabinol level for the oral solution and capsule formulations. The intraindividual coefficient of variation (CV) was calculated for each pharmacokinetic parameter according to 100× (IISD/LN[GM]), where LN was the natural logarithmic function.
Results
A total of 52 patients were included in the safety population and 51 patients were included in the pharmacokinetic analysis population. Twenty-eight (53.9%) of the 52 individuals were female, and 67.3% of individuals were white. The mean (SD) age was 33±10 years (range, 18–53 years) with a mean body mass index of 25.4±2.4 kg/m2 (range, 21.0–29.9 kg/m2). One participant withdrew prior to completion of the first two study periods and was excluded from the pharmacokinetic analysis population. Fifty of the 51 individuals in the pharmacokinetic analysis population completed all four periods (ie, received two doses each of test and reference products, with each dose separated by a 7-day washout period), and one participant withdrew after period 2.
Pharmacokinetic profile
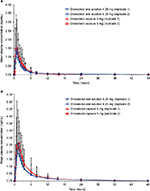
The mean plasma concentration–time curves of dronabinol and 11-OH-Δ9-THC are presented in Figure 1A and B, respectively. Pharmacokinetic parameters are summarized in Table 1. The median Tmax of dronabinol was shorter and the mean t1/2 was longer for dronabinol oral solution 4.25 mg versus dronabinol capsule 5 mg. For the primary active metabolite 11-OH-Δ9-THC, the median Tmax and mean t1/2 were similar for the two dronabinol formulations.
For dronabinol, the 90% CIs of the GM ratios for AUC0–t
and AUC0–∞ were within the bioequivalence range of 80%–125% (Table 2). The 90% CI for dronabinol Cmax fell outside the bioequivalence range of 80%–125%. Because the IISD for the Cmax of the reference product (0.364) was ≥0.294 (FDA criterion for a highly variable drug15,16) and the point estimate of the GM ratio of dronabinol Cmax (82.50%) was within the bioequivalence range, the bioequivalence of Cmax was evaluated using reference-scaled criteria. Using the reference-scaled approach, the point estimate of the GM ratio for dronabinol Cmax (81.96%) was within the range of 80%–125%, and the 95% upper confidence bound (–0.01) was ≤0, thereby meeting the FDA criteria for bioequivalence.15 For 11-OH-Δ9-THC (Table 2), the GM values for Cmax, AUC0–t, and AUC0–∞ were lower for dronabinol oral solution than for dronabinol capsule, and the lower limits of the 90% CIs of the GM ratios were <80%.
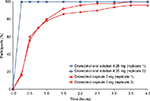
In a post hoc analysis of the intraindividual variability in dronabinol pharmacokinetic parameters (Table 3), the intraindividual CV for AUC0–∞ was lower for dronabinol oral solution 4.25 mg compared with dronabinol capsule 5 mg (13.5% versus 36.8%, respectively). Regarding Cmax, intraindividual variability was greater for dronabinol oral solution versus dronabinol capsule (CV, 66.3% versus 53.8%, respectively). A faster onset of detectable plasma concentrations was observed for dronabinol oral solution, with 100% of individuals with a detectable plasma dronabinol concentration at 15 minutes postdose when compared with 16.8% of individuals after administration of dronabinol capsule (Figure 2).
Adverse events
Single-dose administration of dronabinol oral solution 4.25 mg and dronabinol capsule 5 mg was generally well tolerated. A total of 90 AEs were reported by 25 individuals during the study, with 48.9% and 51.1% of AEs reported with dronabinol oral solution and dronabinol capsule, respectively. The most commonly reported AEs in the safety population were nausea (n=14; dronabinol oral solution, n=8, versus dronabinol capsule, n=6), dizziness (n=13; dronabinol oral solution, n=6, versus dronabinol capsule, n=7), somnolence (n=12; dronabinol oral solution, n=7, versus dronabinol capsule, n=5), and headache (n=11; dronabinol oral solution, n=7, versus dronabinol capsule, n=4). All AEs were considered mild (50%) or moderate (50%) in intensity.
Discussion
This comparative bioavailability study demonstrated the bioequivalence of single-dose dronabinol oral solution 4.25 mg to dronabinol capsule 5 mg in healthy volunteers, under fasted conditions. The 90% CI of the GM ratio (oral solution/capsule) was within the standard bioequivalence range of 80%–125% for AUC0–t and AUC0–∞. Cmax was comparable for the oral solution and capsule formulations based on FDA-recommended reference-scaled criteria. Intraindividual variability in total exposure (AUC0–∞) was >60% lower for the oral solution compared with capsules. In addition, faster absorption was observed for the oral solution formulation.
The bioequivalence of dronabinol oral solution with dronabinol capsules was established using FDA-recommended study design and methodology.17,18 For reference drugs identified as highly variable (ie, IISD ≥0.29 in a bioequivalence study), a reference-scaled approach is recommended.15,16 The IISD for dronabinol capsule 5 mg in the current study was >0.29 for dronabinol Cmax, AUC0–t, and AUC0–∞. Despite this variability, the 90% CIs for the GM ratios (oral solution/capsule) of dronabinol AUC0–t and AUC0–∞ were within the bioequivalence range (80%–125%). The point estimate for the Cmax GM ratio (82.5%) was within the bioequivalence range, but the lower limit of the 90% CI (74.6%) fell outside the range. Therefore, dronabinol Cmax for the oral solution was evaluated using a reference-scaled approach and met the FDA-recommended criteria for bioequivalence.15 For the primary active metabolite of dronabinol (11-OH-Δ9-THC), the mean plasma concentrations were slightly lower after administration of oral solution when compared with capsule and were considered indicative of the similarity between the oral solution and capsule products.
This post hoc analysis demonstrated important attributes for dronabinol oral solution relative to dronabinol capsules, with lower intraindividual CV for AUC0–∞ (13.5% versus 36.8%, respectively) and faster onset of detectable plasma concentrations in 100% and 16.8% of individuals, respectively, at 15 minutes postdose. Variability for Cmax was greater for dronabinol oral solution versus dronabinol capsule (66.3% versus 53.8%, respectively). The frequency of AEs was comparable between dronabinol oral solution and dronabinol capsule. Limitations of the study include that the study population was composed of only healthy adults; the pharmacokinetics of dronabinol may differ in patients with cancer or AIDS and in the presence of antineoplastic agents or other concomitant medications. In addition, this was a single-dose study and does not reflect the steady-state pharmacokinetics of dronabinol. Furthermore, because this study was conducted in healthy adults, additional studies may be needed to assess the clinical relevance of faster onset of detectable plasma concentrations and lower intraindividual variability in a patient population.
The efficacy of dronabinol (Δ9-THC) as an antiemetic for patients with chemotherapy-induced nausea and vomiting and as an appetite stimulant for patients with AIDS is supported by multiple studies of capsule formulations.3–5,7,8,19–24 Potential limitations of prior dronabinol formulations have included variable bioavailability and delayed absorption, which are important considerations that limit the optimal treatment of patients.25 Specifically, it has been noted with prior formulations, as stated by Lucas and Laszlo7 in 1980, that “Δ9-tetrahydrocannabinol is erratically absorbed from the gastrointestinal tract, and dosage individualization may be necessary to control these patients.” Consequently, intraindividual and interindividual variabilities are likely to have important implications for safety and efficacy with dronabinol products, as well as patient adherence. In this regard, the >60% lower intraindividual variability in absorption with the oral solution compared with the capsule formulation is noteworthy. Another important finding of this study was that detectable concentrations of dronabinol oral solution were observed in a substantially greater percentage of patients within 15 minutes postdose with dronabinol oral solution versus dronabinol capsule. The faster onset of detectable concentrations observed with dronabinol oral solution may be an additional consideration for physicians in selecting the appropriate dronabinol formulation for their patients. Furthermore, the oral solution may offer flexibility in dosing calculations in cancer settings (eg, as an antiemetic for patients undergoing chemotherapy, for which the recommended dronabinol dose is based on body surface area9).
Conclusion
This pharmacokinetic study demonstrated the bioequivalence of dronabinol oral solution 4.25 mg and dronabinol capsule 5 mg under fasted conditions in healthy volunteers. The attributes of the dronabinol oral solution formulation, including lower intraindividual variability and faster onset of detectable concentrations relative to dronabinol capsule, may be important considerations in the selection of the optimal formulation of dronabinol for the treatment of patients.
Acknowledgments
Technical editorial and medical writing assistance for the development of this article was provided by Mary Beth Moncrief, PhD, and Nancy Holland, PhD, Synchrony Medical Communications, LLC, West Chester, PA, USA. Funding for this support was provided by INSYS Therapeutics, Inc., which had a role in study design, supervision of data collection, analysis and interpretation of data, and reporting of results. Some of the content herein was published in a “Meeting Abstracts” supplement issue from The American Society of Clinical Oncology annual meeting: Journal of Clinical Oncology. 2016;34(15 suppl):e21593: http://meeting.ascopubs.org/cgi/content/abstract/34/15_suppl/e21593. The abstract was not presented at the meeting.
Disclosure
NP, VK, CCS, and SV are full-time employees of INSYS Therapeutics, Inc. WGK is a paid consultant to INSYS Therapeutics, Inc. The authors report no other conflicts of interest in this work.
References
Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005;168:1–51. | ||
Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327–360. | ||
Beal JE, Olson R, Lefkowitz L, et al. Long-term efficacy and safety of dronabinol for acquired immunodeficiency syndrome-associated anorexia. J Pain Symptom Manage. 1997;14(1):7–14. | ||
Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89–97. | ||
Lane M, Vogel CL, Ferguson J, et al. Dronabinol and prochlorperazine in combination for treatment of cancer chemotherapy-induced nausea and vomiting. J Pain Symptom Manage. 1991;6(6):352–359. | ||
Plasse TF, Gorter RW, Krasnow SH, Lane M, Shepard KV, Wadleigh RG. Recent clinical experience with dronabinol. Pharmacol Biochem Behav. 1991;40(3):695–700. | ||
Lucas VS Jr, Laszlo J. Delta 9-tetrahydrocannabinol for refractory vomiting induced by cancer chemotherapy. JAMA. 1980;243(12):1241–1243. | ||
Orr LE, McKernan JF, Bloome B. Antiemetic effect of tetrahydrocannabinol. Compared with placebo and prochlorperazine in chemotherapy-associated nausea and emesis. Arch Intern Med. 1980;140(11):1431–1433. | ||
Marinol® (dronabinol) capsules [package insert]. North Chicago, IL: AbbVie, Inc.; 2015. | ||
Wall ME, Perez-Reyes M. The metabolism of delta 9-tetrahydrocannabinol and related cannabinoids in man. J Clin Pharmacol. 1981;21(8–9 suppl):178S–189S. | ||
Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R. The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain. 2003;105(1–2):79–88. | ||
Ahmed AI, van den Elsen GA, Colbers A, et al. Safety and pharmacokinetics of oral delta-9-tetrahydrocannabinol in healthy older subjects: a randomized controlled trial. Eur Neuropsychopharmacol. 2014;24(9):1475–1482. | ||
Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15(6):657–680. | ||
Haidar SH, Davit B, Chen ML, et al. Bioequivalence approaches for highly variable drugs and drug products. Pharm Res. 2008;25(1):237–241. | ||
US Department of Health and Human Services, US Food and Drug Administration, Center for Drug Evaluation and Research [webpage on the Internet]. Draft Guidance on Progesterone. 2011. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM209294.pdf. Accessed March 15, 2016. | ||
Davit BM, Chen ML, Conner DP, et al. Implementation of a reference-scaled average bioequivalence approach for highly variable generic drug products by the US Food and Drug Administration. AAPS J. 2012;14(4):915–924. | ||
US Department of Health and Human Services, US Food and Drug Administration, Center for Drug Evaluation and Research [webpage on the Internet]. Draft Guidance on Dronabinol. 2014. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm199636.pdf. Accessed March 15, 2016. | ||
US Department of Health and Human Services, US Food and Drug Administration, Center for Drug Evaluation and Research (CDER) [webpage on the Internet]. Guidance for Industry Bioavailability and Bioequivalance Studies Submitted in NDAs or INDs – General Considerations. 2014. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm389370.pdf. Accessed March 15, 2016. | ||
Ungerleider JT, Andrysiak T, Fairbanks L, Goodnight J, Sarna G, Jamison K. Cannabis and cancer chemotherapy: a comparison of oral delta-9-THC and prochlorperazine. Cancer. 1982;50(4):636–645. | ||
Ekert H, Waters KD, Jurk IH, Mobilia J, Loughnan P. Amelioration of cancer chemotherapy-induced nausea and vomiting by delta-9-tetrahydrocannabinol. Med J Aust. 1979;2(12):657–659. | ||
Frytak S, Moertel CG, O’Fallon JR, et al. Delta-9-tetrahydrocannabinol as an antiemetic for patients receiving cancer chemotherapy. A comparison with prochlorperazine and a placebo. Ann Intern Med. 1979;91(6):825–830. | ||
Kluin-Neleman JC, Neleman FA, Meuwissen OJ, Maes RA. delta 9-tetrahydrocannabinol (THC) as an antiemetic in patients treated with cancerchemotherapy; a double-blind cross-over trial against placebo. Vet Hum Toxicol. 1979;21(5):338–340. | ||
Sallan SE, Cronin C, Zelen M, Zinberg NE. Antiemetics in patients receiving chemotherapy for cancer: a randomized comparison of delta-9-tetrahydrocannabinol and prochlorperazine. N Engl J Med. 1980;302(3):135–138. | ||
Sallan SE, Zinberg NE, Frei EI. Antiemetic effect of delta-9-tetrahydrocannabinol in patients receiving cancer chemotherapy. N Engl J Med. 1975;293(16):795–797. | ||
Chang AE, Shiling DJ, Stillman RC, et al. Delta-9-tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate: a prospective, randomized evaluation. Ann Intern Med. 1979;91(6):819–824. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.